Development of Neurogenic Detrusor Overactivity after Thoracic Spinal Cord Injury Is Accompanied by Time-Dependent Changes in Lumbosacral Expression of Axonal Growth Regulators
Abstract
:1. Introduction
2. Results
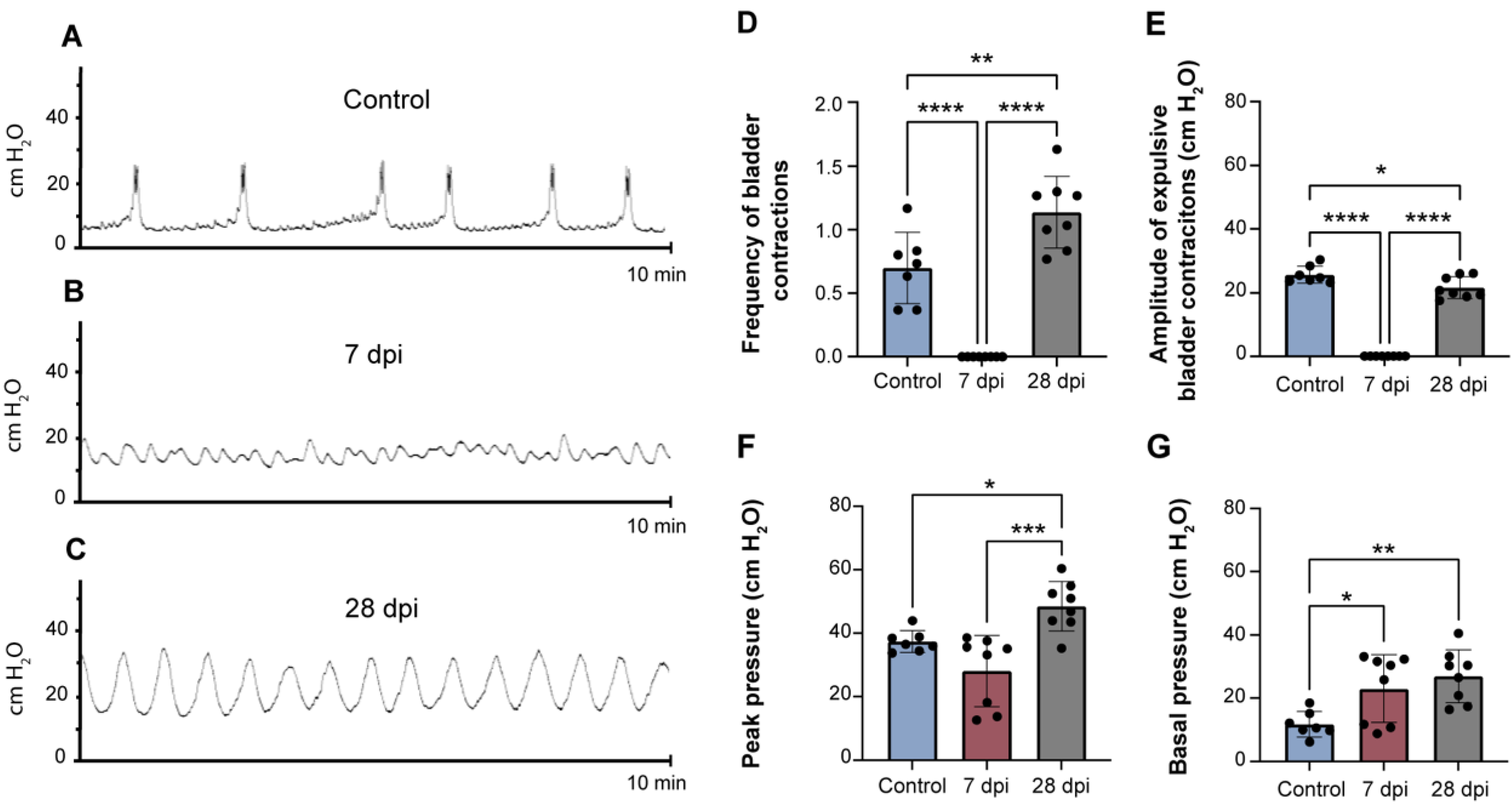
2.1. Spinal Cord Transection Induces Urinary Dysfunction
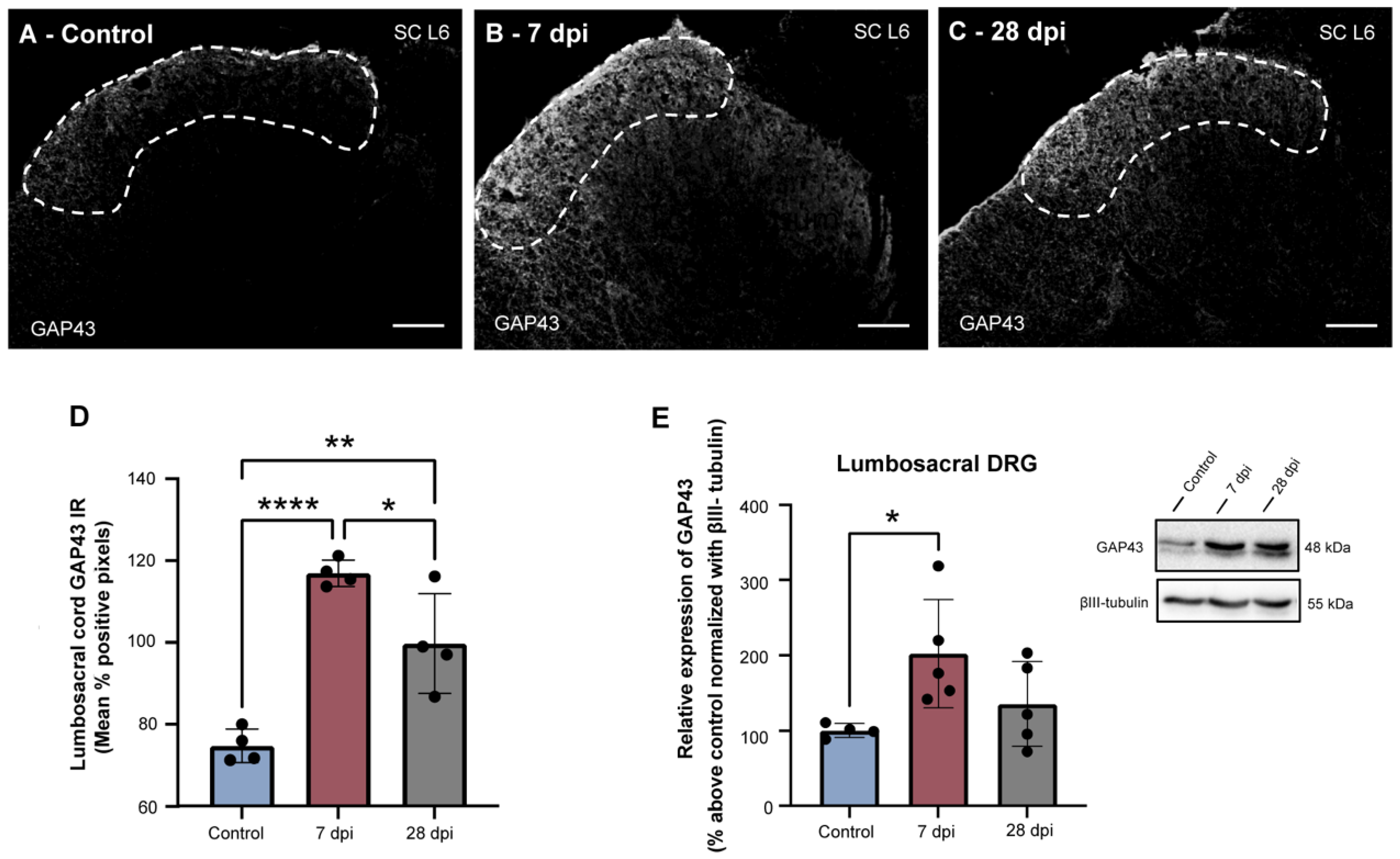
2.2. SCT-Induced Urinary Dysfunction Is Accompanied by Increased Spinal Expression of GAP43
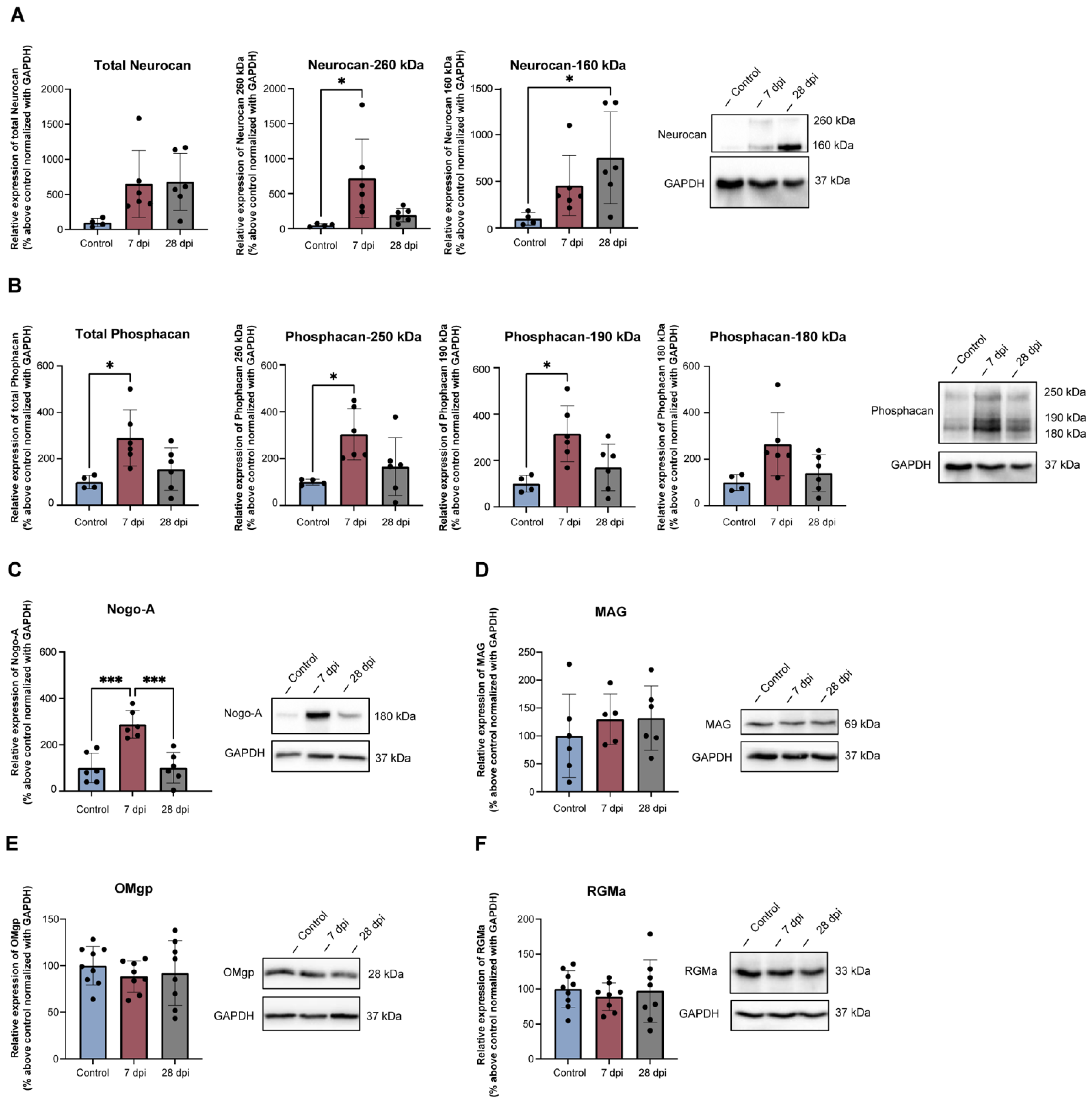
2.3. Thoracic SCT Induces Changes in Lumbosacral Expression of Axonal Growth Regulators
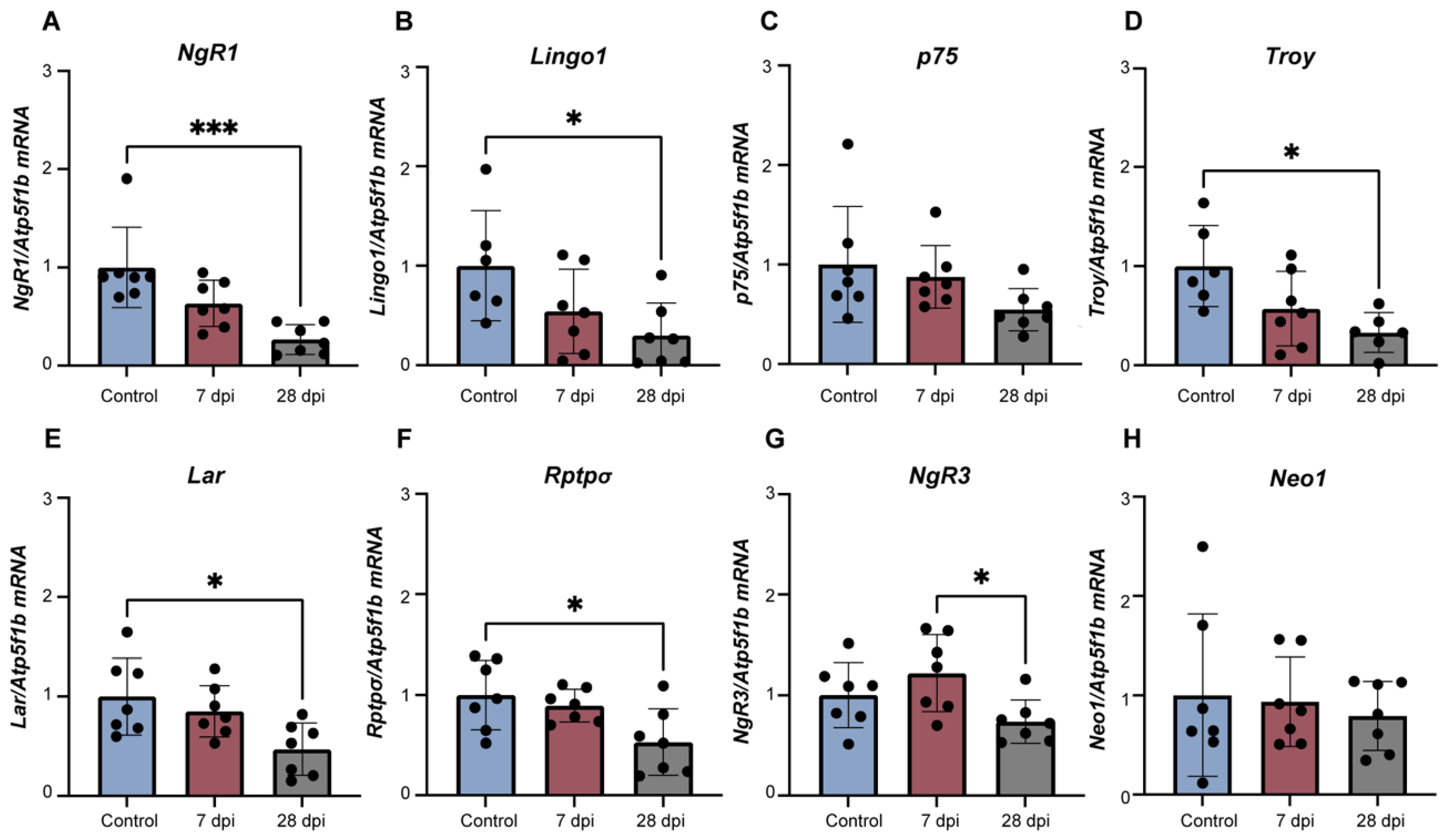
2.4. Alterations in Lumbosacral Expression of Axonal Growth Regulators Is Accompanied by DRG Changes in Levels of Specific Receptors
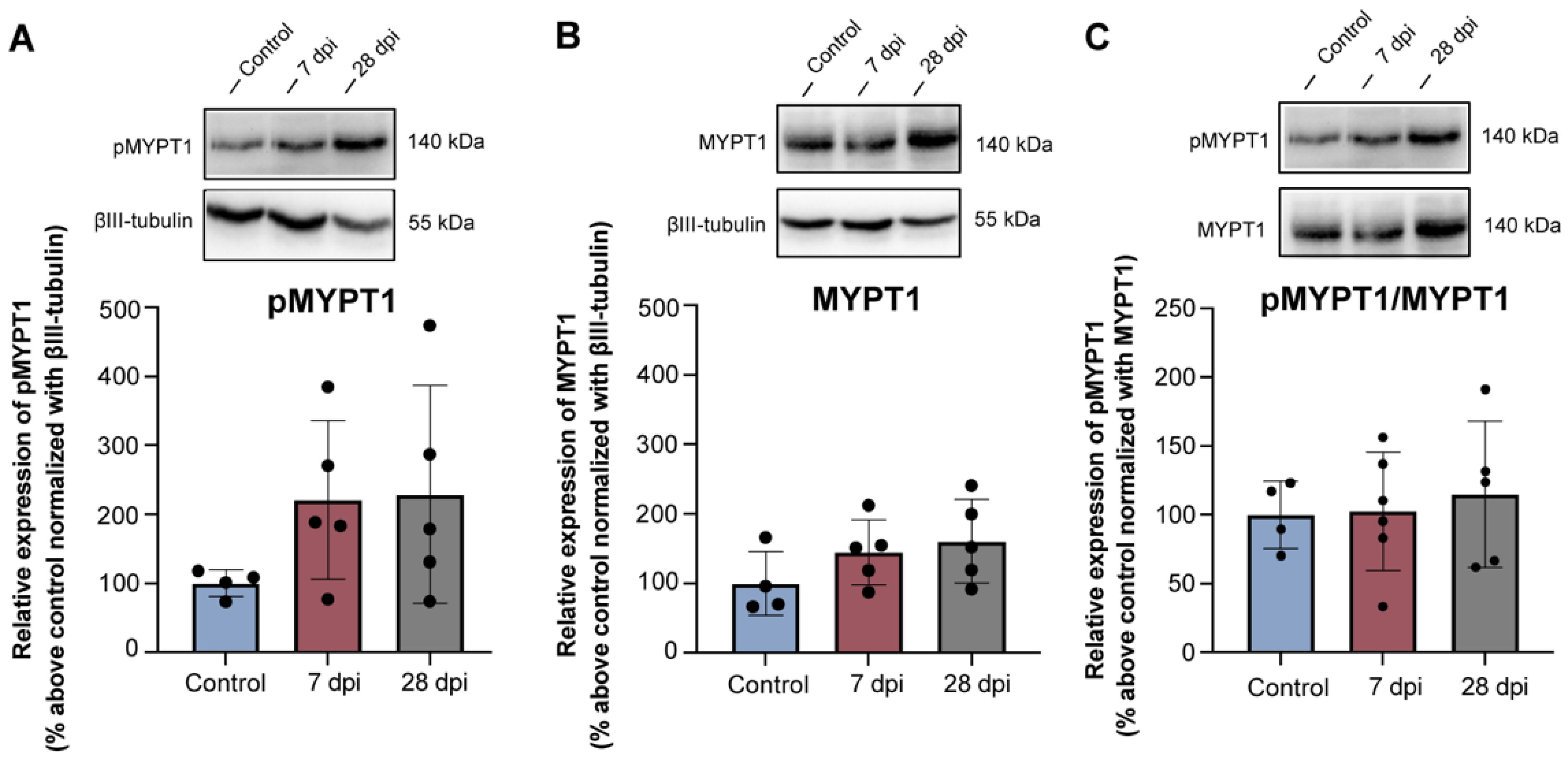
2.5. Rhoa/Rock Module Is Not Altered in Lumbosacral Drg Neurons following Thoracic Spinal Transection
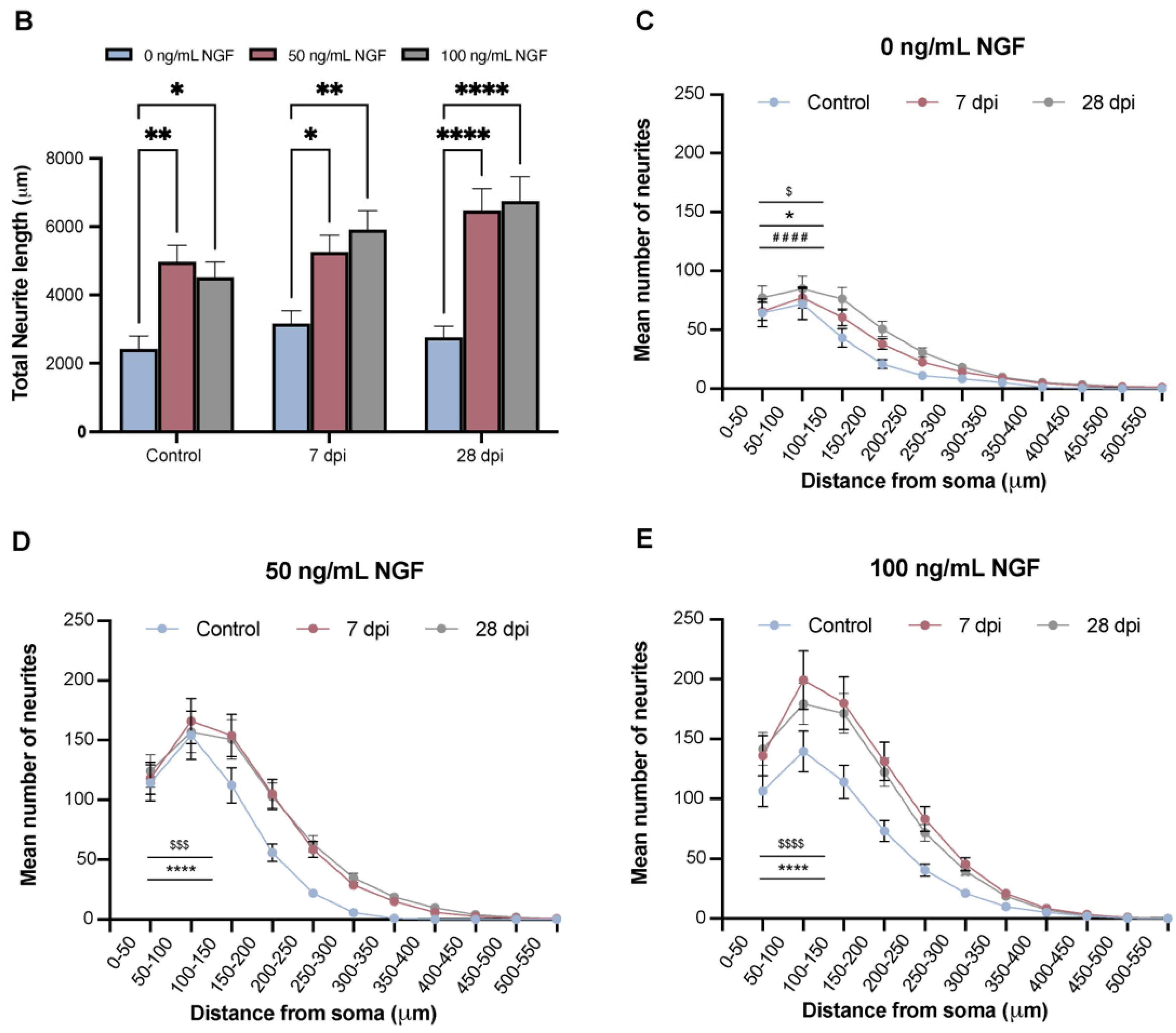
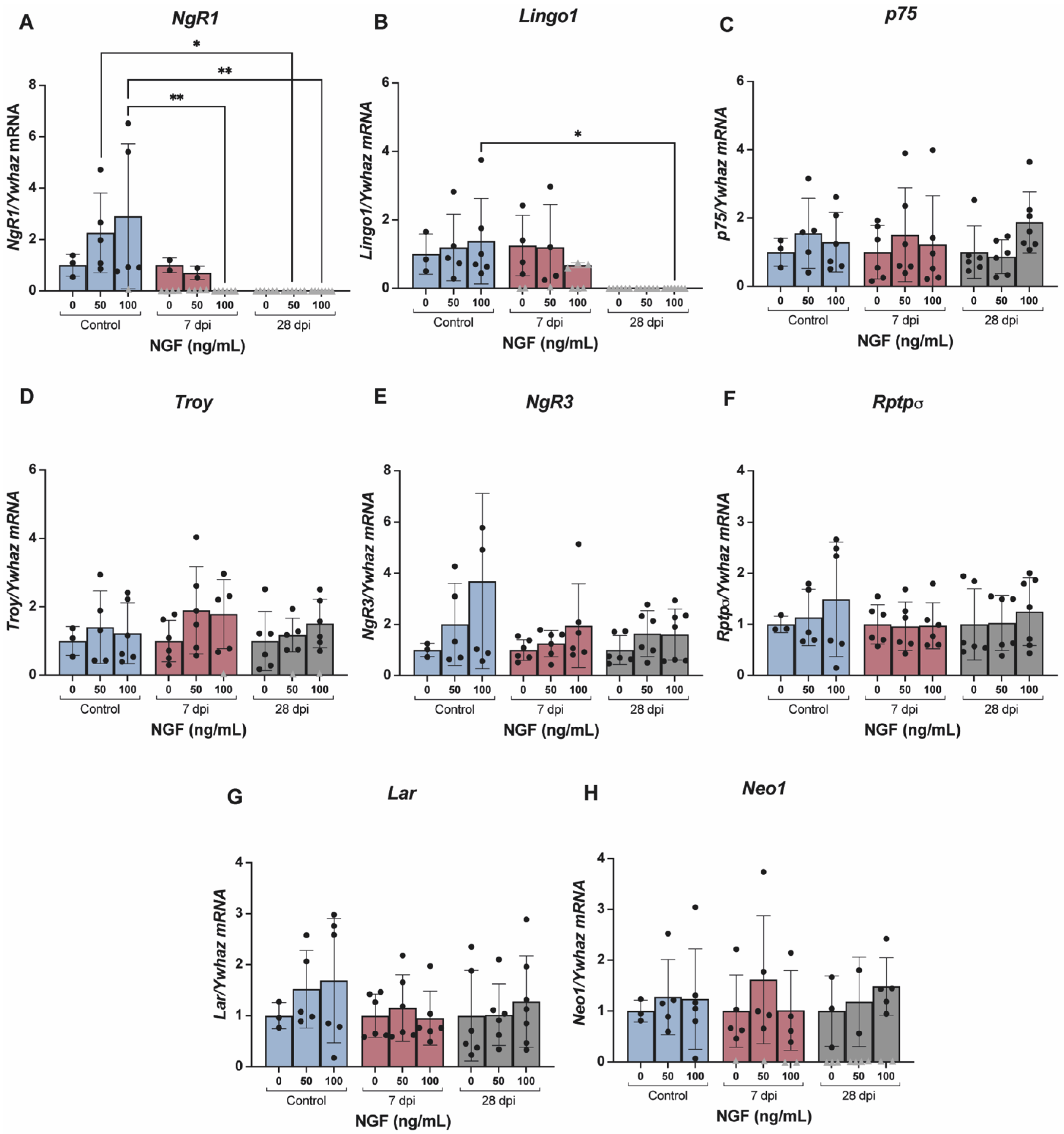
2.6. NGF Regulates the Expression of Receptors for Axonal Guidance Cues in DRG Neurons
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Chemicals and Reagents
4.3. Spinal Cord Transection
4.4. Cystometries
4.5. Western Blotting Analysis
4.6. Perfusion and Tissue Processing
4.7. Immunohistochemistry Assays
4.8. RNA Extraction and cDNA Synthesis
4.9. Quantitative Real-Time PCR (qPCR)
4.10. Primary DRG Cell Culture
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaudet, A.D.; Popovich, P.G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003, 4, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galtrey, C.M.; Fawcett, J. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007, 54, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Snow, D.M.; Mullins, N.; Hynds, D.L. Nervous system-derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: A light, epifluorescence, and electron microscopy study. Microsc. Res. Technol. 2001, 54, 273–286. [Google Scholar] [CrossRef]
- Massey, J.M.; Amps, J.; Viapiano, M.S.; Matthews, R.T.; Wagoner, M.R.; Whitaker, C.M.; Alilain, W.; Yonkof, A.L.; Khalyfa, A.; Cooper, N.G.; et al. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp. Neurol. 2008, 209, 426–445. [Google Scholar] [CrossRef] [Green Version]
- Mironova, Y.A.; Giger, R.J. Where no synapses go: Gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci. 2013, 36, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef] [Green Version]
- Fisher, D.; Xing, B.; Dill, J.; Li, H.; Hoang, H.H.; Zhao, Z.; Yang, X.-L.; Bachoo, R.; Cannon, S.; Longo, F.M.; et al. Leukocyte Common Antigen-Related Phosphatase Is a Functional Receptor for Chondroitin Sulfate Proteoglycan Axon Growth Inhibitors. J. Neurosci. 2011, 31, 14051–14066. [Google Scholar] [CrossRef] [Green Version]
- Dickendesher, T.L.; Baldwin, K.; Mironova, Y.A.; Koriyama, Y.; Raiker, S.J.; Askew, K.L.; Wood, A.; Geoffroy, C.G.; Zheng, B.; Liepmann, C.D.; et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 2012, 15, 703–712. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; Van Der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef] [PubMed]
- McKerracher, L.; David, S.; Jackson, D.; Kottis, V.; Dunn, R.; Braun, P. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 1994, 13, 805–811. [Google Scholar] [CrossRef]
- Wang, K.C.; Koprivica, V.; Kim, J.A.; Sivasankaran, R.; Guo, Y.; Neve, R.L.; He, Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 2002, 417, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Mikol, D.D.; Stefansson, K. A phosphatidylinositol-linked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes. J. Cell Biol. 1988, 106, 1273–1279. [Google Scholar] [CrossRef] [Green Version]
- Caroni, P.; Schwab, M.E. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 1988, 106, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Caroni, P.; Schwab, M.E. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 1988, 1, 85–96. [Google Scholar] [CrossRef]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef]
- Fournier, A.E.; Grandpre, T.; Strittmatter, S. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001, 409, 341–346. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Shao, Z.; Thill, G.; Ji, B.; Relton, J.K.; Levesque, M.; Allaire, N.; Perrin, S.; Sands, B.; et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004, 7, 221–228. [Google Scholar] [CrossRef]
- Wang, K.C.; Kim, J.A.; Sivasankaran, R.; Segal, R.; He, Z. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 2002, 420, 74–78. [Google Scholar] [CrossRef]
- Shao, Z.; Browning, J.L.; Lee, X.; Scott, M.L.; Shulga-Morskaya, S.; Allaire, N.; Thill, G.; Levesque, M.; Sah, D.; McCoy, J.M.; et al. TAJ/TROY, an Orphan TNF Receptor Family Member, Binds Nogo-66 Receptor 1 and Regulates Axonal Regeneration. Neuron 2005, 45, 353–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.B.; Yiu, G.; Kaneko, S.; Wang, J.; Chang, J.; He, X.L.; Garcia, K.C.; He, Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron 2005, 45, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Kempf, A.; Tews, B.; Arzt, M.E.; Weinmann, O.; Obermair, F.J.; Pernet, V.; Zagrebelsky, M.; Delekate, A.; Iobbi, C.; Zemmar, A.; et al. The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity. PLoS Biol. 2014, 12, e1001763. [Google Scholar] [CrossRef] [PubMed]
- Josephson, A.; Trifunovski, A.; Schéele, C.; Widenfalk, J.; Wahlestedt, C.; Brené, S.; Olson, L.; Spenger, C. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res. 2003, 311, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.E.; Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 2014, 27, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Zagrebelsky, M.; Lonnemann, N.; Fricke, S.; Kellner, Y.; Preuß, E.; Michaelsen-Preusse, K.; Korte, M. Nogo-A regulates spatial learning as well as memory formation and modulates structural plasticity in the adult mouse hippocampus. Neurobiol. Learn. Mem. 2017, 138, 154–163. [Google Scholar] [CrossRef]
- Quarles, R.H. Myelin-associated glycoprotein (MAG): Past, present and beyond. J. Neurochem. 2007, 100, 1431–1448. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Lopez, P.H. Myelin-associated glycoprotein and its axonal receptors. J. Neurosci. Res. 2009, 87, 3267–3276. [Google Scholar] [CrossRef] [Green Version]
- Vourc’h, P.; Andres, C. Oligodendrocyte myelin glycoprotein (OMgp): Evolution, structure and function. Brain Res. Rev. 2004, 45, 115–124. [Google Scholar] [CrossRef]
- Lee, J.K.; Case, L.C.; Chan, A.F.; Zhu, Y.; Tessier-Lavigne, M.; Zheng, B. Generation of an OMgp allelic series in mice. Genesis 2009, 47, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafferty, W.B.J.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp Synergize with Nogo-A to Restrict Axonal Growth and Neurological Recovery after Spinal Cord Trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Case, L.C.; Liu, K.; Shao, Z.; Lee, X.; Yang, Z.; Wang, J.; Tian, T.; Shulga-Morskaya, S.; Scott, M.; et al. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol. Cell. Neurosci. 2008, 39, 258–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giger, R.J.; Hollis, E.R., 2nd; Tuszynski, M.H. Guidance molecules in axon regeneration. Cold Spring Harb. Perspect. Biol. 2010, 2, a001867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, E.R. Axon Guidance Molecules and Neural Circuit Remodeling After Spinal Cord Injury. Neurother. J. Am. Soc. Exp. Neurother. 2016, 13, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, J.M.; Conrad, S.; Monnier, P.P.; Julien, S.; Mueller, B.K.; Schluesener, H.J. Spinal cord injury-induced lesional expression of the repulsive guidance molecule (RGM). Eur. J. Neurosci. 2005, 21, 1569–1576. [Google Scholar] [CrossRef]
- Mothe, A.J.; Tassew, N.G.; Shabanzadeh, A.P.; Penheiro, R.; Vigouroux, R.; Huang, L.; Grinnell, C.; Cui, Y.-F.; Fung, E.; Monnier, P.; et al. RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci. Rep. 2017, 7, 10529. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Deitinghoff, L.; Davis, D.; Conrad, S.; Skutella, T.; Chedotal, A.; Mueller, B.K.; Strittmatter, S.M. Neogenin mediates the action of repulsive guidance molecule. Nature 2004, 6, 756–762. [Google Scholar] [CrossRef]
- Tassew, N.G.; Charish, J.; Seidah, N.G.; Monnier, P.P. SKI-1 and Furin Generate Multiple RGMa Fragments that Regulate Axonal Growth. Dev. Cell 2012, 22, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T. Neogenin is a Determining Factor for Regenerating Neurons Following Spinal Cord Injury. Neuroscience 2019, 408, 448–449. [Google Scholar] [CrossRef]
- Andrews, E.M.; Richards, R.J.; Yin, F.Q.; Viapiano, M.; Jakeman, L.B. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp. Neurol. 2012, 235, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Collinger, J.L.; Boninger, M.; Bruns, T.; Curley, K.; Wang, W.; Weber, D.J. Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J. Rehabil. Res. Dev. 2013, 50, 145–160. [Google Scholar] [CrossRef]
- Frias, B.; Santos, J.; Morgado, M.; Sousa, M.; Gray, S.M.; McCloskey, K.D.; Allen, S.; Cruz, F.; Cruz, C.D. The role of brain-derived neurotrophic factor (BDNF) in the development of neurogenic detrusor overactivity (NDO). J. Neurosci. 2015, 35, 2146–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizzard, M.A. Alterations in growth-associated protein (GAP-43) expression in lower urinary tract pathways following chronic spinal cord injury. Somatosens. Mot. Res. 1999, 16, 369–381. [Google Scholar] [CrossRef]
- Zinck, N.; Rafuse, V.; Downie, J. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 2007, 204, 777–790. [Google Scholar] [CrossRef]
- Oliveira, R.; Coelho, A.; Franquinho, F.; Sousa, M.M.; Cruz, F.; Cruz, C.D. Effects of early intravesical administration of resiniferatoxin to spinal cord-injured rats in neurogenic detrusor overactivity. Neurourol. Urodyn. 2019, 38, 1540–1550. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef]
- Schwab, M.E. Structural plasticity of the adult CNS. Negative control by neurite growth inhibitory signals. Int. J. Dev. Neurosci. 1996, 14, 379–385. [Google Scholar] [CrossRef]
- De Biasi, S.; Rustioni, A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc. Natl. Acad. Sci. USA 1988, 85, 7820–7824. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.; Nadelhaft, I.; de Groat, W.C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J. Comp. Neurol. 1981, 201, 415–440. [Google Scholar] [CrossRef]
- Sami, A.; Selzer, M.E.; Li, S. Advances in the Signaling Pathways Downstream of Glial-Scar Axon Growth Inhibitors. Front. Cell. Neurosci. 2020, 14, 174. [Google Scholar] [CrossRef]
- Amano, M.; Chihara, K.; Nakamura, N.; Fukata, Y.; Yano, T.; Shibata, M.; Ikebe, M.; Kaibuchi, K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 1998, 3, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.; Ricci, M.-J.; Weaver, L.C. NGF mRNA is expressed in the dorsal root ganglia after spinal cord injury in the rat. Exp. Neurol. 2007, 205, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Furukawa, S.; Nitta, A.; Furukawa, Y. Accumulation of nerve growth factor protein at both rostral and caudal stumps in the transected rat spinal cord. J. Neurol. Sci. 2002, 198, 63–69. [Google Scholar] [CrossRef]
- Seki, S.; Sasaki, K.; Fraser, M.O.; Igawa, Y.; Nishizawa, O.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. Immunoneutralization of Nerve Growth Factor in Lumbosacral Spinal Cord Reduces Bladder Hyperreflexia in Spinal Cord Injured Rats. J. Urol. 2002, 168, 2269–2274. [Google Scholar] [CrossRef]
- Gundersen, R.W.; Barrett, J.N. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J. Cell Biol. 1980, 87, 546–554. [Google Scholar] [CrossRef]
- Gallo, G.; Lefcort, F.B.; Letourneau, P.C. The trkA Receptor Mediates Growth Cone Turning toward a Localized Source of Nerve Growth Factor. J. Neurosci. 1997, 17, 5445–5454. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Majima, T.; Suzuki, T.; Shimizu, N.; Wada, N.; Kadekawa, K.; Takai, S.; Takaoka, E.; Kwon, J.; Kanai, A.J.; et al. Nerve growth factor-dependent hyperexcitability of capsaicin-sensitive bladder afferent neurones in mice with spinal cord injury. Exp. Physiol. 2018, 103, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Shimizu, T.; Shimizu, N.; de Groat, W.C.; Kanai, A.J.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. The effect of neutralization of nerve growth factor (NGF) on bladder and urethral dysfunction in mice with spinal cord injury. Neurourol. Urodyn. 2018, 37, 1889–1896. [Google Scholar] [CrossRef]
- Anderson, K.D. Targeting Recovery: Priorities of the Spinal Cord-Injured Population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2006, 152, 59–84. [Google Scholar]
- Johnson, P.W.; Abramow-Newerly, W.; Seilheimer, B.; Sadoul, R.; Tropak, M.B.; Arquint, M.; Dunn, R.J.; Schachner, M.; Roder, J.C. Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron 1989, 3, 377–385. [Google Scholar] [CrossRef]
- Shen, Y.J.; DeBellard, M.E.; Salzer, J.L.; Roderc, J.; Filbin, M.T. Myelin-Associated Glycoprotein in Myelin and Expressed by Schwann Cells Inhibits Axonal Regeneration and Branching. Mol. Cell. Neurosci. 1998, 12, 79–91. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.; Kawatani, M.; Hisamitsu, T.; Cheng, C.-L.; Ma, C.-P.; Thor, K.; Steers, W.; Roppolo, J. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990, 30, S71–S77. [Google Scholar] [CrossRef]
- Spillane, M.; Gallo, G. Involvement of Rho-family GTPases in axon branching. Small GTPases 2014, 5, e27974. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.L.; Leo-Macias, A.; Yuen, S.; Khatri, L.; Pfennig, S.; Zhang, Y.; Agullo-Pascual, E.; Caillol, G.; Zhu, M.-S.; Rothenberg, E.; et al. Localized Myosin II Activity Regulates Assembly and Plasticity of the Axon Initial Segment. Neuron 2018, 97, 555–570.e6. [Google Scholar] [CrossRef] [Green Version]
- Dill, J.; Wang, H.; Zhou, F.; Li, S. Inactivation of Glycogen Synthase Kinase 3 Promotes Axonal Growth and Recovery in the CNS. J. Neurosci. 2008, 28, 8914–8928. [Google Scholar] [CrossRef] [PubMed]
- Liz, M.A.; Mar, F.M.; Santos, T.E.; Pimentel, H.I.; Marques, A.M.; Morgado, M.M.; Vieira, S.; Sousa, V.F.; Pemble, H.; Wittmann, T.; et al. Neuronal deletion of GSK3beta increases microtubule speed in the growth cone and enhances axon regeneration via CRMP-2 and independently of MAP1B and CLASP2. BMC Biol. 2014, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Grill, R.; Blesch, A.; Tuszynski, M. Robust Growth of Chronically Injured Spinal Cord Axons Induced by Grafts of Genetically Modified NGF-Secreting Cells. Exp. Neurol. 1997, 148, 444–452. [Google Scholar] [CrossRef]
- Ramer, M.S.; Priestley, J.V.; McMahon, S. Functional regeneration of sensory axons into the adult spinal cord. Nature 2000, 403, 312–316. [Google Scholar] [CrossRef]
- Krenz, N.R.; Weaver, L.C. Nerve Growth Factor in Glia and Inflammatory Cells of the Injured Rat Spinal Cord. J. Neurochem. 2000, 74, 730–739. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A.A.; Smith, G.M.; Randall, D.C.; Brown, D.R.; Rabchevsky, A.G. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J. Neurosci. 2006, 26, 2923–2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, N.; Bennett, N.E.; Hayashi, Y.; Ogawa, T.; Nishizawa, O.; Chancellor, M.B.; De Groat, W.C.; Seki, S. Bladder Overactivity and Hyperexcitability of Bladder Afferent Neurons after Intrathecal Delivery of Nerve Growth Factor in Rats. J. Neurosci. 2006, 26, 10847–10855. [Google Scholar] [CrossRef] [PubMed]
- Krenz, N.R.; Meakin, S.; Krassioukov, A.V.; Weaver, L.C. Neutralizing Intraspinal Nerve Growth Factor Blocks Autonomic Dysreflexia Caused By Spinal Cord Injury. J. Neurosci. 1999, 19, 7405–7414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, N.; Chao, S.; Goritchenko, L.; Horiuchi, A.; Greengard, P.; Nairn, A.C.; Benowitz, L.I. Nerve growth factor controls GAP-43 mRNA stability via the phosphoprotein ARPP-19. Proc. Natl. Acad. Sci. USA 2002, 99, 12427–12431. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.-Q.; Walzer, M.; Wu, Y.-H.; Zhou, J.; Dedhar, S.; Snider, W.D. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J. Cell Sci. 2006, 119, 2787–2796. [Google Scholar] [CrossRef] [Green Version]
- Gavazzi, I.; Kumar, R.D.C.; McMahon, S.B.; Cohen, J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur. J. Neurosci. 1999, 11, 3405–3414. [Google Scholar] [CrossRef]
- Fornaro, M.; Giovannelli, A.; Foggetti, A.; Muratori, L.; Geuna, S.; Novajra, G.; Perroteau, I. Role of neurotrophic factors in enhancing linear axonal growth of ganglionic sensory neurons in vitro. Neural Regen. Res. 2020, 15, 1732–1739. [Google Scholar] [CrossRef]
- Kimpinski, K.; Campenot, R.B.; Mearow, K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J. Neurobiol. 1997, 33, 395–410. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Hua, F.; Zhuang, Y.; Liu, H.; Wang, S. EphA4 Obstructs Spinal Cord Neuron Regeneration by Promoting Excessive Activation of Astrocytes. Cell. Mol. Neurobiol. 2022, 42, 1557–1568. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Serrão, P.; Martins, I.; Tavares, I. Serotoninergic pain modulation from the rostral ventromedial medulla (RVM) in chemotherapy-induced neuropathy: The role of spinal 5-HT3 receptors. Eur. J. Neurosci. 2020, 51, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.K.; Hjorth, J.J.; Joemai, R.M.; Wijntjes, R.; Eijgenraam, S.; de Bruijn, P.; Georgiou, C.; de Jong, A.P.; van Ooyen, A.; Verhage, M.; et al. Automated analysis of neuronal morphology, synapse number and synaptic recruitment. J. Neurosci. Methods 2011, 195, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Primary Antibody Name | Catalogue # | Company | MW | Source | Dilution for WB | Dilution for IHC/ICC |
|---|---|---|---|---|---|---|
| Nogo-A | 11C7 | Kindly gifted by Prof. Martin Schwab | 180 kDa | Mouse | 1:1000 | 1:1000 |
| Phosphacan | MAB5210 | Merck Millipore | 180, 190 and 250 kDa | Mouse | 1:1000 | 1:1000 |
| Neurocan | MAB5212 | Merck Millipore | 245, 160, 130 and 90 kDa | Mouse | 1:1000 | 1:1000 |
| Omgp | ab109746 | Abcam | 28 kDa | Rabbit | 1:5000 | - |
| Mag | ab203060 | Abcam | 69 kDa | Rabbit | 1:1000 | - |
| Rgma | 28045 | IBL Japan | 28 kDa | Rabbit | 1:500 | - |
| GAP43 | ab16053 | Abcam | 43–48 kDa | Rabbit | 1:2000 | 1:2000 |
| GAPDH | ab8245 | Abcam | 37 kDa | Mouse | 1:10,000 | - |
| βIII tubulin | 302302 | Synaptic Systems | 55 kDa | Rabbit | 1:1000 | 1:1000 |
| MYPT1 | #2634 | Cell Signalling | 140 kDa | Rabbit | 1:1000 | - |
| Phospho-MYPT1 (Thr696) | #5163 | Cell Signalling | 140 kDa | Rabbit | 1:1000 | - |
| Secondary Antibody Name | Catalogue # | Company | Source | Dilution for WB | Dilution for IHC/ICC |
|---|---|---|---|---|---|
| Alexa Fluor™ 568 Donkey Anti-Rabbit IgG | A10042 | Invitrogen | Donkey | - | 1:1000 |
| Alexa Fluor™ 488 Donkey Anti-Rabbit IgG | A21206 | Invitrogen | Donkey | - | 1:1000 |
| Alexa Fluor™ 568 Donkey Anti-Mouse IgG | A10037 | Invitrogen | Donkey | - | 1:1000 |
| Goat Anti-Rabbit IgG (HRP) | ab6721 | Abcam | Goat | 1:10,000 | 1:1000 |
| Goat Anti-Mouse IgG (HRP) | ab205719 | Abcam | Goat | 1:10,000 | - |
| Gene Name | Gene Bank Accession Number | Primer Sequence (5′→3′) | Annealing Temperature (°C) |
|---|---|---|---|
| Atp5f1b | NM_134364.1 | Forward: TGC TAT TGC TGA GTT GGG CA Reverse: GGA GAT GGT CAT AGT CAC CTGC | 60 |
| Gap43 | NM_017195.3 | Forward: ACC ACT GAT AAC TCG CCG TC Reverse: TGG CTT CAT CTA CAG CTT CTT TCT | 60 |
| Lar (Ptprf) | NM_019249.1 | Forward: CTC CCA GCG CTT TGA GGT AA Reverse: GAG CCT TCT CCA CCA CCT TC | 59 |
| Lingo1 | NM_001100722.1 | Forward: CTC CTG AGC TCC TGC CCT A Reverse: CTC TCG CTA CCT GCA TCT C | 60 |
| Neo1 | XM_008766432.1 | Forward: GTT GCT AGG GAG CGT GTT GA Reverse: GTA GGC CAC CAC TCG GAA AC | 60 |
| NgR1 (Rtn4r) | NM_053613.1 | Forward: CGA AGA TGA AGA GGG CGT CC Reverse: TAG TTG CTC CAG GAG GGT CA | 60 |
| NgR3 (Rtn4rl1) | NM_181377.3 | Forward: GGG ATT TGA ATC TGG ACC CCA Reverse: CAA TTC CAC ACA GCA CCC TTT G | 59 |
| P75NTR (Ngfr) | NM_012610.2 | Forward: TGA CTC TCC ATT TGG GAC TTT Reverse: TAC GGG TGC GGT TCT TTC | 60 |
| RPTPRσ (Ptprs) | NM_019140.2 | Forward: CAG ACG CCA GGT ATG CTC AG Reverse: ATA GGC GAT GAC ATT GGC GT | 60 |
| Troy (Tnfrsf19) | NM_001044229.2 | Forward: TCT GCA AAC AGT GTG GAC CT Reverse: TCT TCC GGT AAA ATC CTG GCA | 58 |
| Ywhaz | NM_013011.4 | Forward: ACC CAC TCC GGA CAC AGA ATA Reverse: TCG AGA CGA CCC TCC AAG AT | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambel, S.S.; Ferreira, A.; Oliveira, R.; Miranda, R.; Vale, L.; Reguenga, C.; Schwab, M.E.; Cruz, C.D. Development of Neurogenic Detrusor Overactivity after Thoracic Spinal Cord Injury Is Accompanied by Time-Dependent Changes in Lumbosacral Expression of Axonal Growth Regulators. Int. J. Mol. Sci. 2022, 23, 8667. https://doi.org/10.3390/ijms23158667
Chambel SS, Ferreira A, Oliveira R, Miranda R, Vale L, Reguenga C, Schwab ME, Cruz CD. Development of Neurogenic Detrusor Overactivity after Thoracic Spinal Cord Injury Is Accompanied by Time-Dependent Changes in Lumbosacral Expression of Axonal Growth Regulators. International Journal of Molecular Sciences. 2022; 23(15):8667. https://doi.org/10.3390/ijms23158667
Chicago/Turabian StyleChambel, Sílvia Sousa, Ana Ferreira, Raquel Oliveira, Rafael Miranda, Luís Vale, Carlos Reguenga, Martin E. Schwab, and Célia Duarte Cruz. 2022. "Development of Neurogenic Detrusor Overactivity after Thoracic Spinal Cord Injury Is Accompanied by Time-Dependent Changes in Lumbosacral Expression of Axonal Growth Regulators" International Journal of Molecular Sciences 23, no. 15: 8667. https://doi.org/10.3390/ijms23158667






