Motor Neuroplastic Effects of a Novel Paired Stimulation Technology in an Incomplete Spinal Cord Injury Animal Model
Abstract
:1. Introduction
2. Results
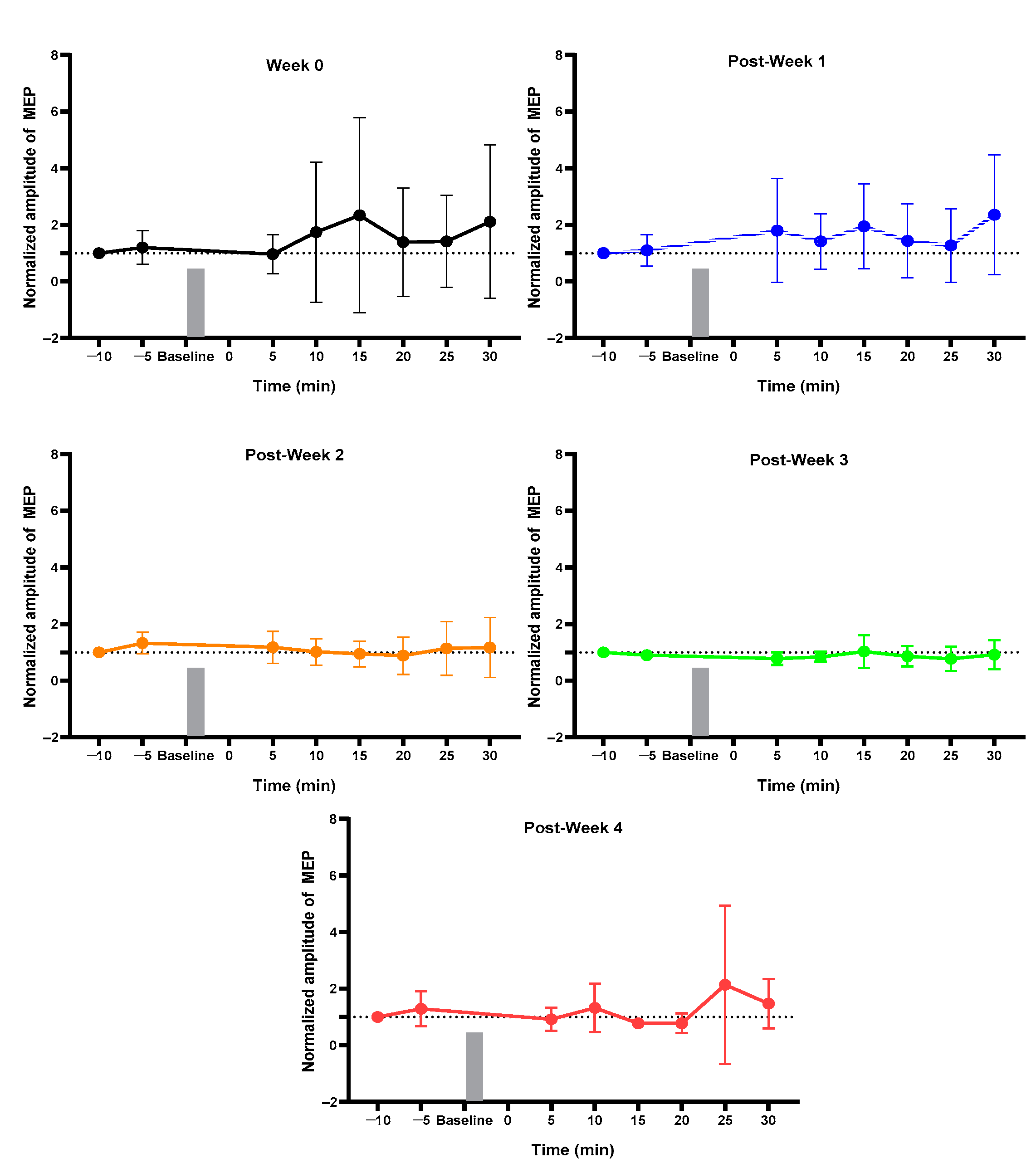
2.1. MEP Assessment after Each Week
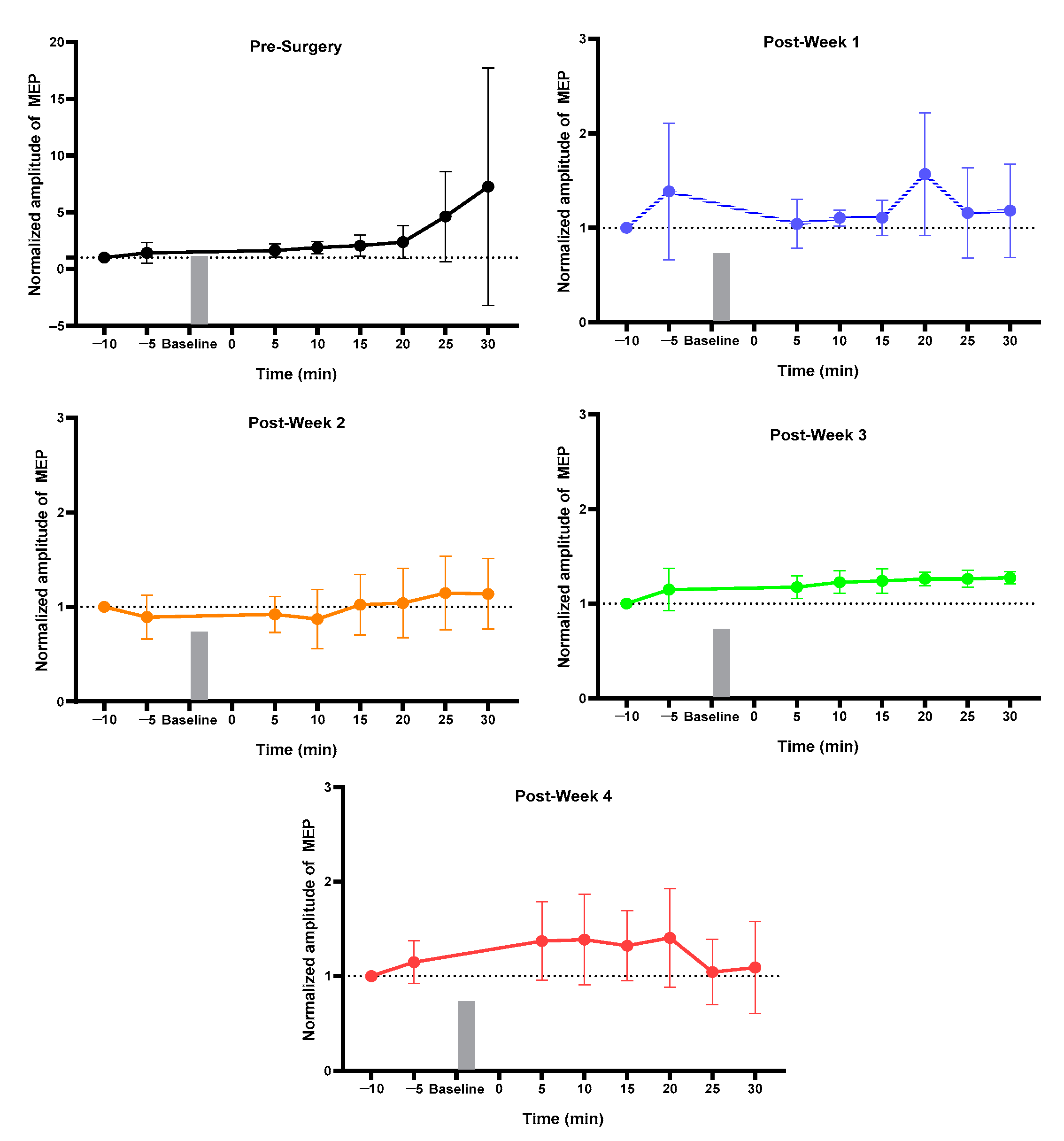
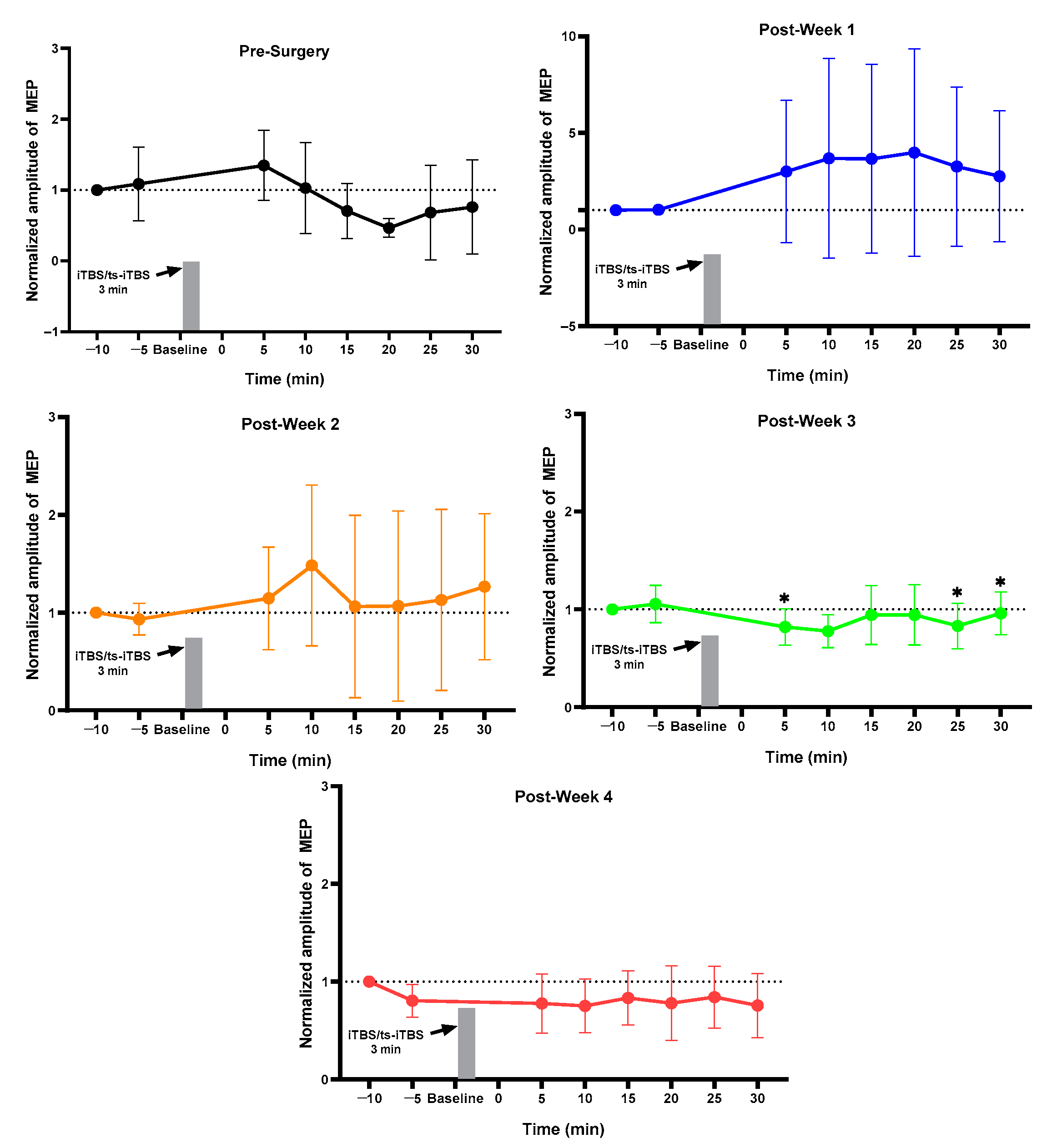
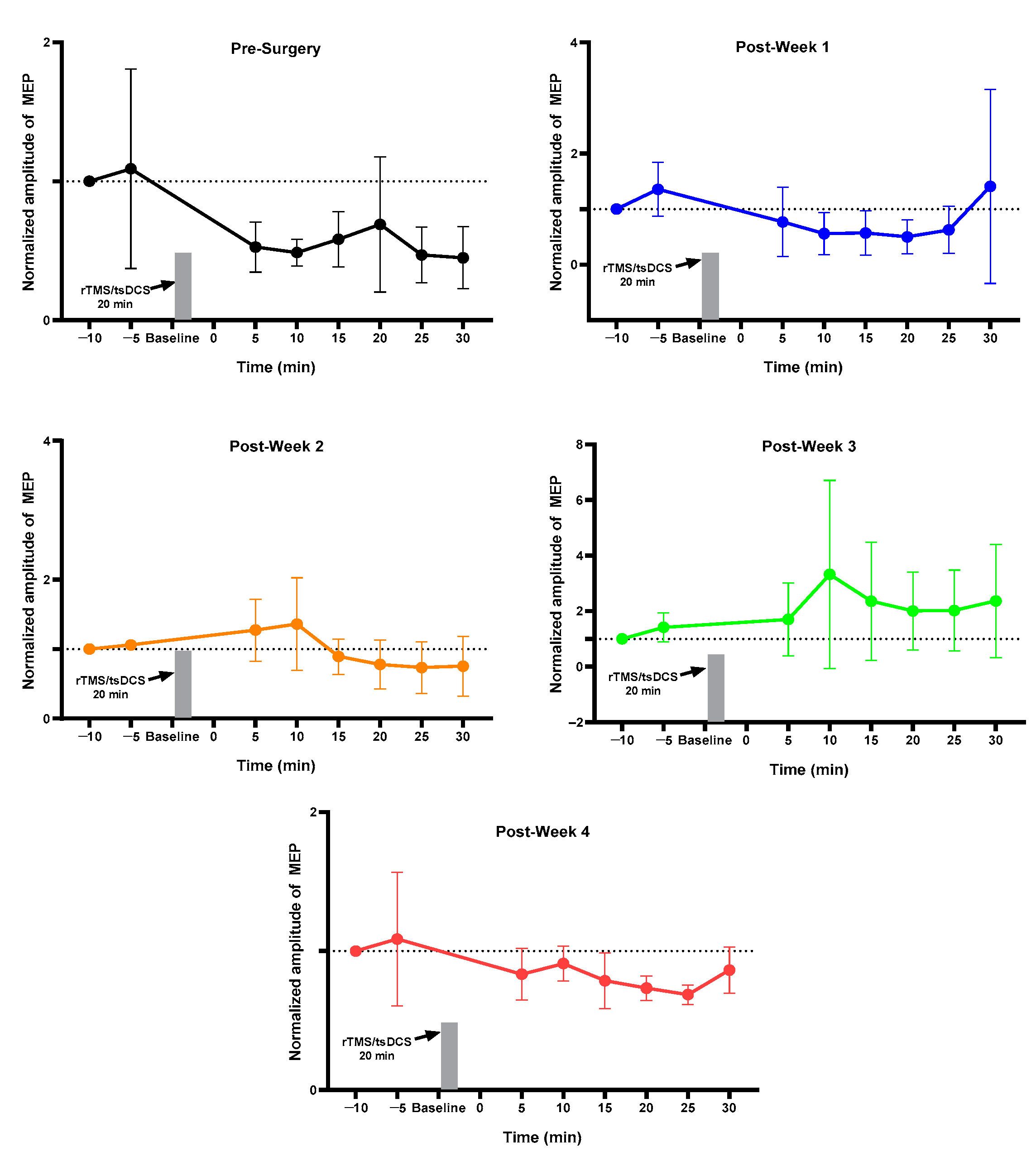
2.2. Week 1 vs. Week 4 MEP of Each Group
2.3. MEP before vs. after Surgery and Stimulation Intervention
2.4. Basso, Beattie, and Bresnahan (BBB) Locomotor Scale Score
3. Discussion
3.1. Strengths of Study
3.2. Limitations of Study
3.3. Clinical Implications
4. Materials and Methods
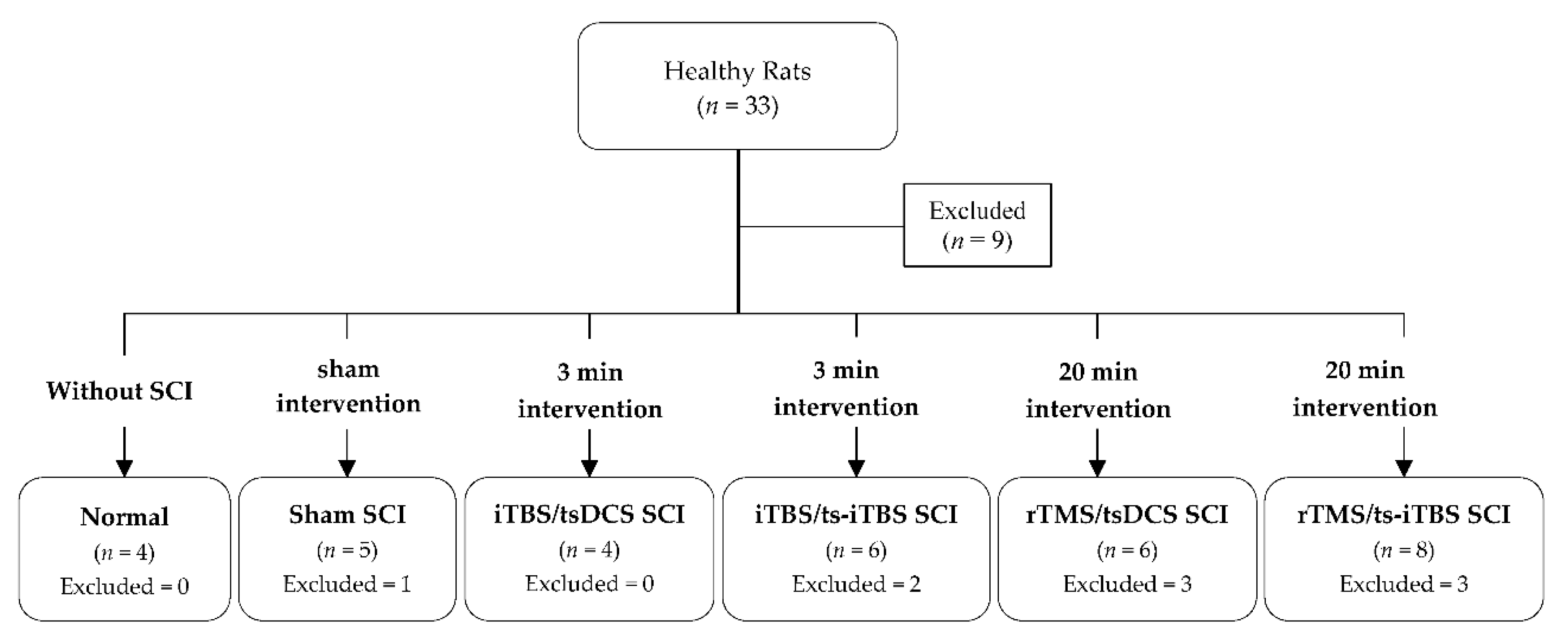
4.1. Animal Handling
4.1.1. Selection and Care of Animals
4.1.2. Animal Grouping
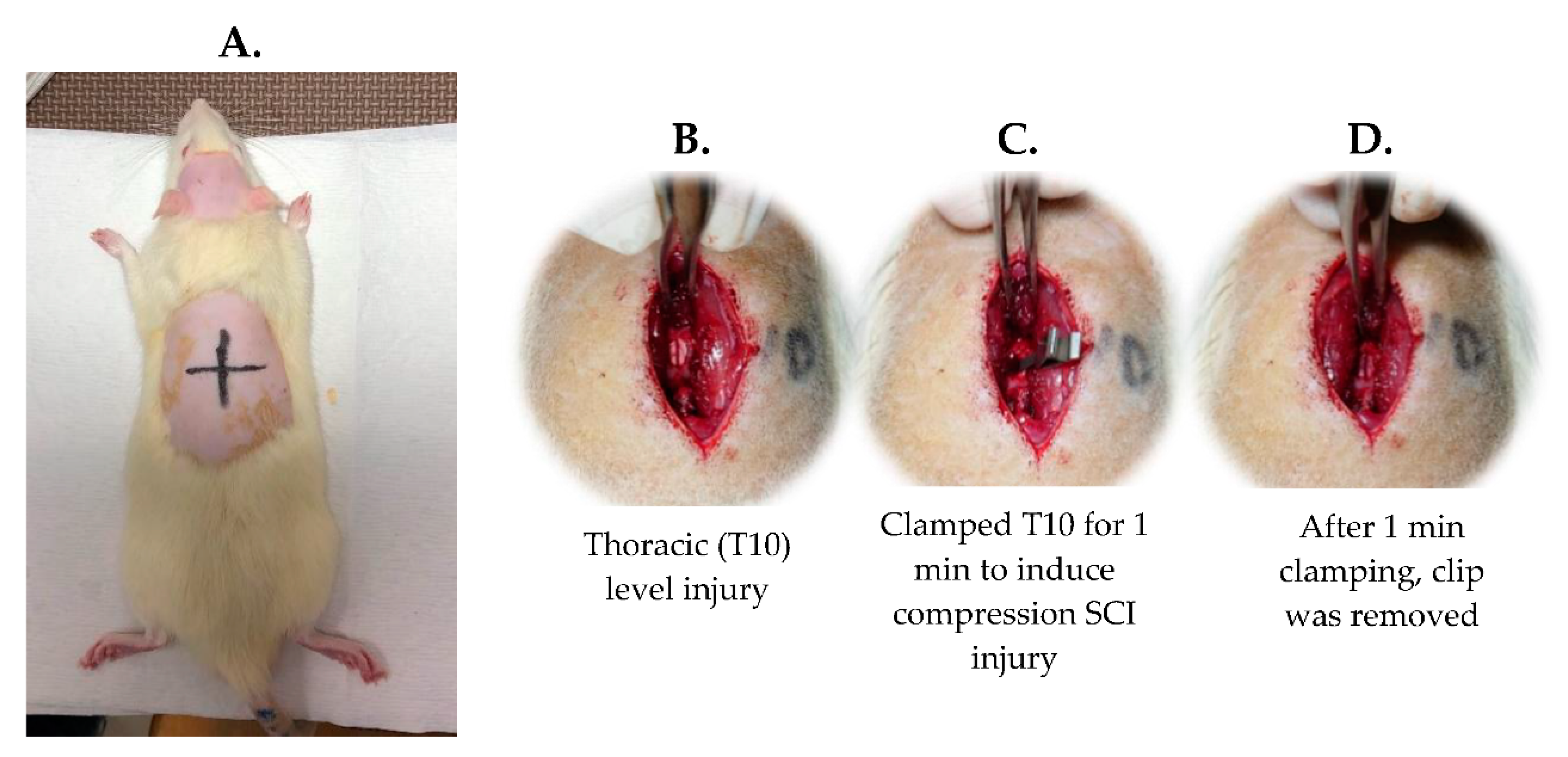
4.1.3. SCI Surgery
4.2. Experiment Intervention
4.2.1. MEP Assessment
4.2.2. Stimulation Protocol
4.2.3. Basso, Beattie, and Bresnahan (BBB) Scoring
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krassioukov, A. Autonomic function following cervical spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Spinal cord injury: Overview of experimental approaches used to restore locomotor activity. Rev. Neurosci. 2015, 26, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Moreno-Duarte, I.; Morse, L.R.; Alam, M.; Bikson, M.; Zafonte, R.; Fregni, F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. NeuroImage 2014, 85 Pt 3, 1003–1013. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; DeVivo, M.J. Changing Demographics and Injury Profile of New Traumatic Spinal Cord Injuries in the United States, 1972–2014. Arch. Phys. Med. Rehabil. 2016, 97, 1610–1619. [Google Scholar] [CrossRef]
- Petrosyan, H.A.; Alessi, V.; Sisto, S.A.; Kaufman, M.; Arvanian, V.L. Transcranial magnetic stimulation (TMS) responses elicited in hindlimb muscles as an assessment of synaptic plasticity in spino-muscular circuitry after chronic spinal cord injury. Neurosci. Lett. 2017, 642, 37–42. [Google Scholar] [CrossRef]
- Freund, P.; Weiskopf, N.; Ward, N.S.; Hutton, C.; Gall, A.; Ciccarelli, O.; Craggs, M.; Friston, K.; Thompson, A.J. Disability, atrophy and cortical reorganization following spinal cord injury. Brain A J. Neurol. 2011, 134, 1610–1622. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Spenger, C.; Tominaga, T.; Brené, S.; Olson, L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain A J. Neurol. 2007, 130, 2951–2961. [Google Scholar] [CrossRef]
- Wrigley, P.J.; Gustin, S.M.; Macey, P.M.; Nash, P.G.; Gandevia, S.C.; Macefield, V.G.; Siddall, P.J.; Henderson, L.A. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb. Cortex 2009, 19, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhao, Z.; Sabirzhanov, B.; Stoica, B.A.; Kumar, A.; Luo, T.; Skovira, J.; Faden, A.I. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: Role of cell cycle pathways. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 10989–11006. [Google Scholar] [CrossRef]
- Wu, J.; Stoica, B.A.; Luo, T.; Sabirzhanov, B.; Zhao, Z.; Guanciale, K.; Nayar, S.K.; Foss, C.A.; Pomper, M.G.; Faden, A.I. Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation. Cell Cycle 2014, 13, 2446–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, P.; Weiskopf, N.; Ashburner, J.; Wolf, K.; Sutter, R.; Altmann, D.R.; Friston, K.; Thompson, A.; Curt, A. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: A prospective longitudinal study. Lancet Neurol. 2013, 12, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Grabher, P.; Callaghan, M.F.; Ashburner, J.; Weiskopf, N.; Thompson, A.J.; Curt, A.; Freund, P. Tracking sensory system atrophy and outcome prediction in spinal cord injury. Ann. Neurol. 2015, 78, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutzeler, C.R.; Huber, E.; Callaghan, M.F.; Luechinger, R.; Curt, A.; Kramer, J.L.; Freund, P. Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 2016, 6, 18534. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Gupta, R.K.; Saksena, S.; Chandra, A.; Srivastava, R.N.; Husain, M.; Rathore, R.; Narayana, P.A. Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J. Neurosci. Res. 2008, 86, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Vallence, A.M.; Ridding, M.C. Non-invasive induction of plasticity in the human cortex: Uses and limitations. Cortex; A J. Devoted Study Nerv. Syst. Behav. 2014, 58, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M.; Lai, C.-H.; Lin, B.-S.; Chan, W.P.; Liou, J.-C.; Wu, C.-W.; Peng, C.-W. Effects of paired stimulation with specific waveforms on cortical and spinal plasticity in subjects with a chronic spinal cord injury. J. Formos. Med. Assoc. 2022, 22, 00097-3. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Zaaimi, B.; Martin, J.H. Competition with Primary Sensory Afferents Drives Remodeling of Corticospinal Axons in Mature Spinal Motor Circuits. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Chari, A.; Hentall, I.D.; Papadopoulos, M.C.; Pereira, E.A. Surgical neurostimulation for spinal cord injury. Brain Sci. 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Lamy, J.C.; Ho, C.; Badel, A.; Arrigo, R.T.; Boakye, M. Modulation of soleus H reflex by spinal DC stimulation in humans. J. Neurophysiol. 2012, 108, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Lungholt, B.K.; Nielsen, J.B. Short-term adaptations in spinal cord circuits evoked by repetitive transcranial magnetic stimulation: Possible underlying mechanisms. Exp. Brain Res. 2005, 162, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cabré, A.; Oliveri, M.; Gangitano, M.; Pascual-Leone, A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport 2001, 12, 3845–3848. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Lu, M.K.; Antal, A.; Classen, J.; Nitsche, M.; Ziemann, U.; Ridding, M.; Hamada, M.; Ugawa, Y.; Jaberzadeh, S.; et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017, 128, 2318–2329. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Chen, R.S.; Rothwell, J.C.; Wen, H.Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007, 118, 1028–1032. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Z.; Rothwell, J.C.; Chen, R.S.; Lu, C.S.; Chuang, W.L. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Cogiamanian, F.; Vergari, M.; Pulecchi, F.; Marceglia, S.; Priori, A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 2636–2640. [Google Scholar] [CrossRef]
- Cogiamanian, F.; Vergari, M.; Schiaffi, E.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain 2011, 152, 370–375. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Beck, M.M.; Therkildsen, E.R.; Svane, C.; Forman, C.; Lorentzen, J.; Conway, B.A.; Lundbye-Jensen, J.; Geertsen, S.S.; Nielsen, J.B. Transcutaneous spinal direct current stimulation increases corticospinal transmission and enhances voluntary motor output in humans. Physiol. Rep. 2020, 8, e14531. [Google Scholar] [CrossRef]
- Ling, Y.T.; Alam, M.; Zheng, Y.P. Spinal Cord Injury: Lessons about Neuroplasticity from Paired Associative Stimulation. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2020, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Yang, L.-Y.; Adeel, M.; Lai, C.-H.; Peng, C.-W. Transcranial electrostimulation with special waveforms enhances upper-limb motor function in patients with chronic stroke: A pilot randomized controlled trial. J. NeuroEng. Rehabil. 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.L.; Campêlo, M.; Mendonça, T.; Fontes, L.A.M.; Brito, R.M.; Monte-Silva, K. Effects of repetitive transcranial magnetic stimulation and trans-spinal direct current stimulation associated with treadmill exercise in spinal cord and cortical excitability of healthy subjects: A triple-blind, randomized and sham-controlled study. PLoS ONE 2018, 13, e0195276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, L.; Ibrahim, M.M.; Santora, D.; Knikou, M. Paired associative transspinal and transcortical stimulation produces plasticity in human cortical and spinal neuronal circuits. J. Neurophysiol. 2016, 116, 904–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeel, M.; Chen, C.-C.; Lin, B.-S.; Chen, H.-C.; Liou, J.-C.; Li, Y.-T.; Peng, C.-W. Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment. Int. J. Mol. Sci. 2022, 23, 6850. [Google Scholar] [CrossRef]
- Li, Y.-T.; Chen, S.-C.; Yang, L.-Y.; Hsieh, T.-H.; Peng, C.-W. Designing and implementing a novel transcranial electrostimulation system for neuroplastic applications: A preliminary study. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 805–813. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Huang, Y.-Z.; Rotenberg, A.; Pascual-Leone, A.; Chiang, Y.-H.; Wang, J.-Y.; Chen, J.-J.J. Functional dopaminergic neurons in substantia nigra are required for transcranial magnetic stimulation-induced motor plasticity. Cereb. Cortex 2015, 25, 1806–1814. [Google Scholar] [CrossRef] [Green Version]
- Marufa, S.A.; Hsieh, T.-H.; Liou, J.-C.; Chen, H.-Y.; Peng, C.-W. Neuromodulatory effects of repetitive transcranial magnetic stimulation on neural plasticity and motor functions in rats with an incomplete spinal cord injury: A preliminary study. PLoS ONE 2021, 16, e0252965. [Google Scholar] [CrossRef]
- Mishra, A.M.; Pal, A.; Gupta, D.; Carmel, J.B. Paired motor cortex and cervical epidural electrical stimulation timed to converge in the spinal cord promotes lasting increases in motor responses. J. Physiol. 2017, 595, 6953–6968. [Google Scholar] [CrossRef] [Green Version]
- Poon, P.C.; Gupta, D.; Shoichet, M.S.; Tator, C.H. Clip compression model is useful for thoracic spinal cord injuries: Histologic and functional correlates. Spine 2007, 32, 2853–2859. [Google Scholar] [CrossRef]
- Rotenberg, A.; Muller, P.A.; Vahabzadeh-Hagh, A.M.; Navarro, X.; López-Vales, R.; Pascual-Leone, A.; Jensen, F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2010, 121, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonoff, E.T.; Dale, C.S.; Pagano, R.L.; Paccola, C.C.; Ballester, G.; Teixeira, M.J.; Giorgi, R. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav. Brain Res. 2009, 196, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aydin-Abidin, S.; Trippe, J.; Funke, K.; Eysel, U.T.; Benali, A. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp. Brain Res. 2008, 188, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Liu, Y.; Zhao, Z.; Feng, J.; Sun, R.; Ma, Z.; Gu, X. Further Standardization in the Aneurysm Clip: The Effects of Occlusal Depth on the Outcome of Spinal Cord Injury in Rats. Spine 2018, 43, E126–E131. [Google Scholar] [CrossRef]
- Onifer, S.M.; Rabchevsky, A.G.; Scheff, S.W. Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J. 2007, 48, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [Green Version]

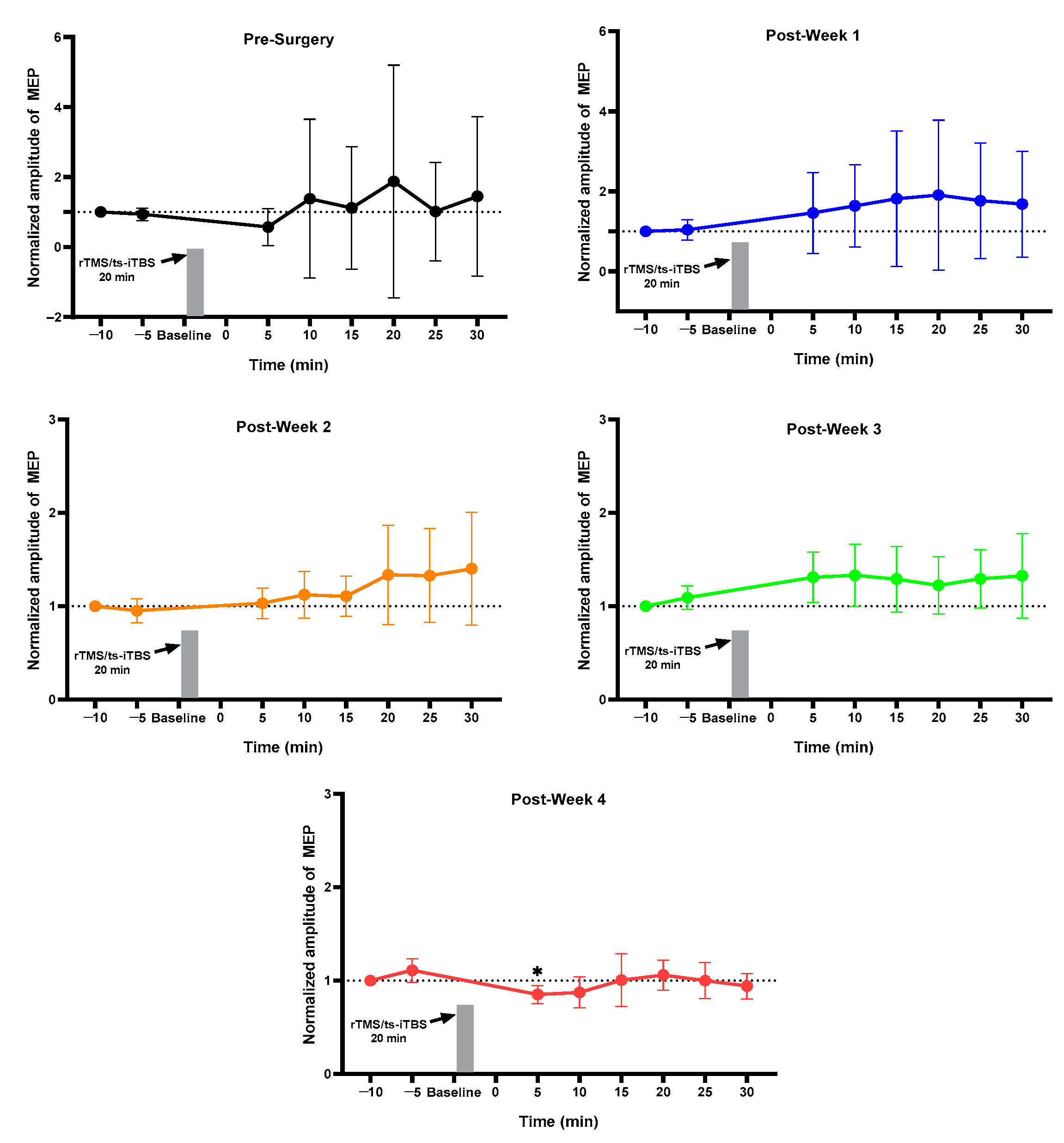
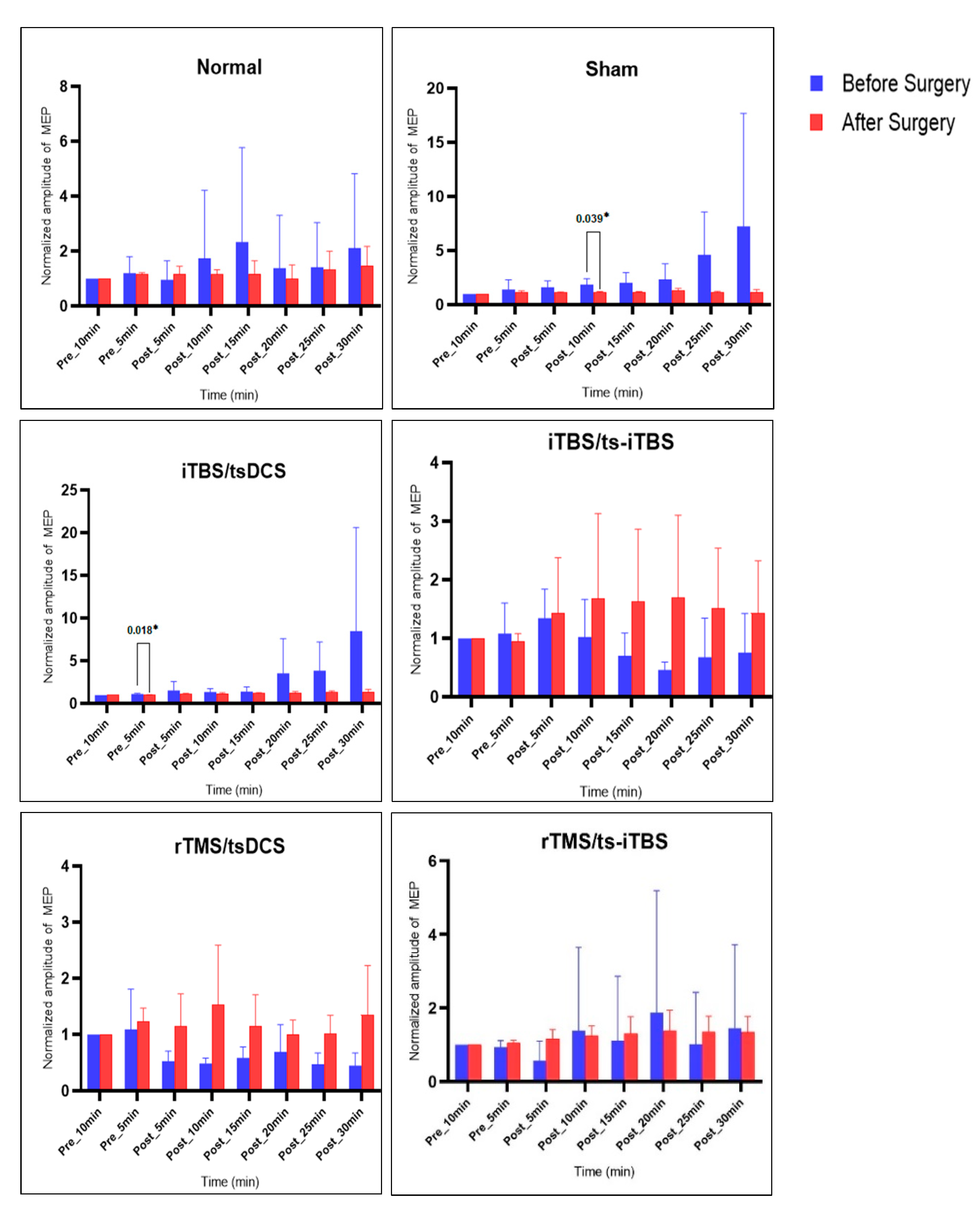

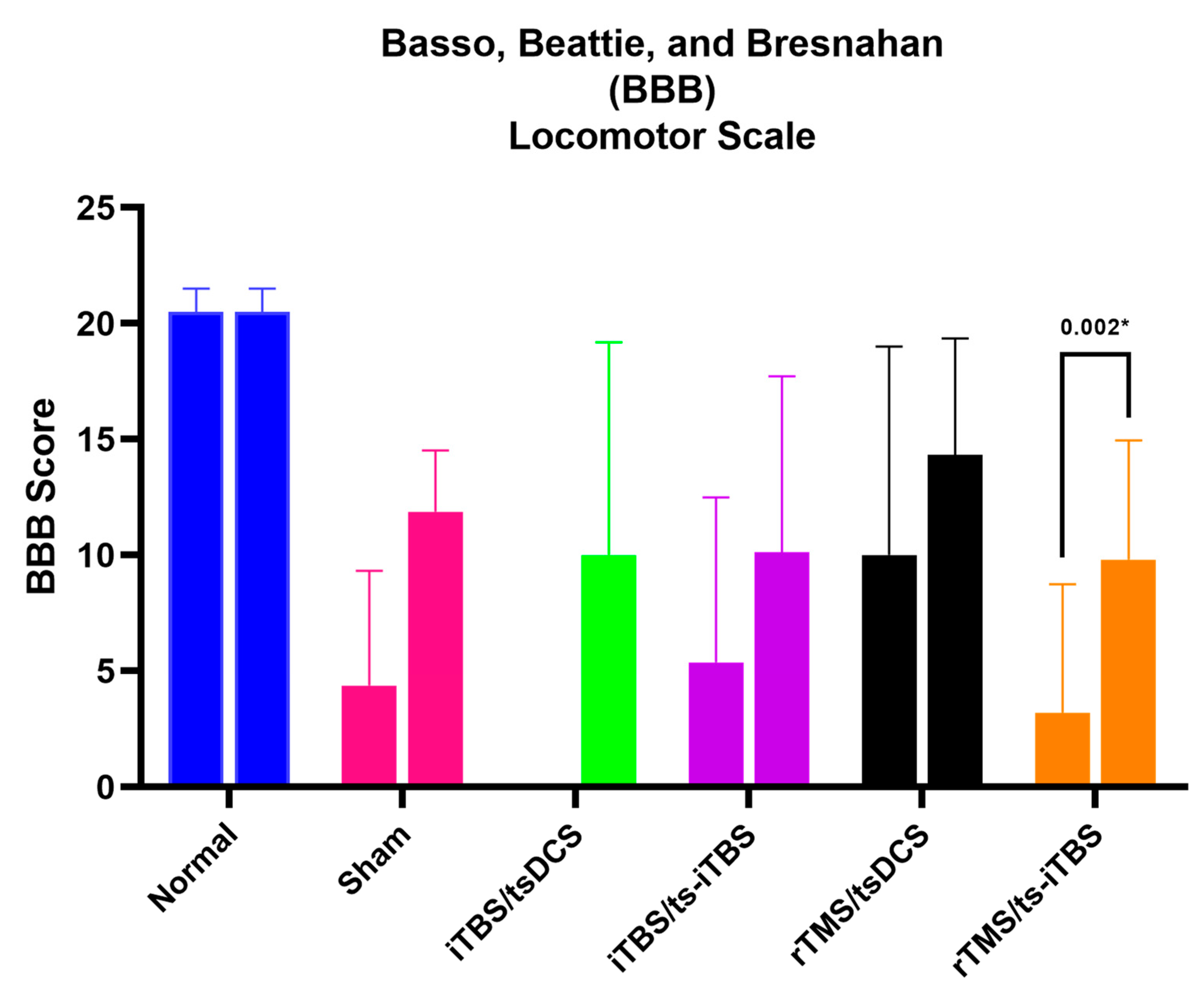

| Groups | Normal (n = 4) | Sham (n = 4) | (iTBS/tsDCS) (n = 4) | (iTBS/ts-iTBS) (n = 4) | (rTMS/tsDCS) (n = 3) | (rTMS/ts-iTBS) (n = 5) |
|---|---|---|---|---|---|---|
| Pre_5 min_w1 | 1.10 ± 0.55 | 1.39 ± 0.72 | 0.99 ± 0.07 | 1.02 ± 0.21 | 1.36 ± 0.48 | 1.04 ± 0.25 |
| Post_5 min_w4 | 0.92 ± 0.41 | 1.37 ± 0.41 | 1.07 ± 0.10 | 0.77 ± 0.30 | 0.83 ± 0.18 | 0.85 ± 0.10 |
| p-value | 0.727 | 0.946 | 0.393 | 0.149 | 0.114 | 0.066 |
| d | 0.19 | 0.04 | −0.50 | 0.96 | 1.56 | 1.12 |
| Pre_5 min_w1 | 1.10 ± 0.55 | 1.39 ± 0.72 | 0.99 ± 0.07 | 1.02 ± 0.21 | 1.36 ± 0.48 | 1.04 ± 0.25 |
| Post_10 min_w4 | 1.32 ± 0.86 | 1.39 ± 0.48 | 1.10 ± 0.11 | 0.75 ± 0.27 | 0.91 ± 0.13 | 0.87 ± 0.16 |
| p-value | 0.745 | 0.999 | 0.279 | 0.119 | 0.214 | 0.058 |
| d | −0.18 | 0.00 | −0.66 | 1.08 | 1.04 | 1.18 |
| Pre_5 min_w1 | 1.10 ± 0.55 | 1.39 ± 0.72 | 0.99 ± 0.07 | 1.02 ± 0.21 | 1.36 ± 0.48 | 1.04 ± 0.25 |
| Post_15 min_w4 | 0.78 ± 0.19 | 1.32 ± 0.37 | 1.12 ± 0.12 | 0.83 ± 0.28 | 0.78 ± 0.20 | 1.01 ± 0.28 |
| p-value | 0.407 | 0.826 | 0.229 | 0.163 | 0.230 | 0.743 |
| d | 0.48 | 0.12 | −0.75 | 0.92 | 0.99 | 0.16 |
| Pre_5 min_w1 | 1.10 ± 0.55 | 1.39 ± 0.72 | 0.99 ± 0.07 | 1.02 ± 0.21 | 1.36 ± 0.48 | 1.04 ± 0.25 |
| Post_20 min_w4 | 0.78 ± 0.35 | 1.41 ± 0.52 | 1.29 ± 0.23 | 0.78 ± 0.38 | 0.73 ± 0.09 | 1.06 ± 0.16 |
| p-value | 0.452 | 0.952 | 0.051 | 0.200 | 0.172 | 0.841 |
| d | 0.43 | −0.03 | −1.58 | 0.82 | 1.21 | −0.10 |
| Pre_5 min_w1 | 1.10 ± 0.55 | 1.39 ± 0.72 | 0.99 ± 0.07 | 1.02 ± 0.21 | 1.36 ± 0.48 | 1.04 ± 0.25 |
| Post_25 min_w4 | 2.14 ± 2.79 | 1.05 ± 0.35 | 1.28 ± 0.13 | 0.84 ± 0.32 | 0.69 ± 0.07 | 1.00 ± 0.20 |
| p-value | 0.565 | 0.528 | 0.012 * | 0.147 | 0.156 | 0.542 |
| d | −0.32 | 0.36 | −2.74 | 0.97 | 1.29 | 0.30 |
| Pre_5 min_w1 | 1.10 ± 0.55 | 1.39 ± 0.72 | 0.99 ± 0.07 | 1.02 ± 0.21 | 1.36 ± 0.48 | 1.04 ± 0.25 |
| Post_30 min_w4 | 1.47 ± 0.87 | 1.09 ± 0.48 | 1.30 ± 0.14 | 0.75 ± 0.33 | 0.86 ± 0.16 | 0.94 ± 0.14 |
| p-value | 0.622 | 0.624 | 0.026 * | 0.177 | 0.117 | 0.309 |
| d | −0.27 | 0.27 | −2.06 | 0.88 | 1.54 | 0.52 |
| Groups | Normal (n = 4) | Sham (n = 4) | (iTBS/tsDCS) (n = 4) | (iTBS/ts-iTBS) (n = 4) | (rTMS/tsDCS) (n = 3) | (rTMS/ts-iTBS) (n = 5) |
|---|---|---|---|---|---|---|
| Pre_5 min | 1.17 ± 0.46 | 1.20 ± 0.52 | 1.03 ± 0.08 | 0.98 ± 0.27 | 1.20 ± 0.45 | 1.03 ± 0.17 |
| Post_5 min | 1.13 ± 0.91 | 1.23 ± 0.41 | 1.23 ± 0.49 | 1.42 ± 1.72 | 1.02 ± 0.72 | 1.05 ± 0.58 |
| p-value | 0.820 | 0.803 | 0.084 | 0.250 | 0.306 | 0.849 |
| Pre_5 min | 1.17 ± 0.46 | 1.20 ± 0.52 | 1.03 ± 0.08 | 0.98 ± 0.27 | 1.20 ± 0.45 | 1.03 ± 0.17 |
| Post_10 min | 1.23 ± 1.18 | 1.29 ± 0.47 | 1.22 ± 0.28 | 1.55 ± 2.39 | 1.33 ± 1.70 | 1.27 ± 1.06 |
| p-value | 0.650 | 0.443 | 0.003 * | 0.294 | 0.760 | 0.265 |
| Pre_5 min | 1.17 ± 0.46 | 1.20 ± 0.52 | 1.03 ± 0.08 | 0.98 ± 0.27 | 1.20 ± 0.45 | 1.03 ± 0.17 |
| Post_15 min | 1.41 ± 1.65 | 1.35 ± 0.57 | 1.25 ± 0.30 | 1.44 ± 2.30 | 1.04 ± 1.08 | 1.27 ± 1.06 |
| p-value | 0.495 | 0.266 | 0.002* | 0.369 | 0.517 | 0.247 |
| Pre_5 min | 1.17 ± 0.46 | 1.20 ± 0.52 | 1.03 ± 0.08 | 0.98 ± 0.27 | 1.20 ± 0.45 | 1.03 ± 0.17 |
| Post_20 min | 1.07 ± 1.02 | 1.53 ± 0.82 | 1.72 ± 1.88 | 1.45 ± 2.55 | 0.94 ± 0.81 | 1.48 ± 1.62 |
| p-value | 0.677 | 0.124 | 0.111 | 0.411 | 0.238 | 0.169 |
| Pre_5 min | 1.17 ± 0.46 | 1.20 ± 0.52 | 1.03 ± 0.08 | 0.98 ± 0.27 | 1.20 ± 0.45 | 1.03 ± 0.17 |
| Post_25 min | 1.35 ± 1.52 | 1.85 ± 2.14 | 1.81 ± 1.72 | 1.35 ± 1.97 | 0.91 ± 0.83 | 1.28 ± 0.91 |
| p-value | 0.587 | 0.211 | 0.053 | 0.402 | 0.153 | 0.154 |
| Pre_5 min | 1.17 ± 0.46 | 1.20 ± 0.52 | 1.03 ± 0.08 | 0.98 ± 0.27 | 1.20 ± 0.45 | 1.03 ± 0.17 |
| Post_30 min | 1.61 ± 1.59 | 2.39 ± 4.86 | 2.81 ± 5.65 | 1.30 ± 1.61 | 1.17 ± 1.25 | 1.36 ± 1.15 |
| p-value | 0.184 | 0.294 | 0.173 | 0.371 | 0.902 | 0.155 |
| Sum of Squares | df | Mean Square | F | p-Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Between groups | 26.68 | 7 | 3.81 | 1.70 | 0.136 |
| Within groups | 89.58 | 40 | 2.24 | |||
| Week 1 | Between groups | 3.32 | 7 | 0.47 | 0.66 | 0.704 |
| Within groups | 28.74 | 40 | 0.72 | |||
| Week 2 | Between groups | 0.29 | 7 | 0.04 | 0.99 | 0.455 |
| Within groups | 1.66 | 40 | 0.04 | |||
| Week 3 | Between groups | 0.95 | 7 | 0.14 | 0.56 | 0.787 |
| Within groups | 9.80 | 40 | 0.25 | |||
| Week 4 | Between groups | 0.18 | 7 | 0.03 | 0.34 | 0.932 |
| Within groups | 3.09 | 40 | 0.08 | |||
| Sum of Squares | df | Mean Square | F | p-Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Between groups | 35.34 | 5 | 7.07 | 3.67 | 0.008 * |
| Within groups | 80.93 | 42 | 1.93 | |||
| Week 1 | Between groups | 18.93 | 5 | 3.79 | 12.12 | 0.0001*** |
| Within groups | 13.12 | 42 | 0.31 | |||
| Week 2 | Between groups | 0.61 | 5 | 0.12 | 3.85 | 0.006 * |
| Within groups | 1.33 | 42 | 0.03 | |||
| Week 3 | Between groups | 6.78 | 5 | 1.36 | 14.31 | 0.0001 *** |
| Within groups | 3.98 | 42 | 0.01 | |||
| Week 4 | Between groups | 1.28 | 5 | 0.26 | 5.41 | 0.001 ** |
| Within groups | 1.99 | 42 | 0.05 | |||
| Sum of Squares | df | Mean Square | F | p-Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Between groups | 0.00 | 5 | 0.00 | -- | -- |
| Within groups | 0.00 | 18 | 0.00 | |||
| Week 1 | Between groups | 1062.28 | 5 | 212.46 | 7.45 | 0.001 ** |
| Within groups | 513.8 | 18 | 28.51 | |||
| Week 2 | Between groups | 610.59 | 5 | 122.12 | 3.80 | 0.016 * |
| Within groups | 578.65 | 18 | 32.15 | |||
| Week 3 | Between groups | 518.01 | 5 | 103.60 | 3.76 | 0.017 * |
| Within groups | 495.73 | 18 | 27.54 | |||
| Week 4 | Between groups | 336.93 | 5 | 67.39 | 2.46 | 0.078 |
| Within groups | 438.84 | 16 | 27.43 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeel, M.; Lin, B.-S.; Chen, H.-C.; Lai, C.-H.; Liou, J.-C.; Wu, C.-W.; Chan, W.P.; Peng, C.-W. Motor Neuroplastic Effects of a Novel Paired Stimulation Technology in an Incomplete Spinal Cord Injury Animal Model. Int. J. Mol. Sci. 2022, 23, 9447. https://doi.org/10.3390/ijms23169447
Adeel M, Lin B-S, Chen H-C, Lai C-H, Liou J-C, Wu C-W, Chan WP, Peng C-W. Motor Neuroplastic Effects of a Novel Paired Stimulation Technology in an Incomplete Spinal Cord Injury Animal Model. International Journal of Molecular Sciences. 2022; 23(16):9447. https://doi.org/10.3390/ijms23169447
Chicago/Turabian StyleAdeel, Muhammad, Bor-Shing Lin, Hung-Chou Chen, Chien-Hung Lai, Jian-Chiun Liou, Chun-Wei Wu, Wing P. Chan, and Chih-Wei Peng. 2022. "Motor Neuroplastic Effects of a Novel Paired Stimulation Technology in an Incomplete Spinal Cord Injury Animal Model" International Journal of Molecular Sciences 23, no. 16: 9447. https://doi.org/10.3390/ijms23169447
APA StyleAdeel, M., Lin, B.-S., Chen, H.-C., Lai, C.-H., Liou, J.-C., Wu, C.-W., Chan, W. P., & Peng, C.-W. (2022). Motor Neuroplastic Effects of a Novel Paired Stimulation Technology in an Incomplete Spinal Cord Injury Animal Model. International Journal of Molecular Sciences, 23(16), 9447. https://doi.org/10.3390/ijms23169447








