The Complement System, Aging, and Aging-Related Diseases
Abstract
1. Introduction
2. The Complement System
2.1. Composition of the Complement System
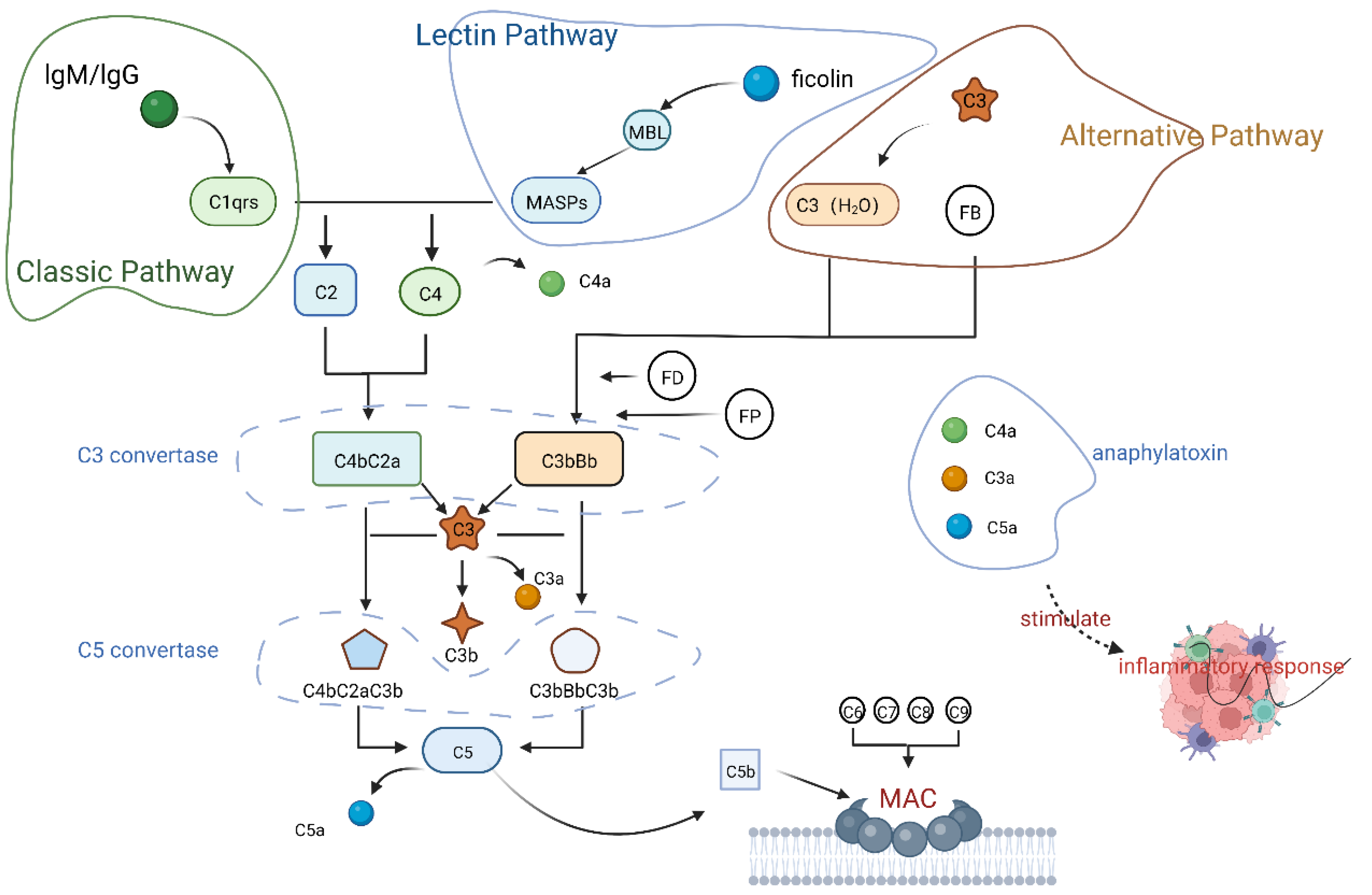
2.2. Activation of the Complement System
2.2.1. Classical Pathway
2.2.2. Lectin Pathway
2.2.3. Alternative Pathway
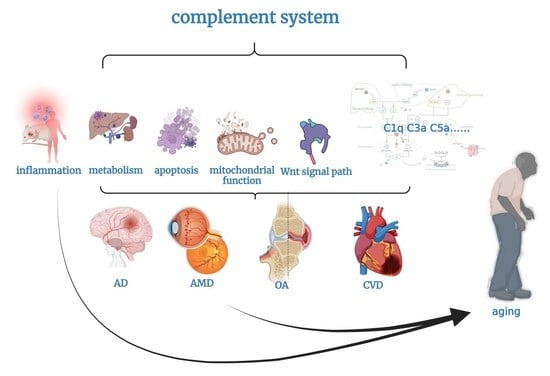
3. Complement and Aging
3.1. Complement and Inflammation
3.2. Complement and Metabolism
3.3. Complement and Apoptosis
3.4. Complement and Mitochondrial Function
3.5. Complement and Wnt Signaling Pathway
4. Complement and Aging-Related Diseases
4.1. Alzheimer’s Disease
4.2. Age-Related Macular Degeneration
4.3. Osteoarthritis
4.4. Cardiovascular Disease
4.5. Other Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AD | Alzheimer’s disease |
| AP | Alternate pathway |
| Aβ | Amyloid β protein |
| ACS | Acute coronary syndromes |
| AMD | Age-related macular degeneration |
| AMI | Acute Myocardial Infarction |
| BA | Biological age |
| CA | Chronological age |
| CP | Classical pathway |
| CNS | Central nervous system |
| CNV | Choroidal neovascularization |
| CRP | C-reactive protein |
| CRT | Calreticulin |
| CVD | Cardiovascular disease |
| CVF | Cobra venom factor |
| C3aR | C3a receptor |
| C1qbp | C1q binding protein |
| C5aR | C5a receptor |
| ERK1/2 | Extracellular signal-regulated protein 1/2 |
| Fb | Factor B |
| Fd | Factor D |
| FP | Properdin |
| ICD | International classification of diseases |
| IC3b | Inactivated C3b |
| LP | Lectin pathway |
| LPS | lipopolysaccharide |
| MAC | Membrane attack complex |
| MBL | Mannose-binding lectin |
| MSCs | Mesenchymal stem cells |
| MASP | MBL-associated serine protease |
| NFTs | Neurofibrillary tangles |
| OA | Osteoarthritis |
| PHF | Paired helical filament |
| PMNs | Polymorphonuclear neutrophils |
| PTX | Pertussis toxin |
| PPA | Pyrophosphate arthritis |
| RA | Rheumatoid arthritis |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelial |
| RTEC | Renal tubular epithelial cell |
| SLE | Systemic lupus erythematosus |
| SASP | Senescence-associated secretory phenotype |
| TED | Thioester structural domain |
| VEGF | Vascular endothelial growth factor |
| WHO | World health organization |
References
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Muscari, A.; Sbano, D.; Bastagli, L.; Poggiopollini, G.; Tomassetti, V.; Forti, P.; Boni, P.; Ravaglia, G.; Zoli, M.; Puddu, P. Effects of weight loss and risk factor treatment in subjects with elevated serum C3, an inflammatory predictor of myocardial infarction. Int. J. Cardiol. 2005, 100, 217–223. [Google Scholar] [CrossRef]
- Vignesh, P.; Rawat, A.; Sharma, M.; Singh, S. Complement in autoimmune diseases. Clin. Chim. Acta 2017, 465, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yao, Y.; Lv, F.; Zhang, F.; Zhao, Y.; Luan, F. Associations of immunological factors with metabolic syndrome and its characteristic elements in Chinese centenarians. J. Transl. Med. 2018, 16, 315. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, D.; Wang, G.; Zhang, J.; Ge, S.; Singh, M.; Wang, H.; Song, M.; Li, D.; Wang, W.; et al. Modelling biological age based on plasma peptides in Han Chinese adults. Aging 2020, 12, 10676–10686. [Google Scholar] [CrossRef]
- Khaltourina, D.; Matveyev, Y.; Alekseev, A.; Cortese, F.; Iovita, A. Aging Fits the Disease Criteria of the International Classification of Diseases. Mech. Ageing Dev. 2020, 189, 111230. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, Y.; Zhang, F.; Luan, F.; Lv, F.; Deng, J.; Zhao, Y.; Yao, Y. Centenarian longevity is positively correlated with IgE levels but negatively correlated with C3/C4 levels, abdominal obesity and metabolic syndrome. Cell Mol. Immunol. 2020, 17, 1196–1197. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, S.; Zhao, M.; Liu, D.; Zhao, Y.; Yao, Y. Associations Between Complement Components and Vitamin D and the Physical Activities of Daily Living Among a Longevous Population in Hainan, China. Front. Immunol. 2020, 11, 1543. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- McGeer, P.L.; Lee, M.; McGeer, E.G. A review of human diseases caused or exacerbated by aberrant complement activation. Neurobiol. Aging 2017, 52, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P. C4d in Native Glomerular Diseases. Am. J. Nephrol. 2019, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Colvin, R.B.; Daha, M.R.; Drachenberg, C.B.; Haas, M.; Nickeleit, V.; Salmon, J.E.; Sis, B.; Zhao, M.H.; Bruijn, J.A.; et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012, 81, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef] [PubMed]
- Toapanta, F.R.; Ross, T.M. Complement-mediated activation of the adaptive immune responses: Role of C3d in linking the innate and adaptive immunity. Immunol. Res. 2006, 36, 197–210. [Google Scholar] [CrossRef]
- Janssen, B.J.; Huizinga, E.G.; Raaijmakers, H.C.; Roos, A.; Daha, M.R.; Nilsson-Ekdahl, K.; Nilsson, B.; Gros, P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 2005, 437, 505–511. [Google Scholar] [CrossRef]
- Gadjeva, M.; Takahashi, K.; Thiel, S. Mannan-binding lectin—A soluble pattern recognition molecule. Mol. Immunol. 2004, 41, 113–121. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S.; Rittirsch, D.; Neff, T.A.; McGuire, S.R.; Lambris, J.D.; Warner, R.L.; Flierl, M.A.; Hoesel, L.M.; et al. Generation of C5a in the absence of C3: A new complement activation pathway. Nat. Med. 2006, 12, 682–687. [Google Scholar] [CrossRef]
- Dodig, S.; Cepelak, I.; Pavic, I. Hallmarks of senescence and aging. Biochem. Med. 2019, 29, 030501. [Google Scholar] [CrossRef]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, A.V.; Deryabin, P.I.; Giukova, A.A.; Nikolsky, N.N. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Nat. 2018, 10, 4–14. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Wu, X.; Lin, L.; Cui, J.; Chen, Y.; Yang, L.; Wan, J. Complement C3 deficiency ameliorates aging related changes in the kidney. Life Sci. 2020, 260, 118370. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, A.G.; Zhou, X.G.; Khan, I.; Zhang, R.L.; Lo, H.H.; Qu, L.Q.; Song, L.L.; Yun, X.Y.; Wang, H.M.; et al. Saponins isolated from Radix polygalae extent lifespan by modulating complement C3 and gut microbiota. Pharmacol. Res. 2021, 170, 105697. [Google Scholar] [CrossRef]
- McGeer, E.G.; Klegeris, A.; McGeer, P.L. Inflammation, the complement system and the diseases of aging. Neurobiol. Aging 2005, 26 (Suppl. S1), 94–97. [Google Scholar] [CrossRef]
- Murakami, Y.; Imamichi, T.; Nagasawa, S. Characterization of C3a anaphylatoxin receptor on guinea-pig macrophages. Immunology 1993, 79, 633–638. [Google Scholar]
- Elsner, J.; Oppermann, M.; Czech, W.; Dobos, G.; Schopf, E.; Norgauer, J.; Kapp, A. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. Eur. J. Immunol. 1994, 24, 518–522. [Google Scholar] [CrossRef]
- Elsner, J.; Oppermann, M.; Czech, W.; Kapp, A. C3a activates the respiratory burst in human polymorphonuclear neutrophilic leukocytes via pertussis toxin-sensitive G-proteins. Blood 1994, 83, 3324–3331. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Xu, J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol. Appl. Biochem. 2019, 66, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Asgari, E.; Le Friec, G.; Yamamoto, H.; Perucha, E.; Sacks, S.S.; Kohl, J.; Cook, H.T.; Kemper, C. C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 2013, 122, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, T.; Vannier, E.; Clark, B.D.; Margolis, N.H.; Dinarello, C.A.; Burke, J.F.; Gelfand, J.A. A new biologic role for C3a and C3a desArg: Regulation of TNF-alpha and IL-1 beta synthesis. J. Immunol. 1996, 156, 3455–3460. [Google Scholar] [PubMed]
- Wu, M.C.; Brennan, F.H.; Lynch, J.P.; Mantovani, S.; Phipps, S.; Wetsel, R.A.; Ruitenberg, M.J.; Taylor, S.M.; Woodruff, T.M. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl. Acad. Sci. USA 2013, 110, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Barbu, A.; Hamad, O.A.; Lind, L.; Ekdahl, K.N.; Nilsson, B. The role of complement factor C3 in lipid metabolism. Mol. Immunol. 2015, 67, 101–107. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Complement C3 and its polymorphism: Biological and clinical consequences. Pathology 2014, 46, 1–10. [Google Scholar] [CrossRef]
- Onat, A.; Hergenc, G.; Can, G.; Kaya, Z.; Yuksel, H. Serum complement C3: A determinant of cardiometabolic risk, additive to the metabolic syndrome, in middle-aged population. Metabolism 2010, 59, 628–634. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, C.; Lin, X.; Zhao, M.; Gong, D.; Liu, L.; Geng, T. Complement C3 participates in the development of goose fatty liver potentially by regulating the expression of FASN and ETNK1. Anim. Sci. J. 2021, 92, e13527. [Google Scholar] [CrossRef]
- Engstrom, G.; Hedblad, B.; Eriksson, K.F.; Janzon, L.; Lindgarde, F. Complement C3 is a risk factor for the development of diabetes: A population-based cohort study. Diabetes 2005, 54, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Can, G.; Rezvani, R.; Cianflone, K. Complement C3 and cleavage products in cardiometabolic risk. Clin. Chim. Acta 2011, 412, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wunder, G.C. Things your most satisfied patients won’t tell you. J. Am. Dent. Assoc. 1992, 123, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K. Apoptosis and aging: Increased resistance to apoptosis enhances the aging process. Cell Mol. Life Sci. 2011, 68, 1021–1031. [Google Scholar] [CrossRef]
- Jha, P.; Banda, H.; Tytarenko, R.; Bora, P.S.; Bora, N.S. Complement mediated apoptosis leads to the loss of retinal ganglion cells in animal model of glaucoma. Mol. Immunol. 2011, 48, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.S.; Ming, L.; Qian, Y.S.; Jing, S.S. Lipopolysaccharide activated complement induces endothelial cell release of adhesion molecules and apoptosis. J. Chin. Bull. Pharmacol. 2011, 27, 1245–1249. [Google Scholar]
- Amarilyo, G.; Verbovetski, I.; Atallah, M.; Grau, A.; Wiser, G.; Gil, O.; Ben-Neriah, Y.; Mevorach, D. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-kappaB-dependent blockade. Eur. J. Immunol. 2010, 40, 699–709. [Google Scholar] [CrossRef]
- Hai, J.Z.; Wan, J.Z.; Fan, W.; Ping, W. Experimental study on the effect of complement C3a on human macrophage apoptosis. J. Chin. J. Mod. Med. 2010, 20, 548–551. [Google Scholar]
- Zheng, Q.Y.; Liang, S.J.; Xu, F.; Yang, Y.; Feng, J.L.; Shen, F.; Zhong, Y.; Wu, S.; Shu, Y.; Sun, D.D.; et al. Complement component 3 prevents imiquimod-induced psoriatic skin inflammation by inhibiting apoptosis in mice. Int. Immunopharmacol. 2020, 85, 106692. [Google Scholar] [CrossRef]
- King, B.C.; Renstrom, E.; Blom, A.M. Intracellular cytosolic complement component C3 regulates cytoprotective autophagy in pancreatic beta cells by interaction with ATG16L1. Autophagy 2019, 15, 919–921. [Google Scholar] [CrossRef]
- Dos Santos, R.S.; Marroqui, L.; Grieco, F.A.; Marselli, L.; Suleiman, M.; Henz, S.R.; Marchetti, P.; Wernersson, R.; Eizirik, D.L. Protective Role of Complement C3 Against Cytokine-Mediated beta-Cell Apoptosis. Endocrinology 2017, 158, 2503–2521. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C.L.; Lesnefsky, E.J.; Chen, Q.; Tandler, B. Mitochondrial Dysfunction in Cardiovascular Aging. Adv. Exp. Med. Biol. 2017, 982, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Barna, J.; Dimen, D.; Puska, G.; Kovacs, D.; Csikos, V.; Olah, S.; Udvari, E.B.; Pal, G.; Dobolyi, A. Complement component 1q subcomponent binding protein in the brain of the rat. Sci. Rep. 2019, 9, 4597. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, C.R.; Jensen, L.; Dagnaes-Hansen, F.; Holm, I.E.; Endo, Y.; Fujita, T.; Thiel, S.; Jensenius, J.C.; Degn, S.E. Mitochondria and the lectin pathway of complement. J. Biol. Chem. 2013, 288, 8016–8027. [Google Scholar] [CrossRef]
- Tan, S.M.; Ziemann, M.; Thallas-Bonke, V.; Snelson, M.; Kumar, V.; Laskowski, A.; Nguyen, T.V.; Huynh, K.; Clarke, M.V.; Libianto, R.; et al. Complement C5a Induces Renal Injury in Diabetic Kidney Disease by Disrupting Mitochondrial Metabolic Agility. Diabetes 2020, 69, 83–98. [Google Scholar] [CrossRef]
- Martinus, R.D.; Cook, C.J. The effect of complement C5a on mitochondrial functions of PC12 cells. Neuroreport 2011, 22, 581–585. [Google Scholar] [CrossRef]
- Ishii, M.; Beeson, G.; Beeson, C.; Rohrer, B. Mitochondrial C3a Receptor Activation in Oxidatively Stressed Epithelial Cells Reduces Mitochondrial Respiration and Metabolism. Front. Immunol. 2021, 12, 628062. [Google Scholar] [CrossRef]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef]
- Castellano, G.; Franzin, R.; Sallustio, F.; Stasi, A.; Banelli, B.; Romani, M.; De Palma, G.; Lucarelli, G.; Divella, C.; Battaglia, M.; et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/betacatenin signaling after ischemia/reperfusion injury. Aging 2019, 11, 4382–4406. [Google Scholar] [CrossRef]
- Horii, N.; Uchida, M.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Hashimoto, T.; Iemitsu, M. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018, 32, 3547–3559. [Google Scholar] [CrossRef]
- Wang, X.D.; Huang, X.F.; Yan, Q.R.; Bao, C.D. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis. PLoS ONE 2014, 9, e84852. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 2013, 24, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Dai, H.; Gao, Y.; Feng, Z.; Liu, W.; Liu, F.; Zhang, Z.; Ma, F.; Xie, X.; Zhu, Z.; et al. Inhibition of the Wnt/beta-catenin signaling pathway reduces autophagy levels in complement treated podocytes. Exp. Ther. Med. 2021, 22, 737. [Google Scholar] [CrossRef] [PubMed]
- Al-Sofiani, M.E.; Ganji, S.S.; Kalyani, R.R. Body composition changes in diabetes and aging. J. Diabetes Complicat. 2019, 33, 451–459. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Izzo, C.; Carrizzo, A.; Alfano, A.; Virtuoso, N.; Capunzo, M.; Calabrese, M.; De Simone, E.; Sciarretta, S.; Frati, G.; Oliveti, M.; et al. The Impact of Aging on Cardio and Cerebrovascular Diseases. Int. J. Mol. Sci. 2018, 19, 481. [Google Scholar] [CrossRef]
- Tenner, A.J. Complement-Mediated Events in Alzheimer’s Disease: Mechanisms and Potential Therapeutic Targets. J. Immunol. 2020, 204, 306–315. [Google Scholar] [CrossRef]
- Shah, A.; Kishore, U.; Shastri, A. Complement System in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 3647. [Google Scholar] [CrossRef]
- Rogers, J.; Cooper, N.R.; Webster, S.; Schultz, J.; McGeer, P.L.; Styren, S.D.; Civin, W.H.; Brachova, L.; Bradt, B.; Ward, P.; et al. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10016–10020. [Google Scholar] [CrossRef]
- Walker, D.G.; McGeer, P.L. Complement gene expression in human brain: Comparison between normal and Alzheimer disease cases. Brain Res. Mol. Brain Res. 1992, 14, 109–116. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Veerhuis, R. Histological and direct evidence for the role of complement in the neuroinflammation of AD. Curr. Alzheimer Res. 2011, 8, 34–58. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Zhou, J.; Botto, M.; Tenner, A.J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 2004, 24, 6457–6465. [Google Scholar] [CrossRef]
- Wu, T.; Dejanovic, B.; Gandham, V.D.; Gogineni, A.; Edmonds, R.; Schauer, S.; Srinivasan, K.; Huntley, M.A.; Wang, Y.; Wang, T.M.; et al. Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep. 2019, 28, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Hettmann, T.; Gillies, S.D.; Kleinschmidt, M.; Piechotta, A.; Makioka, K.; Lemere, C.A.; Schilling, S.; Rahfeld, J.U.; Lues, I. Development of the clinical candidate PBD-C06, a humanized pGlu3-Abeta-specific antibody against Alzheimer’s disease with reduced complement activation. Sci. Rep. 2020, 10, 3294. [Google Scholar] [CrossRef]
- Lee, J.D.; Kumar, V.; Fung, J.N.; Ruitenberg, M.J.; Noakes, P.G.; Woodruff, T.M. Pharmacological inhibition of complement C5a-C5a1 receptor signalling ameliorates disease pathology in the hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2017, 174, 689–699. [Google Scholar] [CrossRef]
- Smith, W.; Assink, J.; Klein, R.; Mitchell, P.; Klaver, C.C.; Klein, B.E.; Hofman, A.; Jensen, S.; Wang, J.J.; de Jong, P.T. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001, 108, 697–704. [Google Scholar] [CrossRef]
- Tan, P.L.; Bowes Rickman, C.; Katsanis, N. AMD and the alternative complement pathway: Genetics and functional implications. Hum. Genom. 2016, 10, 23. [Google Scholar] [CrossRef]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lechanteur, Y.T.E.; Lores-Motta, L.; Schick, T.; Daha, M.R.; Altay, L.; Liakopoulos, S.; Smailhodzic, D.; den Hollander, A.I.; Hoyng, C.B.; et al. Complement Activation Levels Are Related to Disease Stage in AMD. Investig. Ophthalmol. Vis. Sci. 2020, 61, 18. [Google Scholar] [CrossRef] [PubMed]
- Lohman, R.J.; Hamidon, J.K.; Reid, R.C.; Rowley, J.A.; Yau, M.K.; Halili, M.A.; Nielsen, D.S.; Lim, J.; Wu, K.C.; Loh, Z.; et al. Exploiting a novel conformational switch to control innate immunity mediated by complement protein C3a. Nat. Commun. 2017, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; McLeod, D.S.; Jing, T.; Sunness, J.S.; Seddon, J.M.; Lutty, G.A. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Cashman, S.M.; Desai, A.; Ramo, K.; Kumar-Singh, R. Expression of complement component 3 (C3) from an adenovirus leads to pathology in the murine retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3436–3445. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cipriani, V.; Yates, J.R.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Hoh Kam, J.; Lenassi, E.; Malik, T.H.; Pickering, M.C.; Jeffery, G. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. Am. J. Pathol. 2013, 183, 480–492. [Google Scholar] [CrossRef]
- Kassa, E.; Ciulla, T.A.; Hussain, R.M.; Dugel, P.U. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol. Ther 2019, 19, 335–342. [Google Scholar] [CrossRef]
- Felson, D.T. Developments in the clinical understanding of osteoarthritis. Arthritis Res. Ther. 2009, 11, 203. [Google Scholar] [CrossRef]
- Geyer, M.; Schonfeld, C. Novel Insights into the Pathogenesis of Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 98–107. [Google Scholar] [CrossRef]
- Struglics, A.; Okroj, M.; Sward, P.; Frobell, R.; Saxne, T.; Lohmander, L.S.; Blom, A.M. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Res. Ther. 2016, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Mariani, E.; Lisignoli, G.; Addimanda, O.; Meliconi, R. Complement Expression and Activation in Osteoarthritis Joint Compartments. Front. Immunol. 2020, 11, 535010. [Google Scholar] [CrossRef] [PubMed]
- Friscic, J.; Bottcher, M.; Reinwald, C.; Bruns, H.; Wirth, B.; Popp, S.J.; Walker, K.I.; Ackermann, J.A.; Chen, X.; Turner, J.; et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity 2021, 54, 1002–1021. [Google Scholar] [CrossRef]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Clark, D.J.; Li, X.; Yorek, M.S.; Usachev, Y.M.; Brennan, T.J. Nociceptive sensitization by complement C5a and C3a in mouse. Pain 2010, 148, 343–352. [Google Scholar] [CrossRef]
- Quadros, A.U.; Cunha, T.M. C5a and pain development: An old molecule, a new target. Pharmacol. Res. 2016, 112, 58–67. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Crane, J.W.; Proctor, L.M.; Buller, K.M.; Shek, A.B.; de Vos, K.; Pollitt, S.; Williams, H.M.; Shiels, I.A.; Monk, P.N.; et al. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006, 20, 1407–1417. [Google Scholar] [CrossRef]
- Vergunst, C.E.; Gerlag, D.M.; Dinant, H.; Schulz, L.; Vinkenoog, M.; Smeets, T.J.; Sanders, M.E.; Reedquist, K.A.; Tak, P.P. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology 2007, 46, 1773–1778. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, J.D.; Clark, R.J.; Noakes, P.G.; Taylor, S.M.; Woodruff, T.M. Preclinical Pharmacokinetics of Complement C5a Receptor Antagonists PMX53 and PMX205 in Mice. ACS Omega 2020, 5, 2345–2354. [Google Scholar] [CrossRef]
- Schafer, N.; Grassel, S. Involvement of complement peptides C3a and C5a in osteoarthritis pathology. Peptides 2022, 154, 170815. [Google Scholar] [CrossRef]
- Postmus, A.C.; Sturmlechner, I.; Jonker, J.W.; van Deursen, J.M.; van de Sluis, B.; Kruit, J.K. Senescent cells in the development of cardiometabolic disease. Curr. Opin. Lipidol. 2019, 30, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hertle, E.; Stehouwer, C.D.; van Greevenbroek, M.M. The complement system in human cardiometabolic disease. Mol. Immunol. 2014, 61, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Bongrazio, M.; Pries, A.R.; Zakrzewicz, A. The endothelium as physiological source of properdin: Role of wall shear stress. Mol. Immunol. 2003, 39, 669–675. [Google Scholar] [CrossRef]
- Kostner, K.M.; Fahti, R.B.; Case, C.; Hobson, P.; Tate, J.; Marwick, T.H. Inflammation, complement activation and endothelial function in stable and unstable coronary artery disease. Clin. Chim. Acta 2006, 365, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Takeuchi, K.; Hiruma, M.; Iida, H.; Tahara, A.; Itagane, H.; Toda, I.; Akioka, K.; Teragaki, M.; Oku, H.; et al. The complement system in ischemic heart disease. Circulation 1990, 81, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lagrand, W.K.; Niessen, H.W.; Wolbink, G.J.; Jaspars, L.H.; Visser, C.A.; Verheugt, F.W.; Meijer, C.J.; Hack, C.E. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation 1997, 95, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Iltumur, K.; Karabulut, A.; Toprak, G.; Toprak, N. Complement activation in acute coronary syndromes. APMIS 2005, 113, 167–174. [Google Scholar] [CrossRef]
- Muscari, A.; Massarelli, G.; Bastagli, L.; Poggiopollini, G.; Tomassetti, V.; Drago, G.; Martignani, C.; Pacilli, P.; Boni, P.; Puddu, P. Relationship of serum C3 to fasting insulin, risk factors and previous ischaemic events in middle-aged men. Eur. Heart J. 2000, 21, 1081–1090. [Google Scholar] [CrossRef]
- Szeplaki, G.; Prohaszka, Z.; Duba, J.; Rugonfalvi-Kiss, S.; Karadi, I.; Kokai, M.; Kramer, J.; Fust, G.; Kleiber, M.; Romics, L.; et al. Association of high serum concentration of the third component of complement (C3) with pre-existing severe coronary artery disease and new vascular events in women. Atherosclerosis 2004, 177, 383–389. [Google Scholar] [CrossRef]
- Trouw, L.A.; Seelen, M.A.; Daha, M.R. Complement and renal disease. Mol. Immunol. 2003, 40, 125–134. [Google Scholar] [CrossRef]
- Sullivan, K.E. Complement deficiency and autoimmunity. Curr. Opin. Pediatr. 1998, 10, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.H.; Wilkes, D.S. Complement system in lung disease. Am. J. Respir. Cell Mol. Biol. 2014, 51, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.F.; Xavier, A.M.; Liu, M.; Cassivi, S.D.; Lindsay, T.F.; Marsh, H.C.; Slutsky, A.S.; Keshavjee, S.H. Effect of complement inhibition with soluble complement receptor 1 on pig allotransplant lung function. Transplantation 1998, 66, 723–732. [Google Scholar] [CrossRef]
- Schmid, R.A.; Zollinger, A.; Singer, T.; Hillinger, S.; Leon-Wyss, J.R.; Schob, O.M.; Hogasen, K.; Zund, G.; Patterson, G.A.; Weder, W. Effect of soluble complement receptor type 1 on reperfusion edema and neutrophil migration after lung allotransplantation in swine. J. Thorac. Cardiovasc. Surg. 1998, 116, 90–97. [Google Scholar] [CrossRef][Green Version]
- Keshavjee, S.; Davis, R.D.; Zamora, M.R.; de Perrot, M.; Patterson, G.A. A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J. Thorac. Cardiovasc. Surg. 2005, 129, 423–428. [Google Scholar] [CrossRef]
- Stammberger, U.; Hamacher, J.; Hillinger, S.; Schmid, R.A. sCR1sLe ameliorates ischemia/reperfusion injury in experimental lung transplantation. J. Thorac. Cardiovasc. Surg. 2000, 120, 1078–1084. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Mastellos, D.; Tudoran, R.; DeAngelis, R.A.; Strey, C.W.; Franchini, S.; Wetsel, R.A.; Erdei, A.; Lambris, J.D. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J. Immunol. 2004, 173, 747–754. [Google Scholar] [CrossRef]
- Mastellos, D.; Papadimitriou, J.C.; Franchini, S.; Tsonis, P.A.; Lambris, J.D. A novel role of complement: Mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001, 166, 2479–2486. [Google Scholar] [CrossRef]
- Strey, C.W.; Markiewski, M.; Mastellos, D.; Tudoran, R.; Spruce, L.A.; Greenbaum, L.E.; Lambris, J.D. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 2003, 198, 913–923. [Google Scholar] [CrossRef]
- Qin, X.; Gao, B. The complement system in liver diseases. Cell Mol. Immunol. 2006, 3, 333–340. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Zhang, Y.; Zhang, K.; Yuan, Y.; Jia, S.; Liu, J. The Complement System, Aging, and Aging-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8689. https://doi.org/10.3390/ijms23158689
Zheng R, Zhang Y, Zhang K, Yuan Y, Jia S, Liu J. The Complement System, Aging, and Aging-Related Diseases. International Journal of Molecular Sciences. 2022; 23(15):8689. https://doi.org/10.3390/ijms23158689
Chicago/Turabian StyleZheng, Runzi, Yanghuan Zhang, Ke Zhang, Yang Yuan, Shuting Jia, and Jing Liu. 2022. "The Complement System, Aging, and Aging-Related Diseases" International Journal of Molecular Sciences 23, no. 15: 8689. https://doi.org/10.3390/ijms23158689
APA StyleZheng, R., Zhang, Y., Zhang, K., Yuan, Y., Jia, S., & Liu, J. (2022). The Complement System, Aging, and Aging-Related Diseases. International Journal of Molecular Sciences, 23(15), 8689. https://doi.org/10.3390/ijms23158689