Crosstalk between Growth and Osmoregulation of GHRH-SST-GH-IGF Axis in Triploid Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Results
2.1. Identification of Rainbow Trout sst Genes
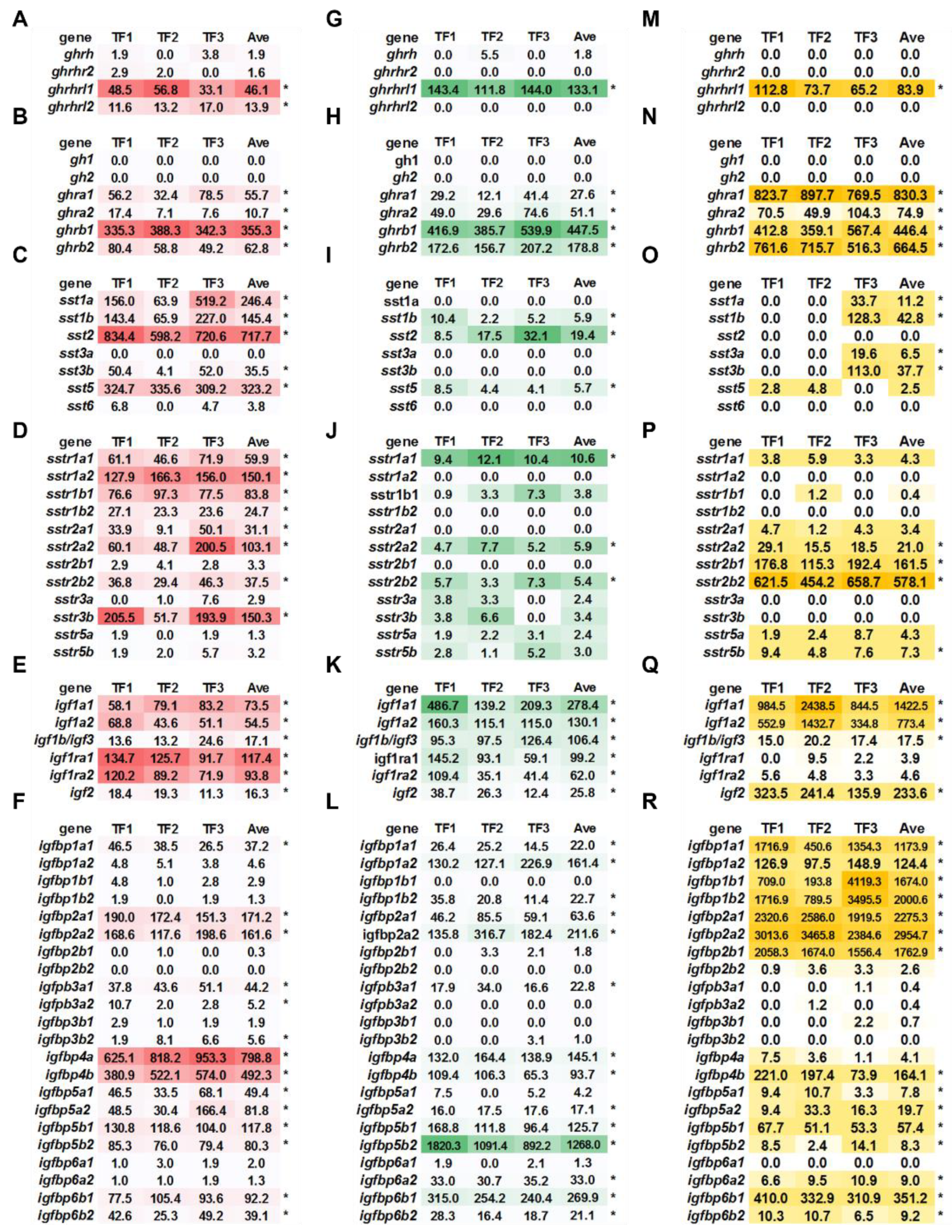
2.2. Basal Expression of ghrh-gh-sst-igf Axis in Triploid Trout
2.3. Comparison of Basal Expression of ghrh-gh-sst-igf Axis between Diploid and Triploid Trout
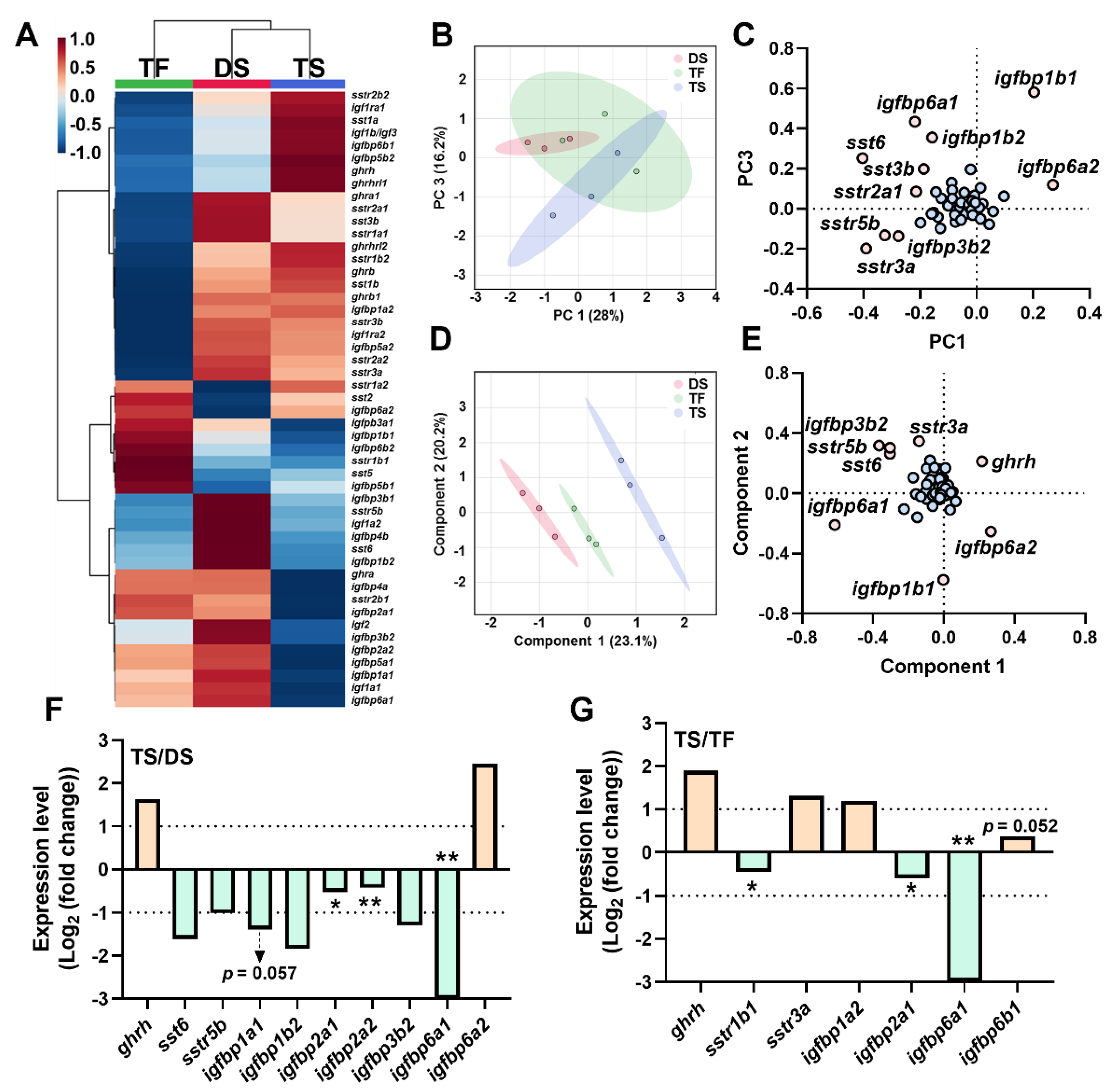
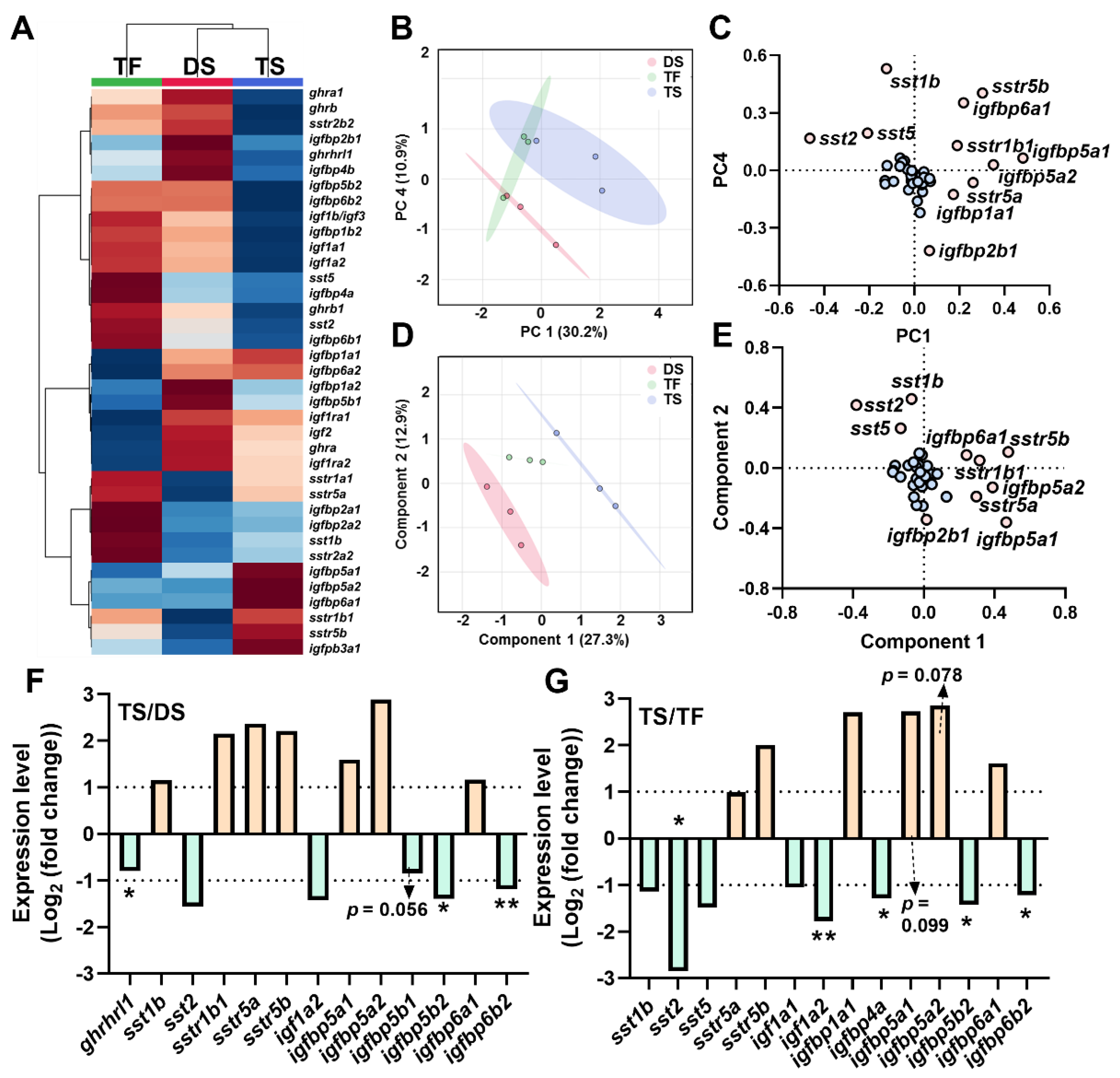
2.4. Transcriptional Signature of Triploid Brain ghrh-gh-sst-igf Axis in Response to Seawater Challenge
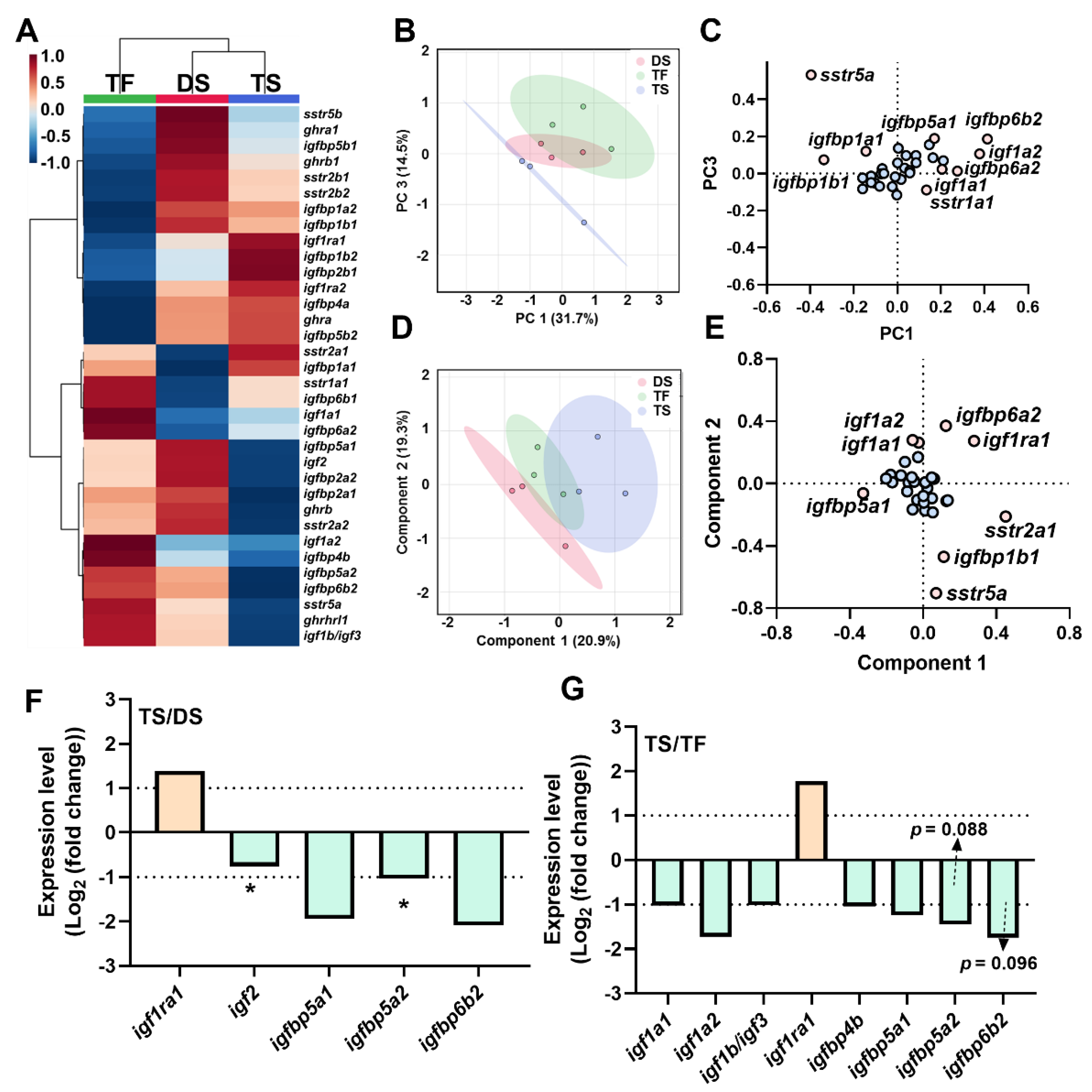
2.5. Transcriptional Signature of Triploid Liver ghrh-gh-sst-igf Axis in Response to Seawater Challenge
2.6. Transcriptional Signature of Triploid Kidney ghrh-gh-sst-igf Axis in Response to Seawater Challenge
2.7. Correlation Analyses of ghrh-gh-sst-igf Axis
3. Discussion
3.1. Complete Repertoire of sst in Rainbow Trout
3.2. Transcriptional Signature of ghrh-sst-gh-igf Axis in Response to Seawater Challenge
3.2.1. The SST System
3.2.2. The IGFBPs System
- The IGFBPs of triploid trout before and after seawater challenge.
- 2.
- Comparison of IGFBPs in diploid and triploid in response to seawater challenge.
4. Materials and Methods
4.1. Ethics Statement
4.2. Ploidy Identification
4.3. Animals Acclimation and Salinity Challenge
4.4. Sampling and RNA-seq
4.5. Identification of Rainbow Trout sst Repertoire
4.6. Quantitative Polymerase Chain Reaction (qPCR)
4.7. Data Visualization and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavlov, D.; Savvaitova, K. On the problem of ratio of anadromy and residence in salmonids (Salmonidae). J. Ichthyol. 2008, 48, 778–791. [Google Scholar] [CrossRef]
- Kendall, N.W.; McMillan, J.R.; Sloat, M.R.; Buehrens, T.W.; Quinn, T.P.; Pess, G.R.; Kuzishchin, K.V.; McClure, M.M.; Zabel, R.W. Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): A review of the processes and patterns. Can. J. Fish. Aquat. Sci. 2015, 72, 319–342. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.-H. Emerging trends in global freshwater availability. Nature 2018, 557, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine heatwaves under global warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Chang. 2017, 7, 718–722. [Google Scholar] [CrossRef]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.; Huijbregts, M.A.; Schipper, A.M. Threats of global warming to the world’s freshwater fishes. Nat. Commun. 2021, 12, 1701. [Google Scholar] [CrossRef]
- Zhang, S.; Han, G.; Xie, Y.; Ruiz, J.J. Data assimilation in numerical weather and climate models. Adv. Meteorol. 2015, 2015, 626893. [Google Scholar] [CrossRef]
- Thorgaard, G.H. 8 Chromosome Set Manipulation and Sex Control in Fish. Fish Physiol. 1983, 9, 405–434. [Google Scholar] [CrossRef]
- Sheehan, R.J.; Shasteen, S.P.; Suresh, A.V.; Kapuscinski, A.R.; Seeb, J.E. Better growth in all-female diploid and triploid rainbow trout. Trans. Am. Fish. Soc. 1999, 128, 491–498. [Google Scholar] [CrossRef]
- Naylor, R.; Hindar, K.; Fleming, I.A.; Goldburg, R.; Williams, S.; Volpe, J.; Whoriskey, F.; Eagle, J.; Kelso, D.; Mangel, M. Fugitive salmon: Assessing the risks of escaped fish from net-pen aquaculture. BioScience 2005, 55, 427–437. [Google Scholar] [CrossRef]
- Fraser, T.W.; Fjelldal, P.G.; Hansen, T.; Mayer, I. Welfare considerations of triploid fish. Rev. Fish. Sci. 2012, 20, 192–211. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef]
- McCormick, S.D. Smolt physiology and endocrinology. Fish Physiol. 2012, 32, 199–251. [Google Scholar] [CrossRef]
- Mancera, J.M.; McCormick, S.D. Role of prolactin, growth hormone, insulin-like growth factor I and cortisol in teleost osmoregulation. In Fish Osmoregulation; CRC Press: Boca Raton, FL, USA, 2019; pp. 497–515. [Google Scholar]
- Daza, D.O.; Sundström, G.; Bergqvist, C.A.; Duan, C.; Larhammar, D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology 2011, 152, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, A.; Castro, R.; Wang, T.; Secombes, C.J.; Boudinot, P.; Macqueen, D.J.; Martin, S.A. Cross talk between growth and immunity: Coupling of the IGF axis to conserved cytokine pathways in rainbow trout. Endocrinology 2016, 157, 1942–1955. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine control of osmoregulation in teleost fish. Am. Zool. 2001, 41, 781–794. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Kim, J.-H.; Macqueen, D.J.; Winton, J.R.; Hansen, J.D.; Park, H.; Devlin, R.H. Effect of growth rate on transcriptomic responses to immune stimulation in wild-type, domesticated, and GH-transgenic coho salmon. BMC Genom. 2019, 20, 1024. [Google Scholar] [CrossRef]
- McCormick, S.D. Effects of growth hormone and insulin-like growth factor I on salinity tolerance and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar): Interaction with cortisol. Gen. Comp. Endocrinol. 1996, 101, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Shepherd, B.S.; Madsen, S.S.; Nishioka, R.S.; Siharath, K.; Richman, N.H., III; Bern, H.A.; Grau, E.G. Osmoregulatory actions of growth hormone and prolactin in an advanced teleost. Gen. Comp. Endocrinol. 1997, 106, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Garcia de la Serrana, D.; Macqueen, D.J. Insulin-Like Growth Factor-Binding Proteins of Teleost Fishes. Front. Endocrinol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Shimizu, M.; Kishimoto, K.; Yamaguchi, T.; Nakano, Y.; Hara, A.; Dickhoff, W.W. Circulating salmon 28- and 22-kDa insulin-like growth factor binding proteins (IGFBPs) are co-orthologs of IGFBP-1. Gen. Comp. Endocrinol. 2011, 174, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Maryoung, L.A.; Lavado, R.; Bammler, T.K.; Gallagher, E.P.; Stapleton, P.L.; Beyer, R.P.; Farin, F.M.; Hardiman, G.; Schlenk, D. Differential gene expression in liver, gill, and olfactory rosettes of coho salmon (Oncorhynchus kisutch) after acclimation to salinity. Mar. Biotechnol. 2015, 17, 703–717. [Google Scholar] [CrossRef]
- Breves, J.P.; Fujimoto, C.K.; Phipps-Costin, S.K.; Einarsdottir, I.E.; Bjornsson, B.T.; McCormick, S.D. Variation in branchial expression among insulin-like growth-factor binding proteins (igfbps) during Atlantic salmon smoltification and seawater exposure. BMC Physiol. 2017, 17, 2. [Google Scholar] [CrossRef]
- Breves, J.P.; Springer-Miller, R.; Chenoweth, D.; Paskavitz, A.; Chang, A.; Regish, A.M.; Einarsdottir, I.; Björnsson, B.T.; McCormick, S.D. Cortisol regulates insulin-like growth-factor binding protein (igfbp) gene expression in Atlantic salmon parr. Mol. Cell. Endocrinol. 2020, 518, 110989. [Google Scholar] [CrossRef]
- Khaw, H.L.; Gjerde, B.; Boison, S.A.; Hjelle, E.; Difford, G.F. Quantitative Genetics of Smoltification Status at the Time of Seawater Transfer in Atlantic Salmon (Salmo Salar). Front. Genet. 2021, 12, 696893. [Google Scholar] [CrossRef]
- Hoar, W. 4 The physiology of smolting salmonids. Fish Physiol. 1988, 11, 275–343. [Google Scholar] [CrossRef]
- Macqueen, D.J.; Garcia de la Serrana, D.; Johnston, I.A. Evolution of ancient functions in the vertebrate insulin-like growth factor system uncovered by study of duplicated salmonid fish genomes. Mol. Biol. Evol. 2013, 30, 1060–1076. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Martin, S.A.; Johnston, I.A.; Macqueen, D.J. Divergent regulation of insulin-like growth factor binding protein genes in cultured Atlantic salmon myotubes under different models of catabolism and anabolism. Gen. Comp. Endocrinol. 2017, 247, 53–65. [Google Scholar] [CrossRef]
- Alzaid, A.; Martin, S.A.; Macqueen, D.J. The complete salmonid IGF-IR gene repertoire and its transcriptional response to disease. Sci. Rep. 2016, 6, 34806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, D.; Zhang, Y.; Li, S.; Liu, X.; Lin, H. The evolution of somatostatin in vertebrates. Gene 2010, 463, 21–28. [Google Scholar] [CrossRef]
- Tostivint, H.; Quan, F.B.; Bougerol, M.; Kenigfest, N.B.; Lihrmann, I. Impact of gene/genome duplications on the evolution of the urotensin II and somatostatin families. Gen. Comp. Endocrinol. 2013, 188, 110–117. [Google Scholar] [CrossRef]
- Tostivint, H.; Gaillard, A.-L.; Mazan, S.; Pézeron, G. Revisiting the evolution of the somatostatin family: Already five genes in the gnathostome ancestor. Gen. Comp. Endocrinol. 2019, 279, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Macqueen, D.J.; Johnston, I.A. A well-constrained estimate for the timing of the salmonid whole genome duplication reveals major decoupling from species diversification. Proc. R. Soc. B 2014, 281, 20132881. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Mommsen, T.P. Effects of nutritional state on in vivo lipid and carbohydrate metabolism of coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 1991, 81, 473–483. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Hagemeister, A.L. Somatostatin and somatostatin receptors in fish growth. Gen. Comp. Endocrinol. 2010, 167, 360–365. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Ehrman, M.M.; Melroe, G.T.; Moore, C.A.; Kittilson, J.D.; Sheridan, M.A. Nutritional regulation of somatostatin expression in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2002, 26, 309–314. [Google Scholar] [CrossRef]
- Kong, C.; Glen, P. The use of the somatostatin analogue octreotide for the conservative management of symptomatic cholecystocolonic fistula: A case report. Ann. R. Coll. Surg. 2019, 101, e59–e61. [Google Scholar] [CrossRef] [PubMed]
- Canosa, L.F.; Chang, J.P.; Peter, R.E. Neuroendocrine control of growth hormone in fish. Gen. Comp. Endocrinol. 2007, 151, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.W.; Duan, G.; Bern, H.A. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 2005, 243, 215–285. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, M.; Björnsson, B.T.; Dickhoff, W.W.; McCormick, S.D.; Navarro, I.; Power, D.M.; Gutiérrez, J. Growth hormone and insulin-like growth factors in fish: Where we are and where to go. Gen. Comp. Endocrinol. 2005, 142, 20–24. [Google Scholar] [CrossRef]
- Gabillard, J.-C.; Kamangar, B.B.; Montserrat, N. Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2006, 191, 15–24. [Google Scholar] [CrossRef]
- Rahman, M.S.; Thomas, P. Characterization of three IGFBP mRNAs in Atlantic croaker and their regulation during hypoxic stress: Potential mechanisms of their upregulation by hypoxia. Am. J. Physiol. 2011, 301, E637–E648. [Google Scholar] [CrossRef]
- Maures, T.J.; Duan, C. Structure, developmental expression, and physiological regulation of zebrafish IGF binding protein-1. Endocrinology 2002, 143, 2722–2731. [Google Scholar] [CrossRef]
- Dai, W.; Bai, Y.; Hebda, L.; Zhong, X.; Liu, J.; Kao, J.; Duan, C. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ. 2014, 21, 568–581. [Google Scholar] [CrossRef]
- Zheng, G.-D.; Zhou, C.-X.; Lin, S.-T.; Chen, J.; Jiang, X.-Y.; Zou, S.-M. Two grass carp (Ctenopharyngodon idella) insulin-like growth factor-binding protein 5 genes exhibit different yet conserved functions in development and growth. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 204, 69–76. [Google Scholar] [CrossRef]
- Himes, R.W.; Smith, C.W. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010, 24, 731–739. [Google Scholar] [CrossRef]
- Chalmers, L.; Thompson, K.D.; Taylor, J.F.; Black, S.; Migaud, H.; North, B.; Adams, A. A comparison of the response of diploid and triploid Atlantic salmon (Salmo salar) siblings to a commercial furunculosis vaccine and subsequent experimental infection with Aeromonas salmonicida. Fish Shellfish Immunol. 2016, 57, 301–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McEwen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.W.; Schlueter, P.J.; Duan, C. Targeted knockdown of insulin-like growth factor binding protein-2 disrupts cardiovascular development in zebrafish embryos. Mol. Endocrinol. 2005, 19, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, W.; Kamei, H.; Duan, C. Duplication of the IGFBP-2 gene in teleost fish: Protein structure and functionality conservation and gene expression divergence. PLoS ONE 2008, 3, e3926. [Google Scholar] [CrossRef][Green Version]
- Bower, N.I.; Johnston, I.A. Transcriptional regulation of the IGF signaling pathway by amino acids and insulin-like growth factors during myogenesis in Atlantic salmon. PLoS ONE 2010, 5, e11100. [Google Scholar] [CrossRef]
- Kusakabe, M.; Ishikawa, A.; Ravinet, M.; Yoshida, K.; Makino, T.; Toyoda, A.; Fujiyama, A.; Kitano, J. Genetic basis for variation in salinity tolerance between stickleback ecotypes. Mol. Ecol. 2017, 26, 304–319. [Google Scholar] [CrossRef]
- Pellissier, T.; Al Nafea, H.; Good, S.V. Divergence of insulin superfamily ligands, receptors and Igf binding proteins in marine versus freshwater stickleback: Evidence of selection in known and novel genes. Comp. Biochem. Physiol. Part D Genom. Proteom 2018, 25, 53–61. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Hou, Z.-S.; Zhao, H.-K.; Xin, Y.-R.; Liu, M.-Q.; Yang, X.-D.; Wen, H.-S.; Li, J.-F. Identification and characterization of caspases genes in rainbow trout (Oncorhynchus mykiss) and their expression profiles after Aeromonas salmonicida and Vibrio anguillarum infection. Dev. Comp. Immunol. 2021, 118, 103987. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Soufan, O.; Xia, J.; Tang, R.; Li, L.; Li, D. Transcriptome and physiological analysis reveal alterations in muscle metabolisms and immune responses of grass carp (Ctenopharyngodon idellus) cultured at different stocking densities. Aquaculture 2019, 503, 186–197. [Google Scholar] [CrossRef]
- Mouton, A.J.; Ma, Y.; Rivera Gonzalez, O.J.; Daseke, M.J.; Flynn, E.R.; Freeman, T.C.; Garrett, M.R.; DeLeon-Pennell, K.Y.; Lindsey, M.L. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res. Cardiol. 2019, 114, 6. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level—The DESeq Package; European Molecular Biology Laboratory (EMBL): Heidelberg, Germany, 2012. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Yang, X.-D.; Hou, Z.-S.; Liu, M.-Q.; Zeng, C.; Zhao, H.-K.; Xin, Y.-R.; Xiang, K.-W.; Yang, Q.; Wen, H.-S.; Li, J.-F. Identification and characterization of mkk genes and their expression profiles in rainbow trout (Oncorhynchus mykiss) symptomatically or asymptomatically infected with Vibrio anguillarum. Fish Shellfish Immunol. 2022, 121, 1–11. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Zeng, C.; Zhao, H.; Li, J.; Hou, Z.; Wen, H. Transcriptional Signatures of Immune, Neural, and Endocrine Functions in the Brain and Kidney of Rainbow Trout (Oncorhynchus mykiss) in Response to Aeromonas salmonicida Infection. Int. J. Mol. Sci. 2022, 23, 1340. [Google Scholar] [CrossRef]

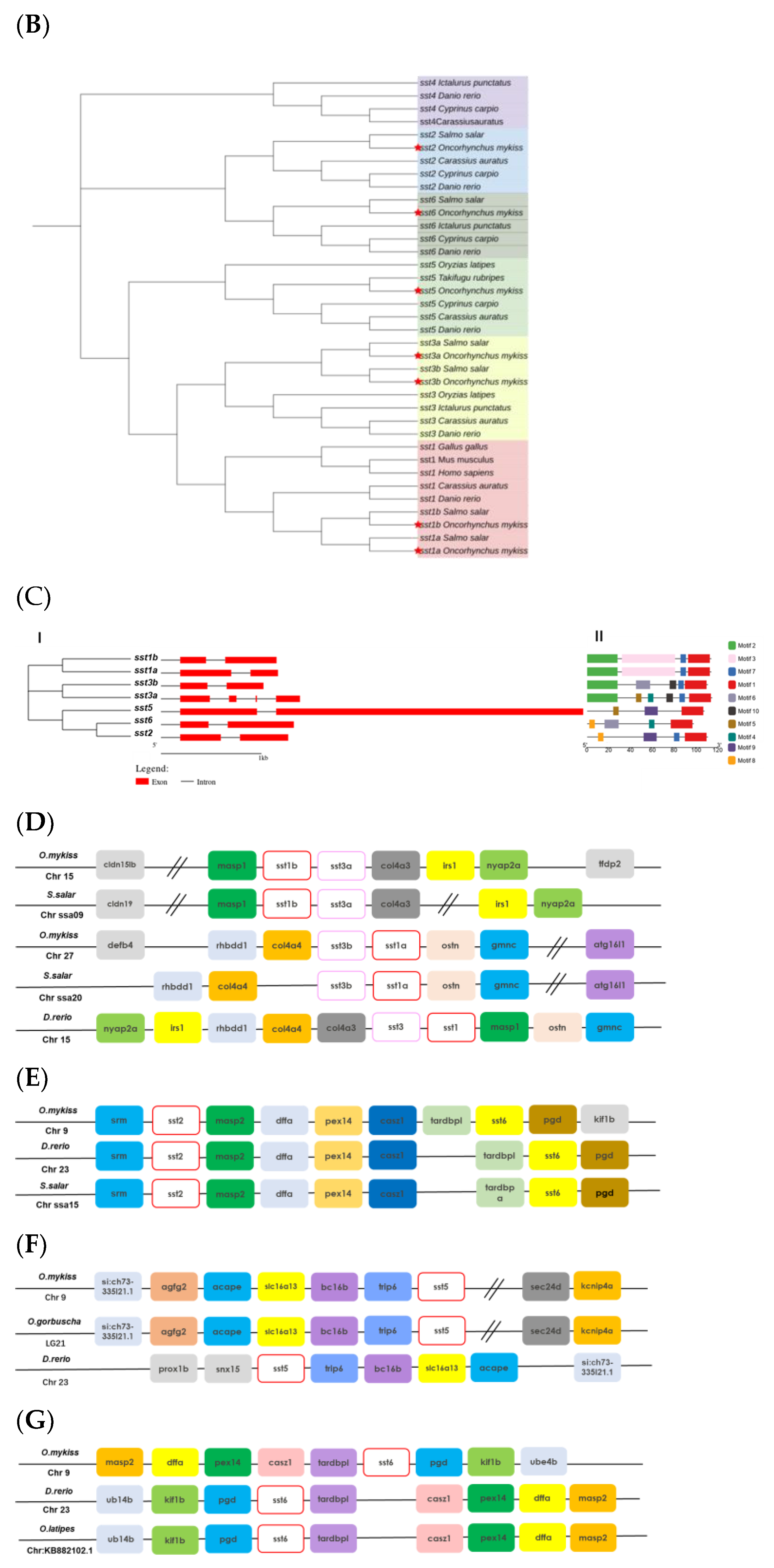

| Gene Name | Chromosome ID | mRNA (bp) | Protein Length (aa) | MW (KDa) | pI | Accession Numbers |
|---|---|---|---|---|---|---|
| sst1a | 27 | 771 | 114 | 12.42032 | 5.51 | XP_021442876.1 |
| sst1b | 15 | 785 | 114 | 12.46135 | 5.14 | XP_021418859.1 |
| sst2 | 9 | 887 | 111 | 12.47253 | 6.31 | XP_021471775.1 |
| sst3a | 15 | 624 | 115 | 12.96316 | 10.16 | NP_001118175.1 |
| sst3b | 27 | 641 | 111 | 12.32508 | 7.66 | XP_021442875.1 |
| sst5 | 9 | 3822 | 108 | 12.06404 | 6.10 | XP_021470745.1 |
| sst6 | 9 | 943 | 98 | 11.50144 | 8.54 | XP_021472825.1 |
| Brain | Liver | Kidney | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Triploid | Diploid | Fold | Gene | Triploid | Diploid | Fold | Gene | Triploid | Diploid | Fold |
| ghrh | 2.19 | 0.57 | 3.82 | ghrb1 | 2761.29 | 6143.88 | 0.45 | ghrh | 1.04 | 0.11 | 9.17 |
| ghrhr2 | 1.89 | 0.61 | 3.11 | sst1b | 264.44 | 116.81 | 2.26 | ghrhrl2 | 0.00 | 0.73 | 0.00 |
| sst1a | 282.66 | 60.84 | 4.65 | sst1a | 69.47 | 124.07 | 0.56 | ghrhrl1 | 75.59 | 21.00 | 3.60 |
| sst3b | 40.72 | 5.74 | 7.10 | sst3b | 233.07 | 61.20 | 3.81 | ghra1 | 15.67 | 7.07 | 2.22 |
| sst5 | 370.77 | 69.89 | 5.30 | sstr1a1 | 26.74 | 8.78 | 3.05 | ghrb1 | 254.22 | 133.56 | 1.90 |
| sstr2a2 | 118.27 | 48.68 | 2.43 | igf1a1 | 8799.16 | 2417.77 | 3.64 | sst1b | 3.36 | 0.74 | 4.57 |
| sstr3b | 172.49 | 32.15 | 5.37 | igf1a2 | 4784.27 | 1005.80 | 4.76 | sst5 | 3.22 | 1.09 | 2.96 |
| sstr5a | 1.46 | 3.89 | 0.38 | igfbp1a1 | 7261.62 | 13,604.07 | 0.53 | sstr1a1 | 6.03 | 3.51 | 1.72 |
| igfbp1b1 | 3.33 | 0.00 | igfbp3a2 | 2.45 | 10.94 | 0.22 | sstr5b | 1.72 | 0.27 | 6.40 | |
| igfbp3a2 | 5.94 | 1.71 | 3.46 | igfbp4a | 25.11 | 44.17 | 0.57 | igf1a1 | 158.15 | 29.80 | 5.31 |
| igfbp3b2 | 6.37 | 13.75 | 0.46 | igfbp4b | 1014.94 | 231.54 | 4.38 | igf1a2 | 73.93 | 9.65 | 7.66 |
| igfbp5a2 | 93.82 | 24.07 | 3.90 | igfbp5a1 | 48.17 | 178.17 | 0.27 | igf1ra1 | 56.33 | 23.54 | 2.39 |
| igfbp6a1 | 2.26 | 4.07 | 0.55 | igfbp6a1 | 0.00 | 0.78 | 0.00 | igfbp1a2 | 91.69 | 30.75 | 2.98 |
| igfbp2a2 | 120.22 | 241.84 | 0.50 | ||||||||
| igfbp3a2 | 0.00 | 0.49 | 0.00 | ||||||||
| igfbp4a | 82.43 | 40.55 | 2.03 | ||||||||
| igfbp6b1 | 153.32 | 83.79 | 1.83 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, K.; Yang, Q.; Liu, M.; Yang, X.; Li, J.; Hou, Z.; Wen, H. Crosstalk between Growth and Osmoregulation of GHRH-SST-GH-IGF Axis in Triploid Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2022, 23, 8691. https://doi.org/10.3390/ijms23158691
Xiang K, Yang Q, Liu M, Yang X, Li J, Hou Z, Wen H. Crosstalk between Growth and Osmoregulation of GHRH-SST-GH-IGF Axis in Triploid Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences. 2022; 23(15):8691. https://doi.org/10.3390/ijms23158691
Chicago/Turabian StyleXiang, Kaiwen, Qian Yang, Mengqun Liu, Xiaodong Yang, Jifang Li, Zhishuai Hou, and Haishen Wen. 2022. "Crosstalk between Growth and Osmoregulation of GHRH-SST-GH-IGF Axis in Triploid Rainbow Trout (Oncorhynchus mykiss)" International Journal of Molecular Sciences 23, no. 15: 8691. https://doi.org/10.3390/ijms23158691
APA StyleXiang, K., Yang, Q., Liu, M., Yang, X., Li, J., Hou, Z., & Wen, H. (2022). Crosstalk between Growth and Osmoregulation of GHRH-SST-GH-IGF Axis in Triploid Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences, 23(15), 8691. https://doi.org/10.3390/ijms23158691





