Adult Exposure to Di-N-Butyl Phthalate (DBP) Induces Persistent Effects on Testicular Cell Markers and Testosterone Biosynthesis in Mice
Abstract
:1. Introduction
2. Results
2.1. General Status
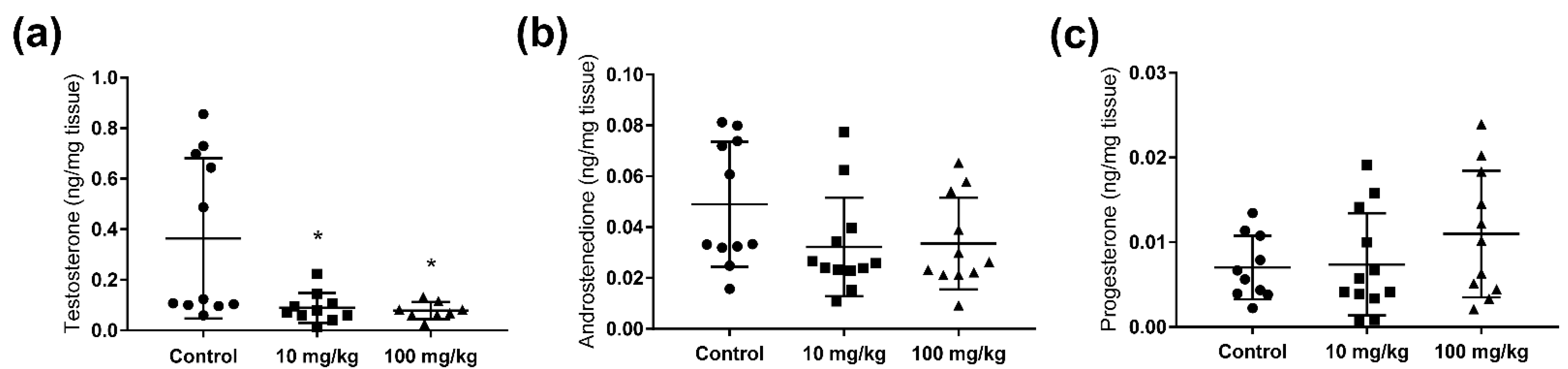
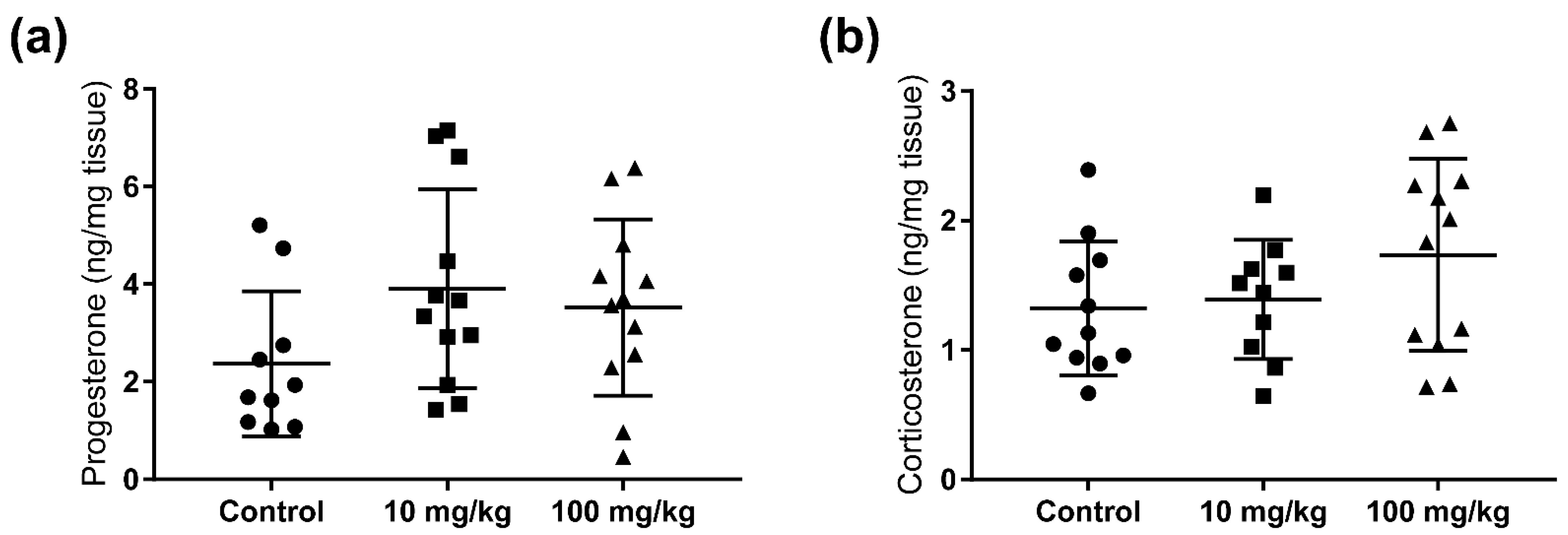
2.2. Steroid Hormone Concentrations in Testis and Adrenal Glands
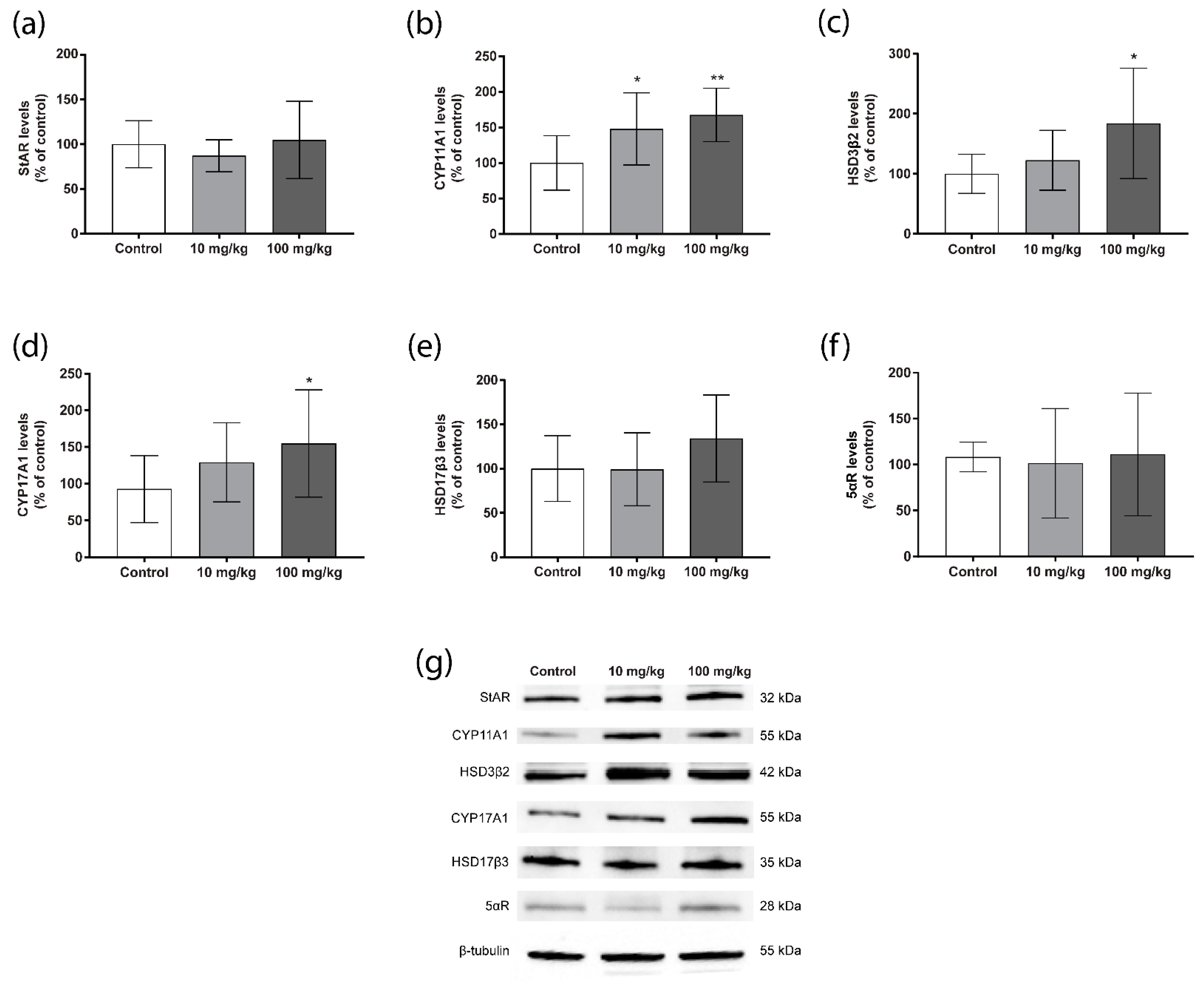
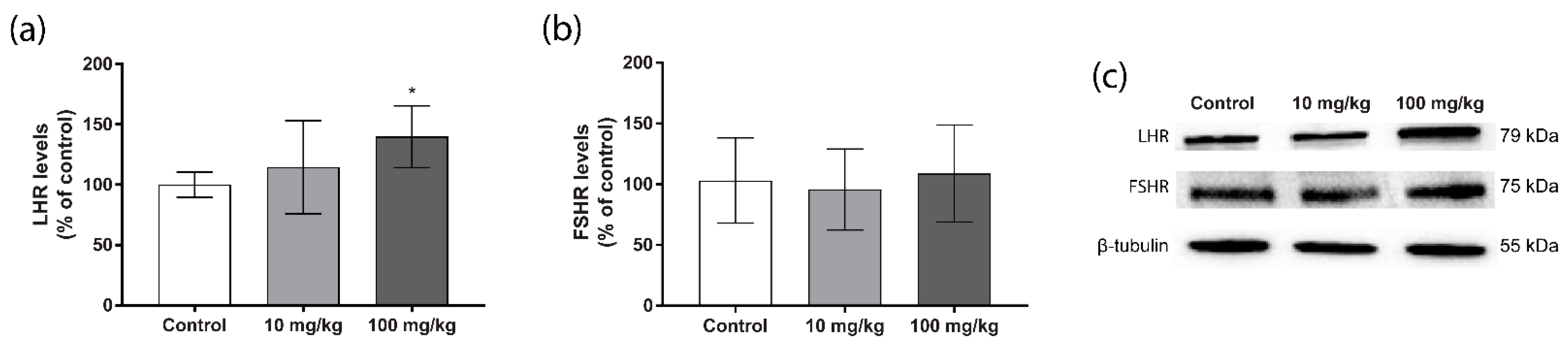
2.3. Steroidogenic Protein Levels
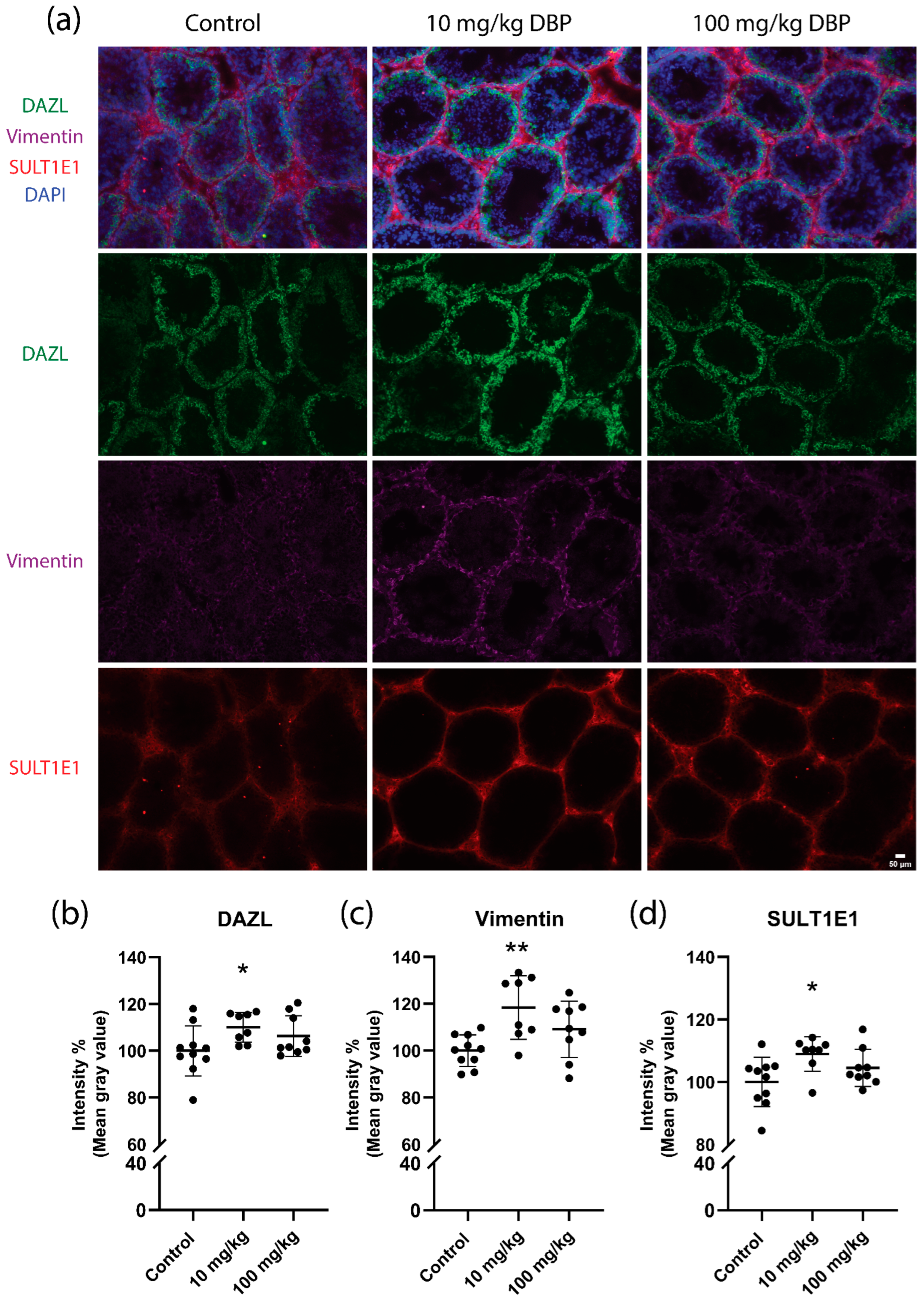
2.4. Testis Histology and Cell Marker Levels
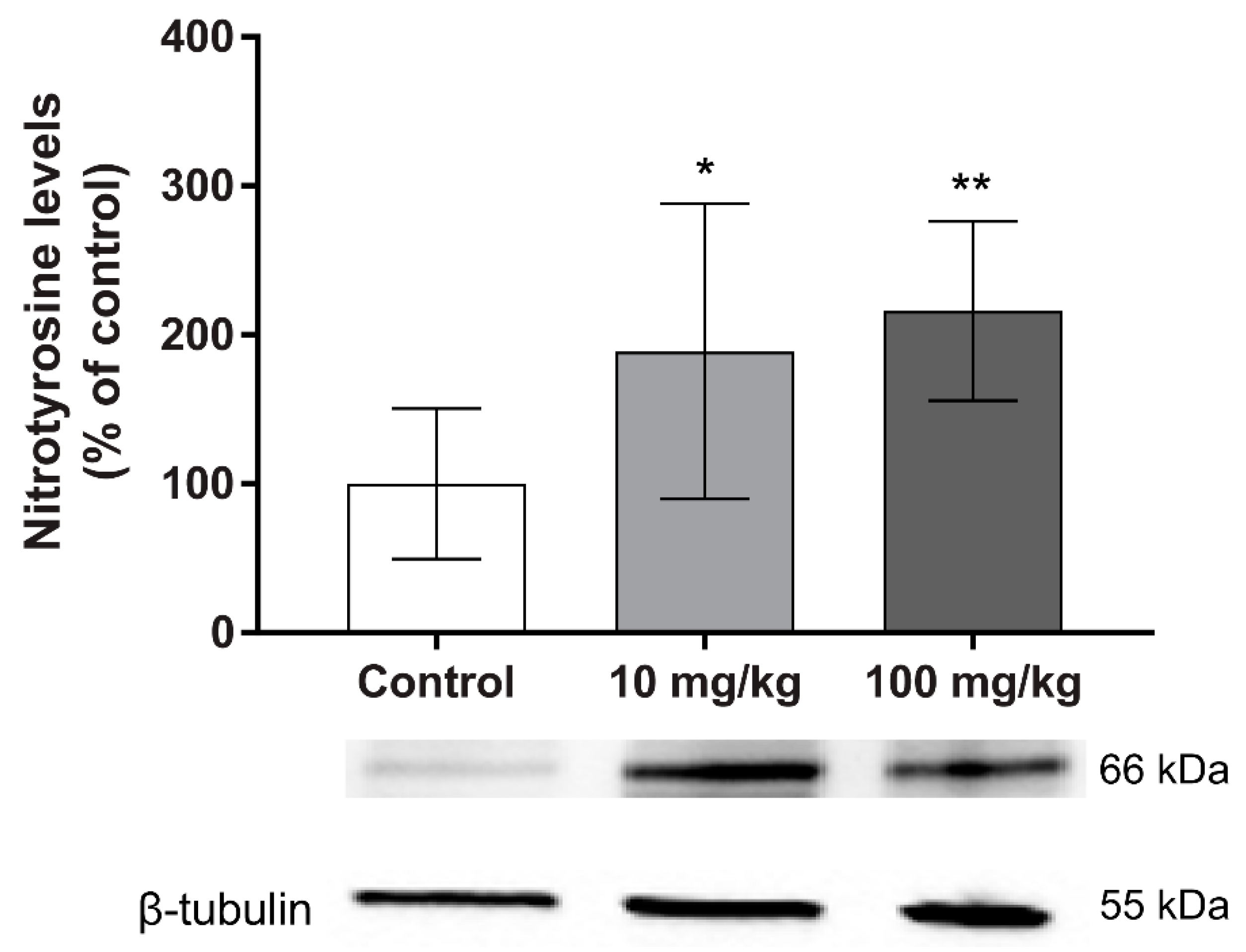
3. Discussion
4. Methods and Materials
4.1. Chemicals and Reagents
4.2. Animal Housing and Exposure
4.3. Steroid Hormone Extraction
4.4. UPLC-HRMS Analysis of Steroid Hormone Levels
4.5. Immunohistochemistry
4.6. Western Blot
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. Comparative Assessment of Human Exposure to Phthalate Esters from House Dust in China and the United States. Environ. Sci. Technol. 2011, 45, 3788–3794. [Google Scholar] [CrossRef]
- Kavlock, R.; Boekelheide, K.; Chapin, R.; Cunningham, M.; Faustman, E.; Foster, P.; Golub, M.; Henderson, R.; Hinberg, I.; Little, R.; et al. NTP Center for the Evaluation of Risks to Human Reproduction: Phthalates Expert Panel Report on the Reproductive and Developmental Toxicity of Di-n-Butyl Phthalate. Reprod. Toxicol. 2002, 16, 489–527. [Google Scholar] [CrossRef]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and Developmental Effects of Phthalate Diesters in Males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate Exposure and Male Reproductive Outcomes: A Systematic Review of the Human Epidemiological Evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef]
- Czubacka, E.; Czerczak, S.; Kupczewska-Dobecka, M.M. The Overview of Current Evidence on the Reproductive Toxicity of Dibutyl Phthalate. Int. J. Occup. Med. Environ. Health 2021, 34, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.C.; Chapin, R.E.; Teague, J.; Davis Lawton, A.; Reel, J.R. Reproductive Effects of Four Phthalic Acid Esters in the Mouse. Toxicol. Appl. Pharmacol. 1987, 88, 255–269. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Y.; Liu, Y.; Tao, L. Exposure to Dibutyl Phthalate and Reproductive-Related Outcomes in Animal Models: Evidence From Rodents Study. Front. Physiol. 2021, 12, 684532. [Google Scholar] [CrossRef]
- Aly, H.A.; Hassan, M.H.; El-Beshbishy, H.A.; Alahdal, A.M.; Osman, A.-M.M. Dibutyl Phthalate Induces Oxidative Stress and Impairs Spermatogenesis in Adult Rats. Toxicol. Ind. Health 2016, 32, 1467–1477. [Google Scholar] [CrossRef]
- Marsman, D. NTP Technical Report on the Toxicity Studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice. Toxic. Rep. Ser. 1995, 30, 1-G5. [Google Scholar] [PubMed]
- Zhou, D.; Wang, H.; Zhang, J.; Gao, X.; Zhao, W.; Zheng, Y. Di-n-Butyl Phthalate (DBP) Exposure Induces Oxidative Damage in Testes of Adult Rats. Syst. Biol. Reprod. Med. 2010, 56, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.S.; Waheeb, R.S. Ginger Attenuated Di (n-Butyl) Phthalate-Induced Reproductive Toxicity in Pubertal Male Rabbits. World Rabbit Sci. 2017, 25, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wang, H.; Zhang, J. Di-n-Butyl Phthalate (DBP) Exposure Induces Oxidative Stress in Epididymis of Adult Rats. Toxicol. Ind. Health 2011, 27, 65–71. [Google Scholar] [CrossRef]
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein Oxidation and Cellular Homeostasis: Emphasis on Metabolism. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007, 1773, 93–104. [Google Scholar] [CrossRef]
- Kaur, P.; Bansal, M.P. Effect of Experimental Oxidative Stress on Steroidogenesis and DNA Damage in Mouse Testis. J. Biomed. Sci. 2004, 11, 391–397. [Google Scholar] [CrossRef]
- Zaidi, S.K.; Shen, W.-J.; Cortez, Y.; Bittner, S.; Bittner, A.; Arshad, S.; Huang, T.-T.; Kraemer, F.B.; Azhar, S. SOD2 Deficiency-Induced Oxidative Stress Attenuates Steroidogenesis in Mouse Ovarian Granulosa Cells. Mol. Cell. Endocrinol. 2021, 519, 110888. [Google Scholar] [CrossRef]
- Bao, A.-M.; Man, X.-M.; Guo, X.-J.; Dong, H.-B.; Wang, F.-Q.; Sun, H.; Wang, Y.-B.; Zhou, Z.-M.; Sha, J.-H. Effects of Di-n-Butyl Phthalate on Male Rat Reproduction Following Pubertal Exposure. Asian J. Androl. 2011, 13, 702–709. [Google Scholar] [CrossRef]
- Matsumoto, A.M.; Bremner, W.J. 4Endocrinology of the Hypothalamic-Pituitary-Testicular Axis with Particular Reference to the Hormonal Control of Spermatogenesis. Baillière’s Clin. Endocrinol. Metab. 1987, 1, 71–87. [Google Scholar] [CrossRef]
- Narayan, P. Genetic Models for the Study of Luteinizing Hormone Receptor Function. Front. Endocrinol. 2015, 6, 152. [Google Scholar] [CrossRef] [Green Version]
- Shultz, V.D. Altered Gene Profiles in Fetal Rat Testes after in Utero Exposure to Di(n-Butyl) Phthalate. Toxicol. Sci. 2001, 64, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Sharma, S.; Afjal, M.A.; Habib, H.; Akhter, J.; Goswami, P.; Parvez, S.; Akhtar, M.; Raisuddin, S. MRNA Expression and Protein-Protein Interaction (PPI) Network Analysis of Adrenal Steroidogenesis in Response to Exposure to Phthalates in Rats. Environ. Toxicol. Pharmacol. 2022, 89, 103780. [Google Scholar] [CrossRef] [PubMed]
- Franke, W.W.; Grund, C.; Schmid, E. Intermediate-Sized Filaments Present in Sertoli Cells Are of the Vimentin Type. Eur. J. Cell Biol. 1979, 19, 269–275. [Google Scholar] [PubMed]
- Ruggiu, M.; Speed, R.; Taggart, M.; McKay, S.J.; Kilanowski, F.; Saunders, P.; Dorin, J.; Cooke, H.J. The Mouse Dazla Gene Encodes a Cytoplasmic Protein Essential for Gametogenesis. Nature 1997, 389, 73–77. [Google Scholar] [CrossRef]
- Song, W.C.; Qian, Y.; Sun, X.; Negishi, M. Cellular Localization and Regulation of Expression of Testicular Estrogen Sulfotransferase. Endocrinology 1997, 138, 5006–5012. [Google Scholar] [CrossRef]
- Li, H.; Liang, Z.; Yang, J.; Wang, D.; Wang, H.; Zhu, M.; Geng, B.; Xu, E.Y. DAZL Is a Master Translational Regulator of Murine Spermatogenesis. Natl. Sci. Rev. 2019, 6, 455–468. [Google Scholar] [CrossRef] [Green Version]
- Zagore, L.L.; Sweet, T.J.; Hannigan, M.M.; Weyn-Vanhentenryck, S.M.; Jobava, R.; Hatzoglou, M.; Zhang, C.; Licatalosi, D.D. DAZL Regulates Germ Cell Survival through a Network of PolyA-Proximal MRNA Interactions. Cell Rep. 2018, 25, 1225–1240.e6. [Google Scholar] [CrossRef] [Green Version]
- Schrans-Stassen, B.H.; Saunders, P.T.; Cooke, H.J.; de Rooij, D.G. Nature of the Spermatogenic Arrest in Dazl-/- Mice. Biol. Reprod. 2001, 65, 771–776. [Google Scholar] [CrossRef]
- Panula, S.; Reda, A.; Stukenborg, J.B.; Ramathal, C.; Sukhwani, M.; Albalushi, H.; Edsgärd, D.; Nakamura, M.; Söder, O.; Orwig, K.E.; et al. Over Expression of NANOS3 and DAZL in Human Embryonic Stem Cells. PLoS ONE 2016, 11, e0165268. [Google Scholar] [CrossRef] [Green Version]
- Mikedis, M.M.; Fan, Y.; Nicholls, P.K.; Endo, T.; Jackson, E.K.; Cobb, S.A.; de Rooij, D.G.; Page, D.C. DAZL Mediates a Broad Translational Program Regulating Expansion and Differentiation of Spermatogonial Progenitors. eLife 2020, 9, e56523. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Caldeira Araújo, H.; Lautenschläger, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogl, A.W.; Vaid, K.S.; Guttman, J.A. The Sertoli Cell Cytoskeleton. Adv. Exp. Med. Biol. 2008, 636, 186–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Dong, C.; Lu, H.; Zhang, C.; Hu, Q.; Li, S.; Qin, H.; Li, Z.; Wang, Y. Vimentin-Mediated Steroidogenesis Induced by Phthalate Esters: Involvement of DNA Demethylation and Nuclear Factor κB. PLoS ONE 2016, 11, e0146138. [Google Scholar] [CrossRef] [Green Version]
- Kleymenova, E.; Swanson, C.; Boekelheide, K.; Gaido, K.W. Exposure in Utero to Di(n-Butyl) Phthalate Alters the Vimentin Cytoskeleton of Fetal Rat Sertoli Cells and Disrupts Sertoli Cell-Gonocyte Contact. Biol. Reprod. 2005, 73, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröjdman, K.; Harley, V.R.; Pelliniemi, L.J. Sox9 Protein in Rat Sertoli Cells Is Age and Stage Dependent. Histochem. Cell Biol. 2000, 113, 31–36. [Google Scholar] [CrossRef]
- Sekido, R.; Lovell-Badge, R. Sex Determination and SRY: Down to a Wink and a Nudge? Trends Genet. 2009, 25, 19–29. [Google Scholar] [CrossRef]
- Barrionuevo, F.J.; Hurtado, A.; Kim, G.J.; Real, F.M.; Bakkali, M.; Kopp, J.L.; Sander, M.; Scherer, G.; Burgos, M.; Jiménez, R. Sox9 and Sox8 Protect the Adult Testis from Male-to-Female Genetic Reprogramming and Complete Degeneration. eLife 2016, 5, e15635. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.A.; Ruthig, V.A.; Ward, M.A. Sry-Independent Overexpression of Sox9 Supports Spermatogenesis and Fertility in the Mouse. Biol. Reprod. 2015, 93, 141. [Google Scholar] [CrossRef] [Green Version]
- Falany, C.N. Enzymology of Human Cytosolic Sulfotransferases. FASEB J. 1997, 11, 206–216. [Google Scholar] [CrossRef]
- Qian, Y.M.; Song, W.C. Regulation of Estrogen Sulfotransferase Expression in Leydig Cells by Cyclic Adenosine 3’,5’-Monophosphate and Androgen. Endocrinology 1999, 140, 1048–1053. [Google Scholar] [CrossRef]
- Runge-Morris, M.; Kocarek, T.A. Expression of the Sulfotransferase 1C Family: Implications for Xenobiotic Toxicity. Drug Metab. Rev. 2013, 45, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase Cytochrome P450, the Enzyme Responsible for Estrogen Biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.T.; Palmer, J.S.; Gray, L.E., Jr.; Veeramachaneni, D.N.R. Effects of Dibutyl Phthalate in Male Rabbits Following in Utero, Adolescent, or Postpubertal Exposure. Toxicol. Sci. 2003, 72, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, K.P.; Phillips, S.; Sar, M.; Foster, P.M.D.; Gaido, K.W. Dose-Dependent Alterations in Gene Expression and Testosterone Synthesis in the Fetal Testes of Male Rats Exposed to Di (n-Butyl) Phthalate. Toxicol. Sci. 2004, 81, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittassek, M.; Angerer, J. Phthalates: Metabolism and Exposure. Int. J. Androl. 2008, 31, 131–138. [Google Scholar] [CrossRef]
- Hait, E.J.; Calafat, A.M.; Hauser, R. Urinary Phthalate Metabolite Concentrations among Men with Inflammatory Bowel Disease on Mesalamine Therapy. Endocr. Disruptors 2014, 1, e25066. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and Other Additives in Plastics: Human Exposure and Associated Health Outcomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef] [Green Version]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.-M. Metabolism of Phthalates in Humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- White, R.D.; Carter, D.E.; Earnest, D.; Mueller, J. Absorption and Metabolism of Three Phthalate Diesters by the Rat Small Intestine. Food Cosmet. Toxicol. 1980, 18, 383–386. [Google Scholar] [CrossRef]
- Clewell, R.A.; Kremer, J.J.; Williams, C.C.; Campbell, J.L.; Sochaski, M.A.; Andersen, M.E.; Borghoff, S.J. Kinetics of Selected Di-n-Butyl Phthalate Metabolites and Fetal Testosterone Following Repeated and Single Administration in Pregnant Rats. Toxicology 2009, 255, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Romero, E.; Scheringer, M. A Review of Phthalate Pharmacokinetics in Human and Rat: What Factors Drive Phthalate Distribution and Partitioning? Drug Metab. Rev. 2019, 51, 314–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.A.C.; Castle, L.; Scotter, M.J.; Massey, R.C.; Springall, C. A Biomarker Approach to Measuring Human Dietary Exposure to Certain Phthalate Diesters. Food Addit. Contam. 2001, 18, 1068–1074. [Google Scholar] [CrossRef]
- Koch, H.M.; Christensen, K.L.Y.; Harth, V.; Lorber, M.; Brüning, T. Di-n-Butyl Phthalate (DnBP) and Diisobutyl Phthalate (DiBP) Metabolism in a Human Volunteer after Single Oral Doses. Arch. Toxicol. 2012, 86, 1829–1839. [Google Scholar] [CrossRef]
- Giribabu, N.; Sainath, S.B.; Sreenivasula Reddy, P. Prenatal Di-n-Butyl Phthalate Exposure Alters Reproductive Functions at Adulthood in Male Rats. Environ. Toxicol. 2014, 29, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Boekelheide, K.; Kleymenova, E.; Liu, K.; Swanson, C.; Gaido, K.W. Dose-Dependent Effects on Cell Proliferation, Seminiferous Tubules, and Male Germ Cells in the Fetal Rat Testis Following Exposure to Di(n-Butyl) Phthalate. Microsc. Res. Tech. 2009, 72, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pierozan, P.; Cattani, D.; Karlsson, O. Hippocampal Neural Stem Cells Are More Susceptible to the Neurotoxin BMAA than Primary Neurons: Effects on Apoptosis, Cellular Differentiation, Neurite Outgrowth, and DNA Methylation. Cell Death Dis. 2020, 11, 910. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting Outliers When Fitting Data with Nonlinear Regression—A New Method Based on Robust Nonlinear Regression and the False Discovery Rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Källsten, L.; Almamoun, R.; Pierozan, P.; Nylander, E.; Sdougkou, K.; Martin, J.W.; Karlsson, O. Adult Exposure to Di-N-Butyl Phthalate (DBP) Induces Persistent Effects on Testicular Cell Markers and Testosterone Biosynthesis in Mice. Int. J. Mol. Sci. 2022, 23, 8718. https://doi.org/10.3390/ijms23158718
Källsten L, Almamoun R, Pierozan P, Nylander E, Sdougkou K, Martin JW, Karlsson O. Adult Exposure to Di-N-Butyl Phthalate (DBP) Induces Persistent Effects on Testicular Cell Markers and Testosterone Biosynthesis in Mice. International Journal of Molecular Sciences. 2022; 23(15):8718. https://doi.org/10.3390/ijms23158718
Chicago/Turabian StyleKällsten, Liselott, Radwa Almamoun, Paula Pierozan, Erik Nylander, Kalliroi Sdougkou, Jonathan W. Martin, and Oskar Karlsson. 2022. "Adult Exposure to Di-N-Butyl Phthalate (DBP) Induces Persistent Effects on Testicular Cell Markers and Testosterone Biosynthesis in Mice" International Journal of Molecular Sciences 23, no. 15: 8718. https://doi.org/10.3390/ijms23158718





