Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC–MS/MS
Abstract
:1. Introduction
2. Results
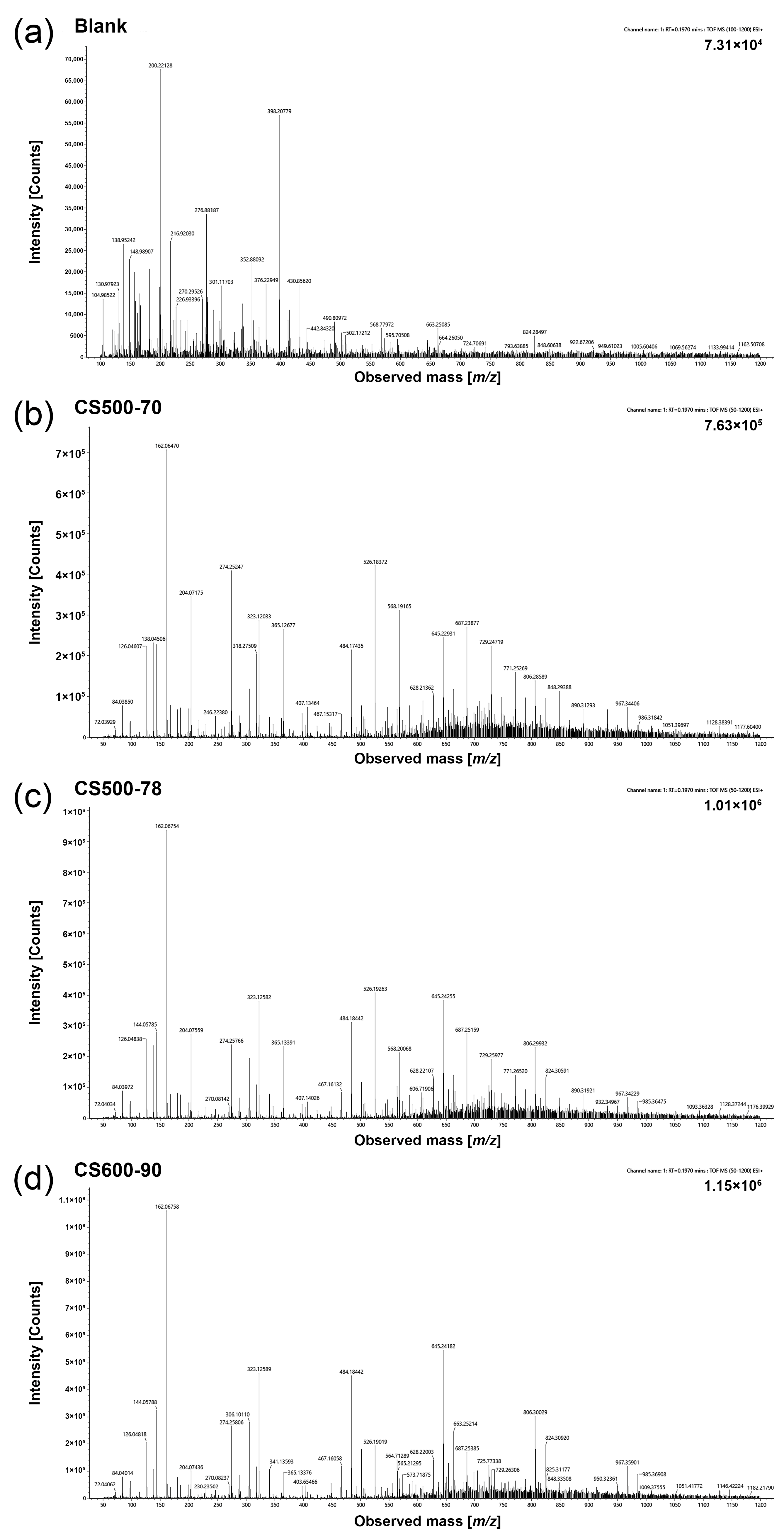
2.1. Mass Spectral Characteristics of Different CSs in Q-TOF
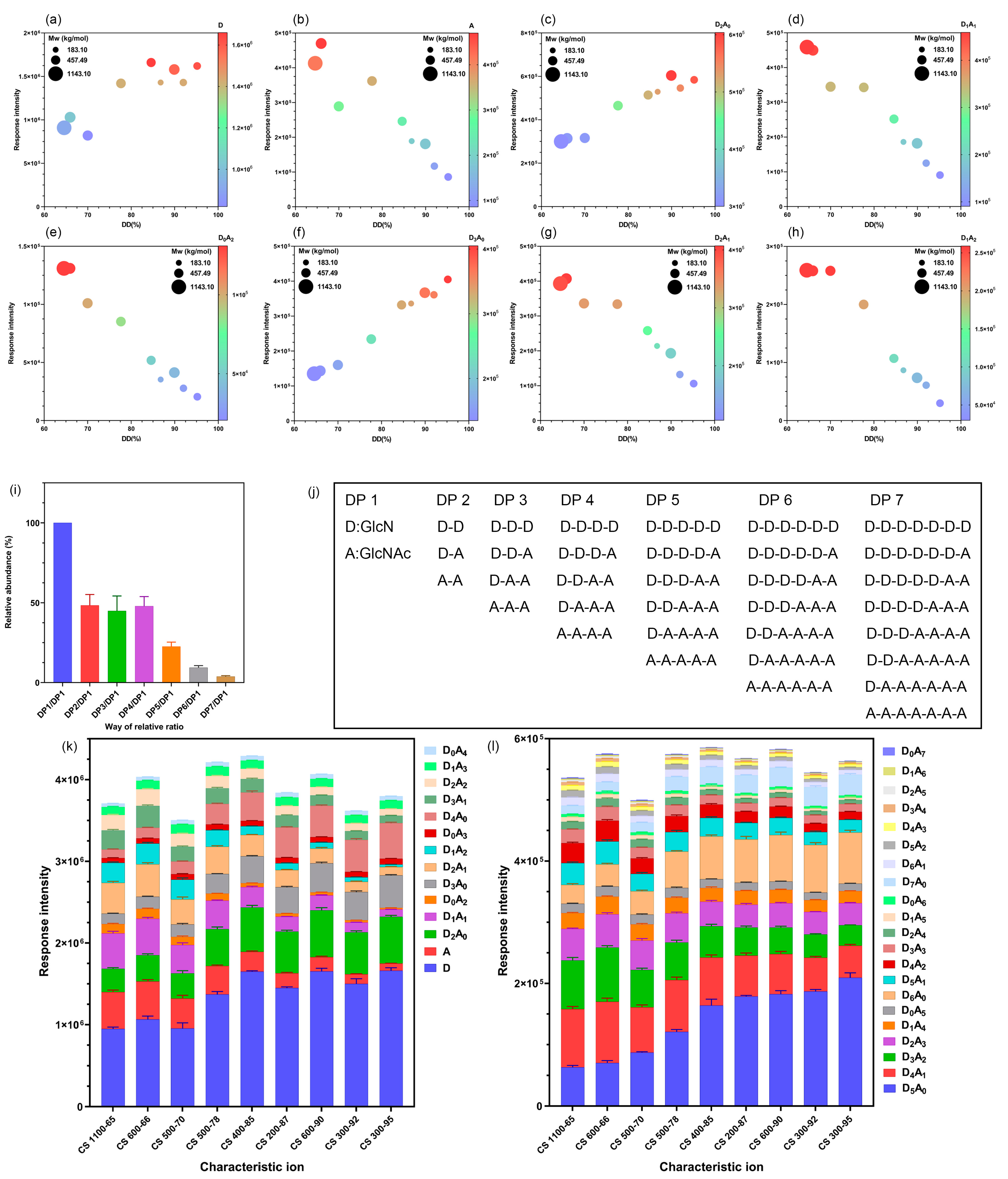
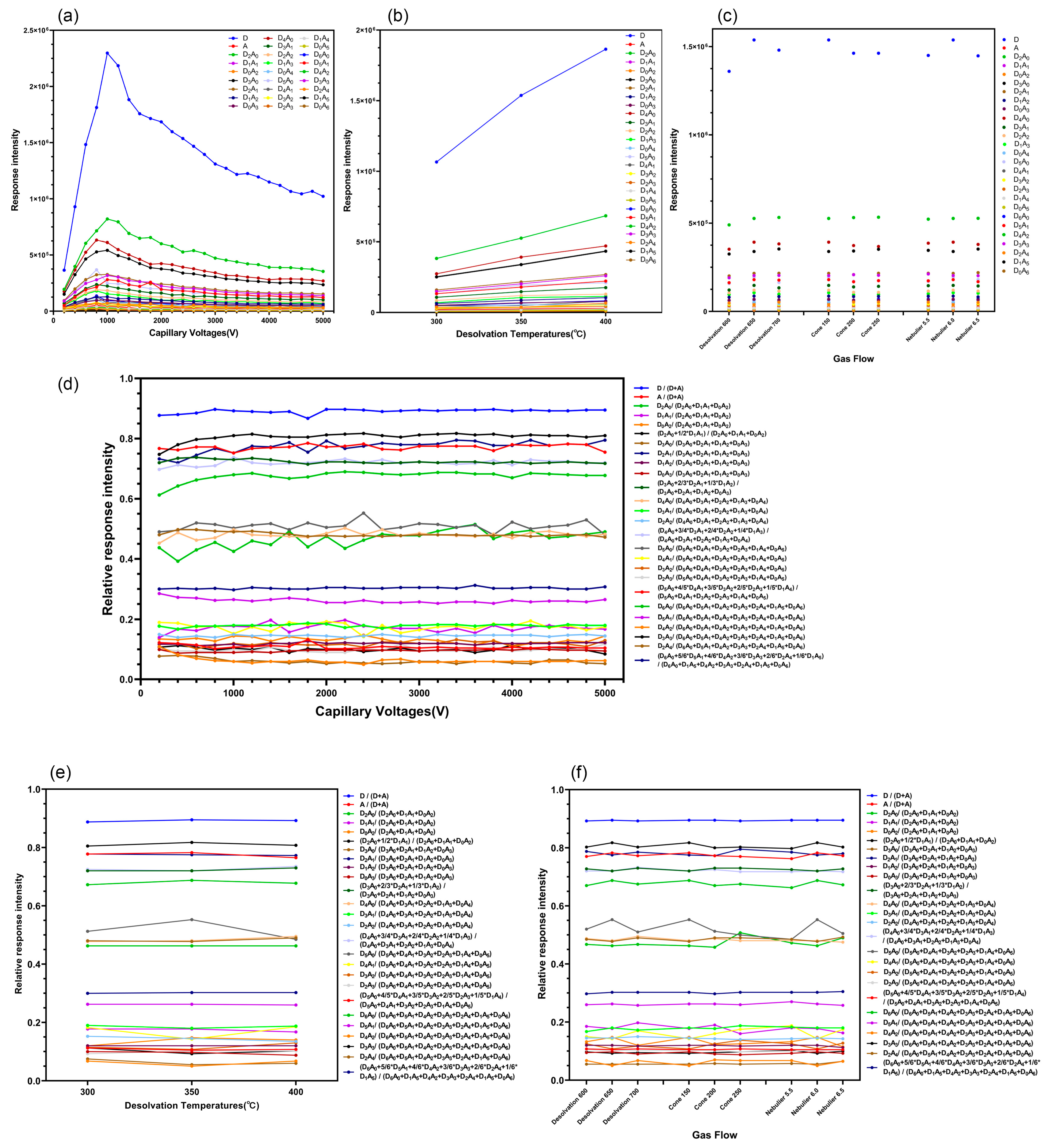
2.2. Triple Quadrupole MS to Detect the Response Intensity of CS Characteristic Ion Pairs
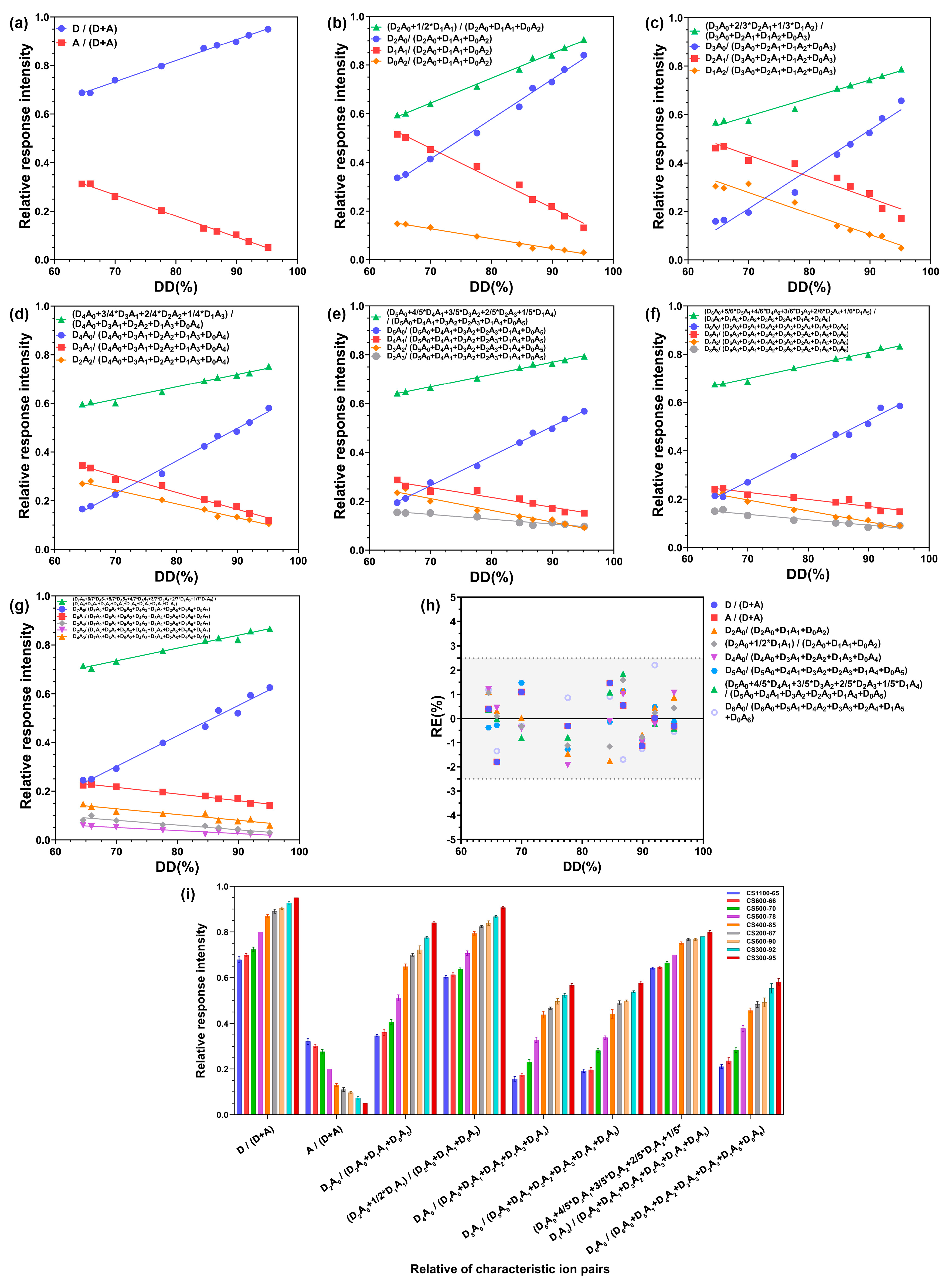
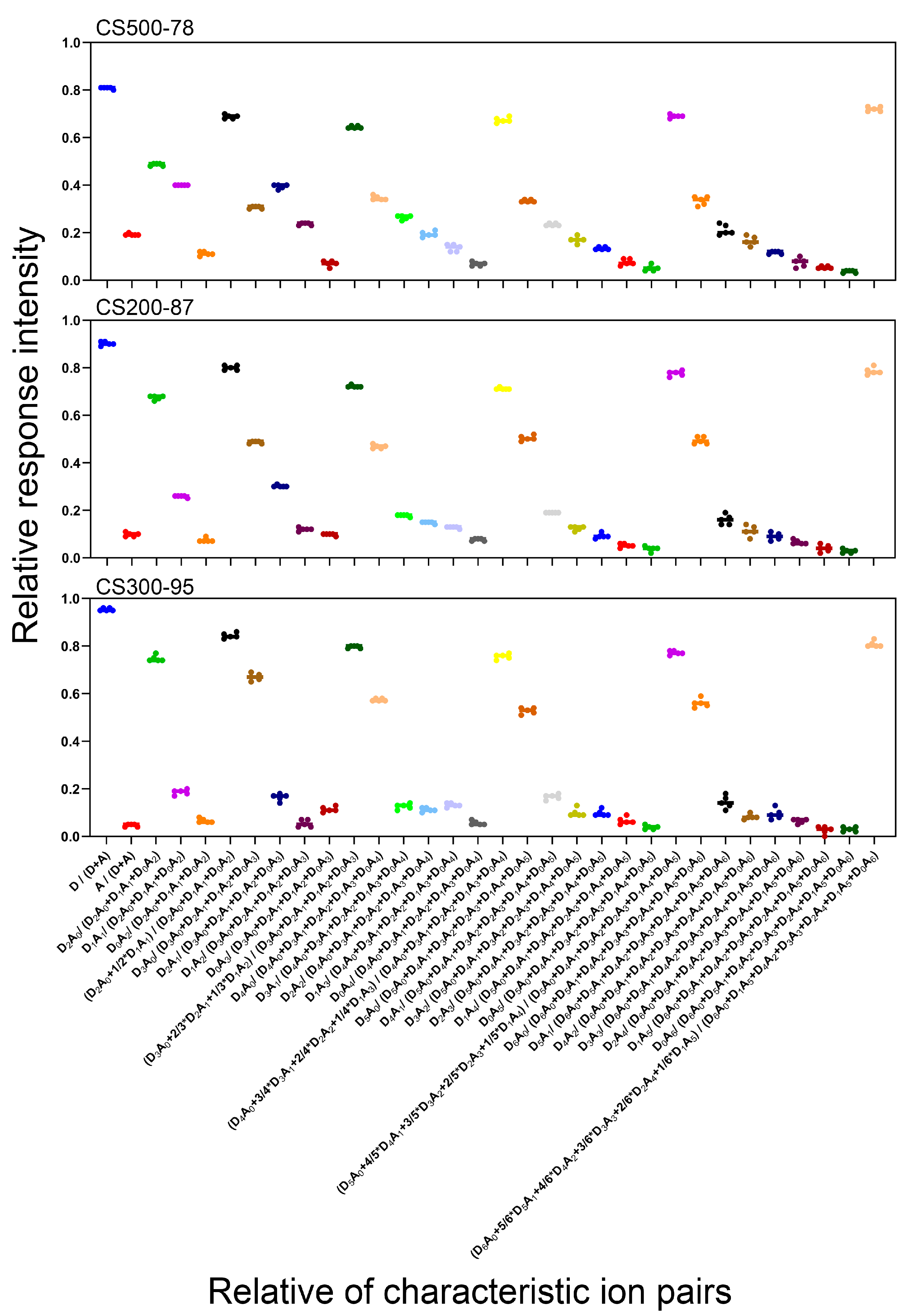
2.3. Linear Relationship between the Relative Response Intensity (RRI) of the Characteristic Ion Pairs and the DD of CS
2.4. The RRI of Characteristic Ions under Different Concentrations of the Same CS Sample Is a Fixed Value
2.5. Effect of the RRI of CS Characteristic Ion Pairs on the Detection Method of Different MS Parameters (Capillary Voltage, Gas Flow, and Temperature)
2.6. Method Reproduction on Different LC-MS/MS Instruments
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. MW Determination of CS Using Size Exclusion Chromatography Multiple Angle Laser Light Scattering (SEC-MALLS)
4.3. CS DD Determination by 1H NMR
4.4. LC-MS/MS
4.4.1. LC
4.4.2. TOF MS
4.4.3. Triple Quadrupole MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011, 84, 696–703. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Solubility, degree of acetylation, and distribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan Volume 1: Preparation and Properties; Connors, K., Ed.; Susan Dennis: Amsterdam, The Netherlands, 2020; pp. 131–164. [Google Scholar]
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; Da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Raymond, L.; Morin, F.G.; Marchessault, R.H. Degree of deacetylation of chitosan using conductometric titration and solid-state NMR. Carbohydr. Res. 1993, 246, 331–336. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhong, W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvão, M.R.; Rocha, J. Determination of the degree of acetylation of chitin materials by 13C CP/MAS NMR spectroscopy. Int. J. Biol. Macromol. 2001, 28, 359–363. [Google Scholar] [CrossRef]
- Dimzon, I.K.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Guinesi, L.S.; Cavalheiro, E.T.G. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tanfani, F.; Scarpini, G.; Laterza, G. The degree of acetylation of chitins by gas chromatography and infrared spectroscopy. J. Biochem. Biophys. Methods 1980, 2, 299–306. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R. Determination of the degree of acetylation of chitosans by first derivative ultraviolet spectrophotometry. Carbohydr. Polym. 1985, 5, 461–472. [Google Scholar] [CrossRef]
- Sashiwa, H.; Saimoto, H.; Shigemasa, Y.; Ogawa, R.; Tokura, S. Distribution of the acetamide group in partially deacetylated chitins. Carbohydr. Polym. 1991, 16, 291–296. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Deacetylation modification techniques of chitin and chitosan. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 5, pp. 117–133. [Google Scholar]
- Chen, M.; Zhu, X.; Li, Z.; Guo, X.; Ling, P. Application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in preparation of chitosan oligosaccharides (COS) with degree of polymerization (DP) 5–12 containing well-distributed acetyl groups. Int. J. Mass Spectrom. 2010, 290, 94–99. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczynski, Z.; Bychowska, A.; Brzozowski, K.; Thoming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Bahrke, S.; Einarsson, J.M.; Gislason, J.; Haebel, S.; Letzel, M.C.; Peter-Katalinic, J.; Peter, M.G. Sequence analysis of chitooligosaccharides by matrix-assisted laser desorption ionization postsource decay mass spectrometry. Biomacromolecules 2002, 3, 696–704. [Google Scholar] [CrossRef]
- Cord-Landwehr, S.; Ihmor, P.; Niehues, A.; Luftmann, H.; Moerschbacher, B.M.; Mormann, M. Quantitative Mass-Spectrometric Sequencing of Chitosan Oligomers Revealing Cleavage Sites of Chitosan Hydrolases. Anal. Chem. 2017, 89, 2893–2900. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Gadre, A.P.; Yi, H.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-Dependent Assembly of the Polysaccharide Chitosan onto an Electrode Surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Arachchige, G.R.P.; Thorstensen, E.B.; Coe, M.; McKenzie, E.J.; O’Sullivan, J.M.; Pook, C.J. LC-MS/MS quantification of fat soluble vitamers—A systematic review. Anal. Biochem. 2021, 613, 113980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Zhang, Y.B.; Yang, X.W.; Xu, W.; Wang, Y.P. Characterization and quantification of ginsenosides from the root of Panax quinquefolius L. by integrating untargeted metabolites and targeted analysis using UPLC-Triple TOF-MS coupled with UFLC-ESI-MS/MS. Food Chem. 2022, 384, 132466. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Ogawa, S. Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: A review. J. Steroid. Biochem. Mol. Biol. 2016, 162, 57–69. [Google Scholar] [CrossRef]
- Suzuki, N. Tissue N-Glycan Analysis Using LC-MS, MS/MS, and MS(n). Curr. Protoc. 2021, 1, e200. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Meng, Z.; Dou, G. Development of a mass spectrometry method for the characterization of a series of chitosan. Int. J. Biol. Macromol. 2019, 121, 89–96. [Google Scholar] [CrossRef]
- Einbu, A.; Varum, K.M. Depolymerization and de-N-acetylation of chitin oligomers in hydrochloric acid. Biomacromolecules 2007, 8, 309–314. [Google Scholar] [CrossRef]
- Abou Zeid, L.; Pell, A.; Tytus, T.; Delangle, P.; Bresson, C. Separation of multiphosphorylated cyclopeptides and their positional isomers by hydrophilic interaction liquid chromatography (HILIC) coupled to electrospray ionization mass spectrometry (ESI-MS). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1177, 122792. [Google Scholar] [CrossRef]
- Tian, C.; Feng, C.; Wang, Q. The identification of Al nanoclusters by electrospray ionization mass spectrometry (ESI-MS). Sci. Total Environ. 2021, 754, 142154. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
| DP | Multimer Combination | Single-Charge Ion | Characteristic Ion (m/z) | Quantitation Transition | Collision Energy (eV) |
|---|---|---|---|---|---|
| Precursor Ion→Product Ion (m/z) | |||||
| 1 | D:GlcN | [D+H]+ | 162.08 | 162.08→162.08 | 5 |
| A:GlcNAc | [A+H]+ | 204.09 | 204.09→204.09 | 5 | |
| 2 | D2A0 | [D2A0+H]+ | 323.15 | 323.15→323.15 | 5 |
| D1A1 | [D1A1+H]+ | 365.16 | 365.16→365.16 | 5 | |
| D0A2 | [D0A2+H]+ | 407.17 | 407.17→407.17 | 5 | |
| 3 | D3A0 | [D3A0+H]+ | 484.21 | 484.21→484.21 | 5 |
| D2A1 | [D2A1+H]+ | 526.22 | 526.22→526.22 | 5 | |
| D1A2 | [D1A2+H]+ | 568.24 | 568.24→568.24 | 5 | |
| D0A3 | [D0A3+H]+ | 610.25 | 610.25→610.25 | 5 | |
| 4 | D4A0 | [D4A0+H]+ | 645.28 | 645.28→645.28 | 5 |
| D3A1 | [D3A1+H]+ | 687.29 | 687.29→687.29 | 5 | |
| D2A2 | [D2A2+H]+ | 729.30 | 729.30→729.30 | 5 | |
| D1A3 | [D1A3+H]+ | 771.31 | 771.31→771.31 | 5 | |
| D0A4 | [D0A4+H]+ | 813.33 | 813.33→813.33 | 5 | |
| 5 | D5A0 | [D5A0+H]+ | 806.35 | 806.35→806.35 | 5 |
| D4A1 | [D4A1+H]+ | 848.36 | 848.36→848.36 | 5 | |
| D3A2 | [D3A2+H]+ | 890.37 | 890.37→890.37 | 5 | |
| D2A3 | [D2A3+H]+ | 932.38 | 932.38→932.38 | 5 | |
| D1A4 | [D1A4+H]+ | 974.39 | 974.39→974.39 | 5 | |
| D0A5 | [D0A5+H]+ | 1016.40 | 1016.40→1016.40 | 5 | |
| 6 | D6A0 | [D6A0+H]+ | 967.42 | 967.42→967.42 | 5 |
| D5A1 | [D5A1+H]+ | 1009.43 | 1009.43→1009.43 | 5 | |
| D4A2 | [D4A2+H]+ | 1051.44 | 1051.44→1051.44 | 5 | |
| D3A3 | [D3A3+H]+ | 1093.45 | 1093.45→1093.45 | 5 | |
| D2A4 | [D2A4+H]+ | 1135.46 | 1135.46→1135.46 | 5 | |
| D1A5 | [D1A5+H]+ | 1177.47 | 1177.47→1177.47 | 5 | |
| D0A6 | [D0A6+H]+ | 1219.48 | 1219.48→1219.48 | 5 | |
| 7 | D7A0 | [D7A0+H]+ | 1128.49 | 1128.49→1128.49 | 5 |
| D6A1 | [D6A1+H]+ | 1170.50 | 1170.50→1170.50 | 5 | |
| D5A2 | [D5A2+H]+ | 1212.51 | 1212.51→1212.51 | 5 | |
| D4A3 | [D4A3+H]+ | 1254.53 | 1254.53→1254.53 | 5 | |
| D3A4 | [D3A4+H]+ | 1296.53 | 1296.53→1296.53 | 5 | |
| D2A5 | [D2A5+H]+ | 1338.54 | 1338.54→1338.54 | 5 | |
| D1A6 | [D1A6+H]+ | 1380.55 | 1380.55→1380.55 | 5 | |
| D0A7 | [D0A7+H]+ | 1422.56 | 1422.56→1422.56 | 5 |
| DP | Relative Response Intensity Ways of Characteristic Ion Pairs | Equation | R2 | Remark |
|---|---|---|---|---|
| 1 | D/(D + A) | Y = 0.008723*X + 0.1223 | 0.9953 | *** |
| A/(D + A) | Y = −0.008723*X + 0.8777 | 0.9953 | *** | |
| 2 | D2A0/(D2A0 + D1A1 + D0A2) | Y = 0.01637*X − 0.7313 | 0.9942 | *** |
| D1A1/(D2A0 + D1A1 + D0A2) | Y = −0.01228*X + 1.318 | 0.9889 | ** | |
| D0A2/(D2A0 + D1A1 + D0A2) | Y = −0.004082*X + 0.4129 | 0.9868 | ** | |
| (D2A0 + 1/2*D1A1)/(D2A0 + D1A1 + D0A2) | Y = 0.01022*X − 0.07196 | 0.9955 | *** | |
| 3 | D3A0/(D3A0 + D2A1 + D1A2 + D0A3) | Y = 0.01623*X − 0.9241 | 0.9760 | ** |
| D2A1/(D3A0 + D2A1 + D1A2 + D0A3) | Y = −0.008817*X + 1.050 | 0.9334 | ** | |
| D1A2/(D3A0 + D2A1 + D1A2 + D0A3) | Y = −0.008707*X + 0.8883 | 0.9627 | ** | |
| (D3A0 + 2/3*D2A1 + 1/3*D1A2)/(D3A0 + D2A1 + D1A2 + D0A3) | Y = 0.007454*X + 0.07184 | 0.9742 | ** | |
| 4 | D4A0/(D4A0 + D3A1 + D2A2 + D1A3 + D0A4) | Y = 0.01343*X − 0.7113 | 0.9948 | *** |
| D3A1/(D4A0 + D3A1 + D2A2 + D1A3 + D0A4) | Y = −0.006953*X + 0.7904 | 0.9872 | ** | |
| D2A2/(D4A0 + D3A1 + D2A2 + D1A3 + D0A4) | Y = −0.005676*X + 0.6412 | 0.9839 | ** | |
| (D4A0 + 3/4*D3A1 + 2/4*D2A2 + 1/4*D1A3)/(D4A0 + D3A1 + D2A2 + D1A3 + D0A4) | Y = 0.005125*X + 0.2580 | 0.9797 | ** | |
| 5 | D5A0/(D5A0 + D4A1 + D3A2 + D2A3 + D1A4 + D0A5) | Y = 0.01217*X − 0.5880 | 0.9963 | *** |
| D4A1/(D5A0 + D4A1 + D3A2 + D2A3 + D1A4 + D0A5) | Y = −0.004052*X + 0.5397 | 0.9390 | ** | |
| D3A2/(D5A0 + D4A1 + D3A2 + D2A3 + D1A4 + D0A5) | Y = −0.004835*X + 0.5495 | 0.9686 | ** | |
| D2A3/(D5A0 + D4A1 + D3A2 + D2A3 + D1A4 + D0A5) | Y = −0.001975*X + 0.2844 | 0.9421 | ** | |
| (D5A0 + 4/5*D4A1 + 3/5*D3A2 + 2/5*D2A3 + 1/5*D1A4)/(D5A0 + D4A1 + D3A2 + D2A3 + D1A4 + D0A5) | Y = 0.005067*X + 0.3140 | 0.9950 | *** | |
| 6 | D6A0/(D6A0 + D5A1 + D4A2 + D3A3 + D2A4 + D1A5 + D0A6) | Y = 0.01268*X − 0.6147 | 0.9905 | *** |
| D5A1/(D6A0 + D5A1 + D4A2 + D3A3 + D2A4 + D1A5 + D0A6) | Y = −0.002922*X + 0.4328 | 0.9233 | ** | |
| D4A2/(D6A0 + D5A1 + D4A2 + D3A3 + D2A4 + D1A5 + D0A6) | Y = −0.004521*X + 0.5133 | 0.9836 | ** | |
| D3A3/(D6A0 + D5A1 + D4A2 + D3A3 + D2A4 + D1A5 + D0A6) | Y = −0.002248*X + 0.2947 | 0.9374 | ** | |
| (D6A0 + 5/6*D5A1 + 4/6*D4A2 + 3/6*D3A3 + 2/6*D2A4 + 1/6*D1A5)/(D6A0 + D1A5 + D4A2 + D3A3 + D2A4 + D1A5 + D0A6) | Y = 0.005395*X + 0.3218 | 0.9874 | ** | |
| 7 | D7A0/(D7A0 + D6A1 + D5A2 + D4A3 + D3A4 + D2A5 + D1A6 + D0A7) | Y = 0.01255*X − 0.5774 | 0.9865 | ** |
| D6A1/(D7A0 + D6A1 + D5A2 + D4A3 + D3A4 + D2A5 + D1A6 + D0A7) | Y = −0.002736*X + 0.4073 | 0.9742 | ** | |
| D4A3/(D7A0 + D6A1 + D5A2 + D4A3 + D3A4 + D2A5 + D1A6 + D0A7) | Y = −0.002362*X + 0.2933 | 0.9032 | ** | |
| D3A4/(D7A0 + D6A1 + D5A2 + D4A3 + D3A4 + D2A5 + D1A6 + D0A7) | Y = −0.001948*X + 0.2171 | 0.9287 | ** | |
| D2A5/(D7A0 + D6A1 + D5A2 + D4A3 + D3A4 + D2A5 + D1A6 + D0A7) | Y = −0.001252*X + 0.1388 | 0.9185 | ** | |
| (D7A0 + 6/7*DA61 + 5/7*DA52 + 4/7*DA43 + 3/7*D3A4 + 2/7*D2A5 + 1/7*D1A6)/(D7A0 + D6A1 + D5A2 + D4A3 + D3A4 + D2A5 + D1A6 + D0A7) | Y = 0.005193*X + 0.3718 | 0.9797 | ** |
| Mass Spectrometry Model | ESI Source Parameter Category | Optimal Conditions | Parameter Range |
|---|---|---|---|
| Xevo TQ-S | Capillary Voltages (kV) | 2.4 | 1.0–3.5 |
| Desolvation Temperatures (°C) | 350 | 300–400 | |
| Desolvation Gas Flow (L/h) | 650 | 600–700 | |
| Cone Gas Flow (L/h) | 150 | 150–250 | |
| Nebulier Gas Flow (bar) | 6.0 | 5.5–6.5 | |
| Concentration (ng/mL) | 2500 | 500–4000 | |
| SCIEX Triple Quad™ 6500plus | Curtain Gas (CUR, psi) | 35 | 30–35 |
| Collision Gas (CAD) | 8 | 6–8 | |
| IonSpray Voltage (IS, V) | 5500 | 4500–5500 | |
| Temperature (TEM, °C) | 550 | 450–550 | |
| Ion Source Gas 1 (GS1, psi) | 50 | 40–55 | |
| Ion Source Gas 2 (GS2, psi) | 50 | 40–60 | |
| Concentration (ng/mL) | 2500 | 500–4000 | |
| API4000 | Curtain Gas (CUR, psi) | 25 | 25 |
| Collision Gas (CAD) | 6 | 6 | |
| IonSpray Voltage (IS, V) | 5000 | 4000–5500 | |
| Temperature (TEM, °C) | 500 | 350–550 | |
| Ion Source Gas 1 (GS1, psi) | 50 | 40–55 | |
| Ion Source Gas 2 (GS2, psi) | 50 | 40–65 | |
| Concentration (ng/mL) | 10,000 | 1000–50,000 |
| Sample | DD (%) | Mw (kDa) | Mn (kDa) | Mw/Mn | Rg,z (nm) |
|---|---|---|---|---|---|
| CS 1100-65 | 64.5 | 1143.1 | 667.6 | 1.71 | 108.0 |
| CS 600-66 | 65.9 | 624.6 | 388.7 | 1.61 | 84.6 |
| CS 500-70 | 70.0 | 545.3 | 313.4 | 1.74 | 74.4 |
| CS 500-78 | 77.6 | 496.4 | 342.1 | 1.45 | 67.7 |
| CS 400-85 | 84.6 | 445.1 | 349.5 | 1.27 | 69.9 |
| CS 200-87 | 86.8 | 183.1 | 141.9 | 1.29 | 41.6 |
| CS 600-90 | 89.9 | 596.4 | 482.4 | 1.24 | 78.2 |
| CS 300-92 | 92.0 | 290.2 | 215.7 | 1.35 | 56.6 |
| CS 300-95 | 95.2 | 313.1 | 241.7 | 1.30 | 62.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Wang, W.; Yang, Z.; Wang, F.; Yang, L.; Li, J.; Gan, H.; Gu, R.; Wu, Z.; Dou, G.; et al. Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC–MS/MS. Int. J. Mol. Sci. 2022, 23, 8810. https://doi.org/10.3390/ijms23158810
Xue T, Wang W, Yang Z, Wang F, Yang L, Li J, Gan H, Gu R, Wu Z, Dou G, et al. Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC–MS/MS. International Journal of Molecular Sciences. 2022; 23(15):8810. https://doi.org/10.3390/ijms23158810
Chicago/Turabian StyleXue, Ting, Wenqing Wang, Zhiyuan Yang, Fanjun Wang, Lei Yang, Jian Li, Hui Gan, Ruolan Gu, Zhuona Wu, Guifang Dou, and et al. 2022. "Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC–MS/MS" International Journal of Molecular Sciences 23, no. 15: 8810. https://doi.org/10.3390/ijms23158810
APA StyleXue, T., Wang, W., Yang, Z., Wang, F., Yang, L., Li, J., Gan, H., Gu, R., Wu, Z., Dou, G., & Meng, Z. (2022). Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC–MS/MS. International Journal of Molecular Sciences, 23(15), 8810. https://doi.org/10.3390/ijms23158810





