Prostate Infiltration by Treg and Th17 Cells as an Immune Response to Propionibacterium acnes Infection in the Course of Benign Prostatic Hyperplasia and Prostate Cancer
Abstract
:1. Introduction
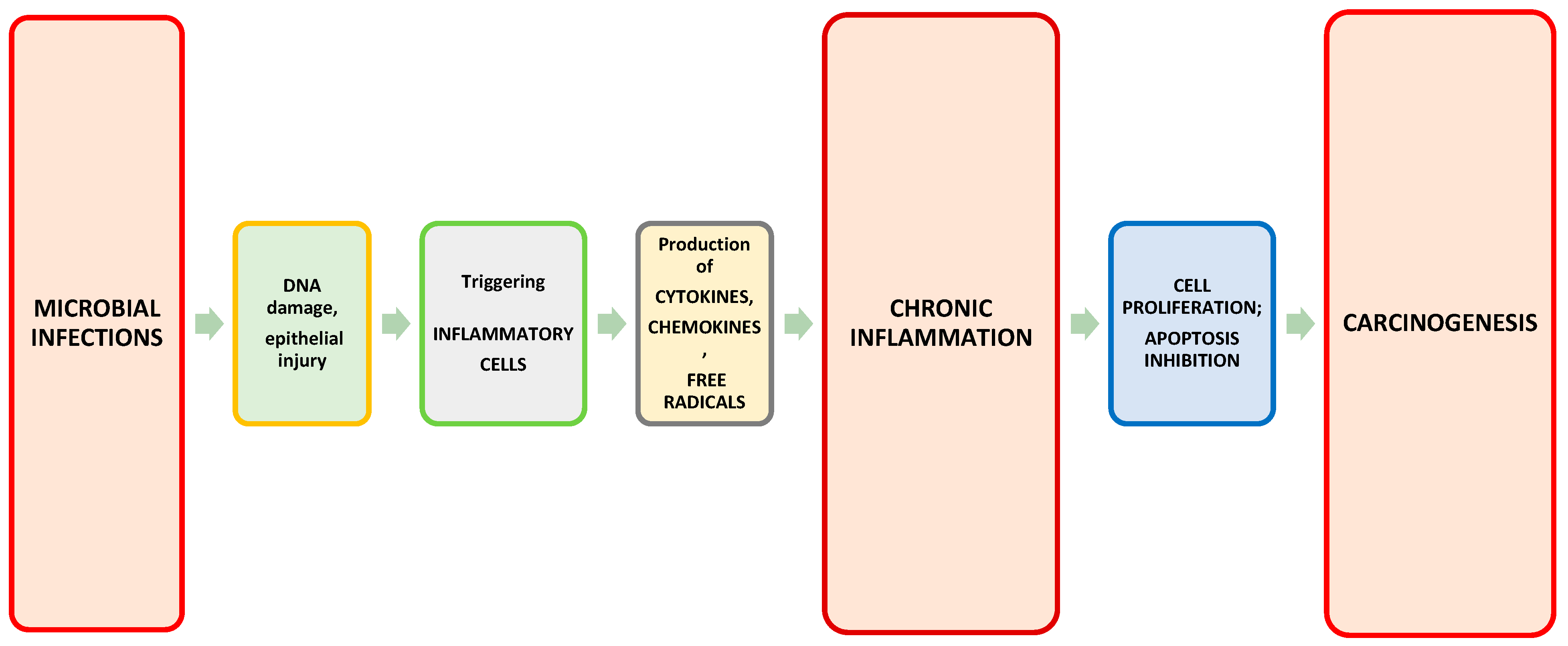
2. The Prostate Microbiome and Chronic Inflammation
3. P. acnes in Patients with BPH and PCa
4. P. acnes May Also Contribute to Other Cancers and Inflammatory Diseases
5. P. acnes Induces Proinflammatory Response in Prostate Gland
6. Treg and Th17 Cells in the Tumor Environment
7. P. acnes Contributes to Prostate Infiltration by Treg and Th17 Cells
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Bin Lim, K. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef]
- Devlin, C.M.; Simms, M.S.; Maitland, N.J. Benign prostatic hyperplasia–what do we know? Br. J. Urol. 2020, 127, 389–399. [Google Scholar] [CrossRef]
- Ficarra, V.; Sekulovic, S.; Zattoni, F.; Zazzera, M.; Novara, G. Why and How to Evaluate Chronic Prostatic Inflammation. Eur. Urol. Suppl. 2013, 12, 110–115. [Google Scholar] [CrossRef]
- Ficarra, V.; Rossanese, M.; Zazzara, M.; Giannarini, G.; Abbinante, M.; Bartoletti, R.; Mirone, V.; Scaglione, F. The Role of Inflammation in Lower Urinary Tract Symptoms (LUTS) due to Benign Prostatic Hyperplasia (BPH) and Its Potential Impact on Medical Therapy. Curr. Urol. Rep. 2014, 15, 463. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Devesa, S.S.; Chang, B.-L.; Bunker, C.H.; Cheng, I.; Cooney, K.; Eeles, R.; Fernandez, P.; Giri, V.N.; Gueye, S.M.; et al. Global Patterns of Prostate Cancer Incidence, Aggressiveness, and Mortality in Men of African Descent. Prostate Cancer 2013, 2013, 560857. [Google Scholar] [CrossRef] [Green Version]
- Foerster, B.; Pozo, C.; Abufaraj, M.; Mari, A.; Kimura, S.; D’Andrea, D.; John, H.; Shariat, S. Association of Smoking Status With Recurrence, Metastasis, and Mortality Among Patients With Localized Prostate Cancer Undergoing Prostatectomy or Radiotherapy: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhao, D.; Spring, D.J.; Depinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current Knowledge of the Potential Links between Inflammation and Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verze, P.; Cai, T.; Lorenzetti, S. The role of the prostate in male fertility, health and disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2017, 15, 11–24. [Google Scholar] [CrossRef]
- Moghadam, S.O.; Momeni, S.A. Human microbiome and prostate cancer development: Current insights into the prevention and treatment. Front. Med. 2020, 15, 11–32. [Google Scholar] [CrossRef]
- Brüggemann, H.; Al-Zeer, M.A. Bacterial signatures and their inflammatory potentials associated with prostate cancer. APMIS 2020, 128, 80–91. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.P.B.; Alluri, L.S.C.; Bissada, N.F.; Gupta, S. Association between oral pathogens and prostate cancer: Building the relationship. Am. J. Clin. Exp. Urol. 2019, 18, 1–10. [Google Scholar]
- Virchow, R. An Address on the Value of Pathological Experiments. BMJ 1881, 2, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Delongchamps, N.B.; de la Roza, G.; Chandan, V.; Jones, R.; Sunheimer, R.; Threatte, G.; Jumbelic, M.; Haas, G.P. Evaluation of Prostatitis in Autopsied Prostates—Is Chronic Inflammation More Associated With Benign Prostatic Hyperplasia or Cancer? J. Urol. 2008, 179, 1736–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 2015, 4, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Guner, E.; Danacioglu, Y.O.; Arikan, Y.; Seker, K.G.; Polat, S.; Baytekin, H.F.; Simsek, A. The presence of chronic inflammation in positive prostate biopsy is associated with upgrading in radical prostatectomy. Arch. Ital. Urol. Androl. 2021, 93, 280–284. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, R.-Y. Review of the Roles and Interaction of Androgen and Inflammation in Benign Prostatic Hyperplasia. Mediat. Inflamm. 2020, 2020, 7958316. [Google Scholar] [CrossRef]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef]
- Bultman, S.J. Emerging roles of the microbiome in cancer. Carcinogenesis 2013, 35, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Rosean, C.M.B.; Rutkowski, M.R. The influence of the commensal microbiota on distal tumor-promoting inflammation. Semin. Immunol. 2017, 32, 62–73. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [Green Version]
- Sfanos, K.S.; Sauvageot, J.; Fedor, H.L.; Dick, J.D.; De Marzo, A.M.; Isaacs, W.B. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate 2007, 68, 306–320. [Google Scholar] [CrossRef]
- Banerjee, S.; Alwine, J.C.; Wei, Z.; Tian, T.; Shih, N.; Sperling, C.; Guzzo, T.; Feldman, M.D.; Robertson, E.S. Microbiome signatures in prostate cancer. Carcinogenesis 2019, 40, 749–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexeyev, O.; Bergh, J.; Marklund, I.; Thellenberg-Karlsson, C.; Wiklund, F.; Grönberg, H.; Bergh, A.; Elgh, F. Association between the presence of bacterial 16S RNA in prostate specimens taken during transurethral resection of prostate and subsequent risk of prostate cancer (Sweden). Cancer Causes Control. 2006, 17, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson-Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Markowski, M.C.; Peiffer, L.; Ernst, S.E.; White, J.R.; Pienta, K.J.; Antonarakis, E.S.; Ross, A.E. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018, 21, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavarretta, I.; Ferrarese, R.; Cazzaniga, W.; Saita, D.; Lucianò, R.; Ceresola, E.R.; Locatelli, I.; Visconti, L.; Lavorgna, G.; Briganti, A.; et al. The Microbiome of the Prostate Tumor Microenvironment. Eur. Urol. 2017, 72, 625–631. [Google Scholar] [CrossRef]
- Feng, Y.; Ramnarine, V.R.; Bell, R.; Volik, S.; Davicioni, E.; Hayes, V.M.; Ren, S.; Collins, C.C. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. BMC Genom. 2019, 20, 146. [Google Scholar] [CrossRef] [Green Version]
- Yow, M.A.; Tabrizi, S.N.; Severi, G.; Bolton, D.M.; Pedersen, J.; Australian Prostate Cancer BioResource; Giles, G.G.; Southey, M.C. Characterisation of microbial communities within aggressive prostate cancer tissues. Infect. Agents Cancer 2017, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Platsidaki, E.; Dessinioti, C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, 1953. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.N.; Yu, S.-H.; De Marzo, A.; Brüggemann, H.; Sfanos, K.S. Multilocus sequence typing (MLST) analysis ofPropionibacteriumacnesisolates from radical prostatectomy specimens. Prostate 2012, 73, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, E.; White, J.R.; Yu, S.-H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Manente, L.; Gargiulo, U.; Gargiulo, P.; Dovinola, G. Propionibacterium acnes in urine and semen samples from men with urinary infection. Arch. Ital. Urol. Androl. 2022, 94, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Goldstein, E.J.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From Commensal to Opportunistic Biofilm-Associated Implant Pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capoor, M.N.; Birkenmaier, C.; Wang, J.C.; McDowell, A.; Ahmed, F.S.; Brüggemann, H.; Coscia, E.; Davies, D.G.; Ohrt-Nissen, S.; Raz, A.; et al. A review of microscopy-based evidence for the association of Propionibacterium acnes biofilms in degenerative disc disease and other diseased human tissue. Eur. Spine J. 2019, 28, 2951–2971. [Google Scholar] [CrossRef] [Green Version]
- Beijer, E.; Seldenrijk, K.; Eishi, Y.; Uchida, K.; Damen, J.; Grutters, J.C.; Veltkamp, M. Presence of Propionibacterium acnes in granulomas associates with a chronic disease course in Dutch sarcoidosis patients. ERJ Open Res. 2020, 7, 486. [Google Scholar] [CrossRef]
- Uchida, K.; Furukawa, A.; Yoneyama, A.; Furusawa, H.; Kobayashi, D.; Ito, T.; Yamamoto, K.; Sekine, M.; Miura, K.; Akashi, T.; et al. Propionibacterium acnes-Derived Circulating Immune Complexes in Sarcoidosis Patients. Microorganisms 2021, 9, 2194. [Google Scholar] [CrossRef]
- Davidsson, S.; Mölling, P.; Rider, J.; Unemo, M.; Karlsson, M.G.; Carlsson, J.; Andersson, S.-O.; Elgh, F.; Söderquist, B.; Andrén, O. Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect. Agents Cancer 2016, 11, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FassiFehri, L.; Mak, T.N.; Laube, B.; Brinkmann, V.; Ogilvie, L.A.; Mollenkopf, H.; Lein, M.; Schmidt, T.; Meyer, T.F.; Brüggemann, H. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int. J. Med. Microbiol. 2011, 301, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, M.; Eslami, G.; Taghavi, A.; Goudarzi, H.; Hajikhani, B.; Goudarzi, M.; Ghazi, M. Is Propionibacterium acnes a Causative Agent in Benign Prostate Hyperplasia and Prostate Cancer? Arch. Clin. Infect. Dis. 2018, 13, e58947. [Google Scholar] [CrossRef]
- Radej, S.; Płaza, P.; Olender, A.; Szewc, M.; Bar, K.; Maciejewski, R. Infiltrating Treg and Th17 Cells of the Prostate Hypertrophy Gland Associated with Propionibacterium Acnes Infection. Res. Rep. Urol. 2020, 12, 593–597. [Google Scholar] [CrossRef]
- Cohen, R.J.; Shannon, B.A.; McNEAL, J.E.; Shannon, T.; Garrett, K.L. Propionibacterium acnes Associated with Inflammation in Radical Prostatectomy Specimens: A Possible Link to Cancer Evolution? J. Urol. 2005, 173, 1969–1974. [Google Scholar] [CrossRef] [Green Version]
- Kakegawa, T.; Bae, Y.; Ito, T.; Uchida, K.; Sekine, M.; Nakajima, Y.; Furukawa, A.; Suzuki, Y.; Kumagai, J.; Akashi, T.; et al. Frequency of Propionibacterium acnes Infection in Prostate Glands with Negative Biopsy Results Is an Independent Risk Factor for Prostate Cancer in Patients with Increased Serum PSA Titers. PLoS ONE 2017, 12, e0169984. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Zaffuto, E.; Fossati, N.; Cucchiara, V.; Mirone, V.; Montorsi, F.; Briganti, A. The role of prostatic inflammation in the development and progression of benign and malignant diseases. Curr. Opin. Urol. 2017, 27, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, W.; Gong, D.; Shang, R.; Wang, J.; Yu, H. Propionibacterium acnes overabundance in gastric cancer promote M2 polarization of macrophages via a TLR4/PI3K/Akt signaling. Gastric Cancer 2021, 24, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, L.; Liu, X.; Ji, F.; Mei, Y.; Cheng, Y.; Liu, F.; Yan, C.; Li, L.; Ling, Z. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. eBioMedicine 2018, 40, 336–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.-C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Gunathilake, M.N.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Ahn, Y.; Park, C.; Kim, J. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: A case-control study. Sci. Rep. 2019, 9, 13589–13611. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage m1-m2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Han, K.; Han, Y.; Kang, N.; Shin, T.-S.; Park, H.; Kim, H.; Kwon, W.; Lee, S.; Kim, Y.-K.; et al. Microbiome Markers of Pancreatic Cancer Based on Bacteria-Derived Extracellular Vesicles Acquired from Blood Samples: A Retrospective Propensity Score Matching Analysis. Biology 2021, 10, 219. [Google Scholar] [CrossRef]
- Suprewicz, Ł.; Tokajuk, G.; Cieśluk, M.; Deptuła, P.; Sierpińska, T.; Wolak, P.; Wollny, T.; Tokajuk, J.; Głuszek, S.; Piktel, E.; et al. Bacteria Residing at Root Canals Can Induce Cell Proliferation and Alter the Mechanical Properties of Gingival and Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7914. [Google Scholar] [CrossRef]
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012, 25, 1284–1297. [Google Scholar] [CrossRef]
- Isshiki, T.; Homma, S.; Eishi, Y.; Yabe, M.; Koyama, K.; Nishioka, Y.; Yamaguchi, T.; Uchida, K.; Yamamoto, K.; Ohashi, K.; et al. Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms 2021, 9, 1668. [Google Scholar] [CrossRef]
- Schupp, J.C.; Tchaptchet, S.; Lützen, N.; Engelhard, P.; Müller-Quernheim, J.; Freudenberg, M.A.; Prasse, A. Immune response to Propionibacterium acnes in patients with sarcoidosis–in vivo and in vitro. BMC Pulm. Med. 2015, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Nelson, D.E.; Toh, E.; Diao, L.; Gao, X.; Fortenberry, J.D.; Van Der Pol, B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE 2011, 6, e19709. [Google Scholar] [CrossRef] [Green Version]
- Alexeyev, O.A.; Marklund, I.; Shannon, B.; Golovleva, I.; Olsson, J.; Andersson, C.; Eriksson, I.; Cohen, R.; Elgh, F. Direct Visualization of Propionibacterium acnes in Prostate Tissue by Multicolor Fluorescent In Situ Hybridization Assay. J. Clin. Microbiol. 2007, 45, 3721–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, J.; Schokrpur, S.; Liu, C.; Priceman, S.J.; Moughon, D.; Jiang, Z.; Pouliot, F.; Magyar, C.; Sung, J.L.; Xu, J.; et al. CSF1 Receptor Targeting in Prostate Cancer Reverses Macrophage-Mediated Resistance to Androgen Blockade Therapy. Cancer Res. 2015, 75, 950–962. [Google Scholar] [CrossRef] [Green Version]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes Promotes Th17 and Th17/Th1 Responses in Acne Patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, D.B.; Vaghasia, A.M.; Yu, S.-H.; Mak, T.N.; Brüggemann, H.; Nelson, W.G.; De Marzo, A.M.; Yegnasubramanian, S.; Sfanos, K.S. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate 2013, 73, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Palayoor, S.T.; Youmell, M.Y.; Calderwood, S.K.; Coleman, C.N.; Price, B.D. Constitutive activation of IκB kinase α and NF-κB in prostate cancer cells is inhibited by ibuprofen. Oncogene 1999, 18, 7389–7394. [Google Scholar] [CrossRef]
- Lin, Q.; Lai, R.; Chirieac, L.R.; Li, C.; Thomazy, V.A.; Grammatikakis, I.; Rassidakis, G.Z.; Zhang, W.; Fujio, Y.; Kunisada, K.; et al. Constitutive Activation of JAK3/STAT3 in Colon Carcinoma Tumors and Cell Lines: Inhibition of JAK3/STAT3 Signaling Induces Apoptosis and Cell Cycle Arrest of Colon Carcinoma Cells. Am. J. Pathol. 2005, 167, 969–980. [Google Scholar] [CrossRef]
- Durant, L.; Watford, W.T.; Ramos, H.L.; Laurence, A.; Vahedi, G.; Wei, L.; Takahashi, H.; Sun, H.-W.; Kanno, Y.; Powrie, F.; et al. Diverse Targets of the Transcription Factor STAT3 Contribute to T Cell Pathogenicity and Homeostasis. Immunity 2010, 32, 605–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The balance of Th17 versus treg cells in autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.-B.; Luo, M.-M.; Chen, Z.-Y.; He, B.-H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Shitara, K.; Nishikawa, H. Regulatory T cells: A potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. 2018, 1417, 104–115. [Google Scholar] [CrossRef]
- Chougnet, C.; Hildeman, D. Helios—controller of Treg stability and function. Transl. Cancer Res. 2016, 5, S338–S341. [Google Scholar] [CrossRef]
- Yu, W.-Q.; Ji, N.-F.; Gu, C.-J.; Wang, Y.-L.; Huang, M.; Zhang, M.-S. Coexpression of Helios in Foxp3+ Regulatory T Cells and Its Role in Human Disease. Dis. Markers 2021, 2021, 5574472. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin. Cancer Biol. 2019, 65, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Lu, J.; Korty, P.E.; Kim, Y.C.; Martens, C.; Sun, P.D.; Shevach, E.M. Helios+ and Helios− Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur. J. Immunol. 2019, 49, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell Biol. 2018, 96, 21–33. [Google Scholar] [CrossRef]
- Mayne, C.G.; Williams, C.B. Induced and Natural Regulatory T Cells in the Development of Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2013, 19, 1772–1788. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e5. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; Xie, D.; Wazir, J.; Shrestha, S.; Ullah, R.; Baig, M.M.F.A.; Ahmed, A.; Srivastava, P.K.; Thapa, K.B.; et al. Chronic prostatitis/chronic pelvic pain syndrome and prostate cancer: Study of immune cells and cytokines. Fundam. Clin. Pharmacol. 2019, 34, 160–172. [Google Scholar] [CrossRef]
- Chang, S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharmacal. Res. 2019, 42, 549–559. [Google Scholar] [CrossRef]
- Liu, S.; Liu, F.; Zhang, B.; Yan, P.; Rowan, B.G.; Dvm, A.B.A.; Steele, C.; Jazwinski, S.M.; Moroz, K.; Norton, E.B.; et al. CD4 + T helper 17 cell response of aged mice promotes prostate cancer cell migration and invasion. Prostate 2020, 80, 764–776. [Google Scholar] [CrossRef]
- You, Z.; Ge, D.; Liu, S.; Zhang, Q.; Borowsky, A.D.; Melamed, J. Interleukin-17 Induces Expression of Chemokines and Cytokines in Prostatic Epithelial Cells but Does Not Stimulate Cell Growth In Vitro. Int. J. Med. Biol. Front. 2012, 18, 629–644. [Google Scholar] [PubMed]
- Li, Q.; Liu, L.; Zhang, Q.; Liu, S.; Ge, D.; You, Z. Interleukin-17 Indirectly Promotes M2 Macrophage Differentiation through Stimulation of COX-2/PGE2 Pathway in the Cancer Cells. Cancer Res. Treat. 2014, 46, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Patel, J.; Marengo, S.R.; Resnick, M.L.; Gupta, S. Nuclear Factor-κB/p65 (Rel A) Is Constitutively Activated in Human Prostate Adenocarcinoma and Correlates with Disease Progression. Neoplasia 2004, 6, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Steiner, G.E.; Newman, M.E.; Paikl, D.; Stix, U.; Memaran-Dagda, N.; Lee, C.; Marberger, M.J. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 2003, 56, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, J.C.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic Analysis of Prostate-Infiltrating Lymphocytes Reveals TH17 and Treg Skewing. Clin. Cancer Res. 2008, 14, 3254–3261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, S.; Parajuli, K.R.; Zhang, W.; Zhang, K.; Mo, Z.; Liu, J.; Chen, Z.; Yang, S.; Wang, A.R.; et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene 2016, 36, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, S.; Ge, D.; Cunningham, D.M.; Huang, F.; Ma, L.; Burris, T.P.; You, Z. Targeting Th17-IL-17 Pathway in Prevention of Micro-Invasive Prostate Cancer in a Mouse Model. Prostate 2017, 77, 888–899. [Google Scholar] [CrossRef]
- Cunningham, D.; Zhang, Q.; Liu, S.; Parajuli, K.R.; Nie, Q.; Ma, L.; Zhang, A.; Chen, Z.; You, Z. Interleukin-17 promotes metastasis in an immunocompetent orthotopic mouse model of prostate cancer. Am. J. Clin. Exp. Urol. 2018, 6, 114–122. [Google Scholar]
- Miller, A.M.; Lundberg, K.; Özenci, V.; Banham, A.H.; Hellström, M.; Egevad, L.; Pisa, P. CD4+CD25high T Cells Are Enriched in the Tumor and Peripheral Blood of Prostate Cancer Patients. J. Immunol. 2006, 177, 7398–7405. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Jin, H.; Xu, Y. T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D. Role of imbalance between Th17 and regulatory T-cells in sarcoidosis. Curr. Opin. Pulm. Med. 2018, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Agak, G.W.; Kao, S.; Ouyang, K.; Qin, M.; Moon, D.; Butt, A.; Kim, J. Phenotype and Antimicrobial Activity of Th17 Cells Induced by Propionibacterium acnes Strains Associated with Healthy and Acne Skin. J. Investig. Dermatol. 2018, 138, 316–324. [Google Scholar] [CrossRef] [PubMed]
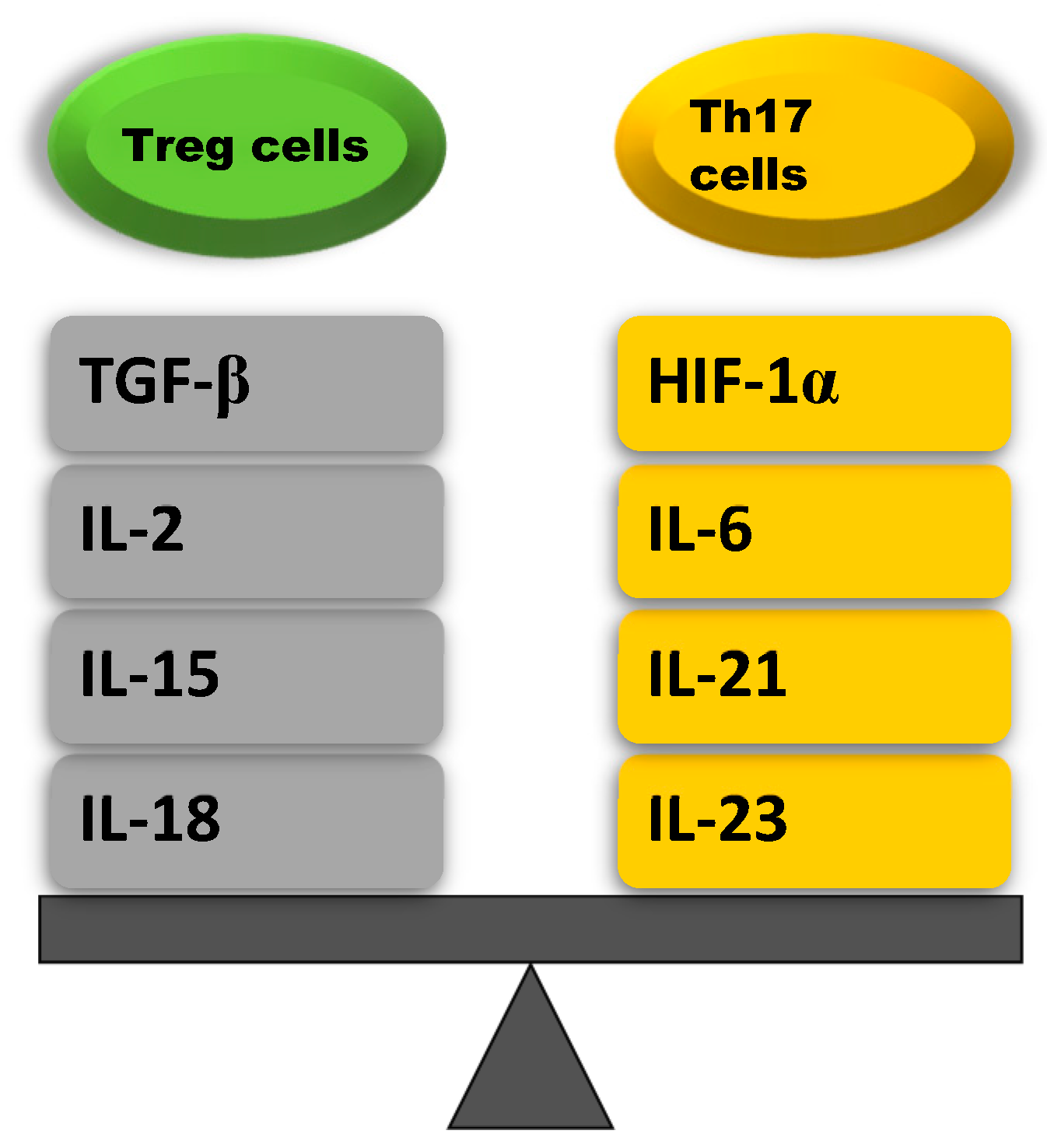
| Treg Cells Upregulating Cytokines | Th17 Cells Upregulating Cytokines |
|---|---|
| TGF-β: stimulates naïve CD4+ T cells that induce SMAD2 and SMAD3 that activate the transcription factor Foxp3 | HIF-1α: promotes the differentation of Th17 cells by inducing ROR-γt transcription and inhibits the differentation of Treg cell in an active process aimed at degradation of the Foxp3 protein |
| IL-2: increases Foxp3 expression by phosphorylation of STAT5 which binds to the Foxp3 locus | IL-6: stimulates naïve CD4+ T cells to differentiate into Th17 via STAT3 phosphorylation which induces the upregulation of Th17-specific genes (ROR-γt, IL-17, IL-23) |
| IL-15: increases Foxp3 expression by activating STAT5 and inhibits Th17 cell differentation by reducing IL-17 secretion | IL-21: stimulates Th17 cell differentation by activating STAT3, which increases ROR-γt expression |
| IL-18: inhibits Th17 cell differentation by inhibiting MyD88- dependent IL-1R downstream signal | IL-23: maintains Th17 cell differentation by enhancing the transcription of Th17 specyfic cytokines such as ROR-γt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radej, S.; Szewc, M.; Maciejewski, R. Prostate Infiltration by Treg and Th17 Cells as an Immune Response to Propionibacterium acnes Infection in the Course of Benign Prostatic Hyperplasia and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 8849. https://doi.org/10.3390/ijms23168849
Radej S, Szewc M, Maciejewski R. Prostate Infiltration by Treg and Th17 Cells as an Immune Response to Propionibacterium acnes Infection in the Course of Benign Prostatic Hyperplasia and Prostate Cancer. International Journal of Molecular Sciences. 2022; 23(16):8849. https://doi.org/10.3390/ijms23168849
Chicago/Turabian StyleRadej, Sebastian, Monika Szewc, and Ryszard Maciejewski. 2022. "Prostate Infiltration by Treg and Th17 Cells as an Immune Response to Propionibacterium acnes Infection in the Course of Benign Prostatic Hyperplasia and Prostate Cancer" International Journal of Molecular Sciences 23, no. 16: 8849. https://doi.org/10.3390/ijms23168849






