Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization
Abstract
1. Introduction
2. Results and Discussion
2.1. Biophysical Properties of Liposomes and Proteoliposomes
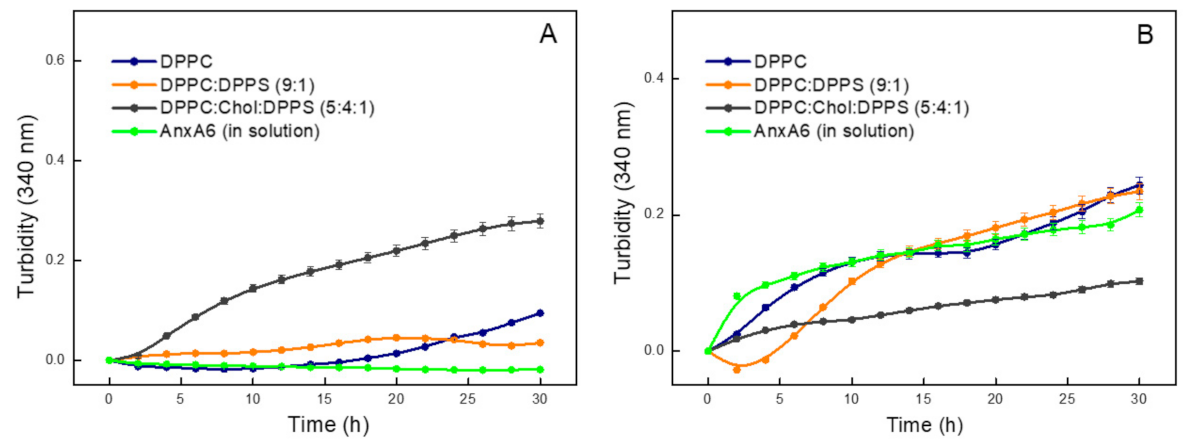
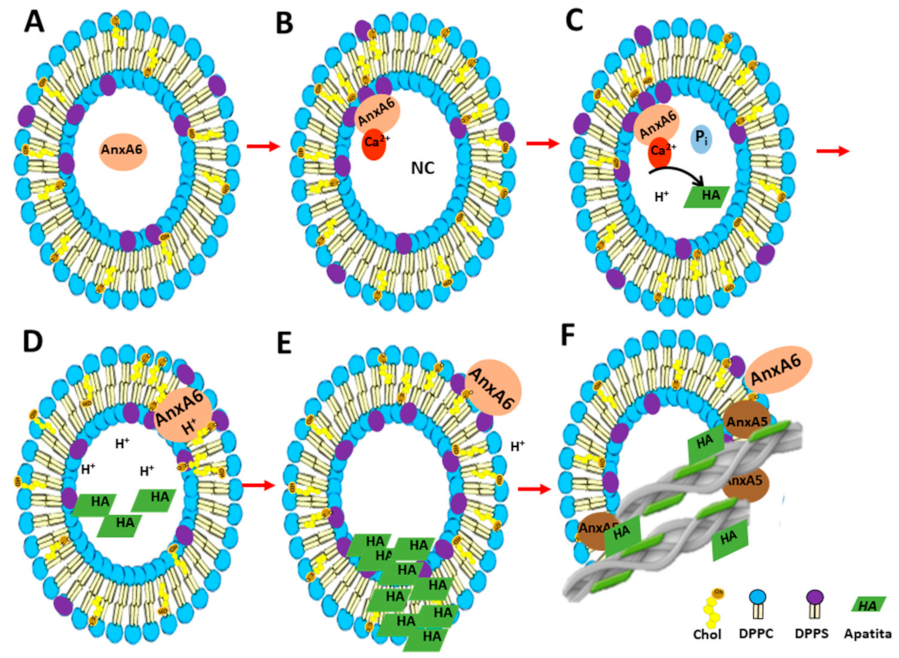
2.2. Biomineralization Mediated by AnxA6-Harbouring Proteoliposomes with Amorphous Calcium Phosphate
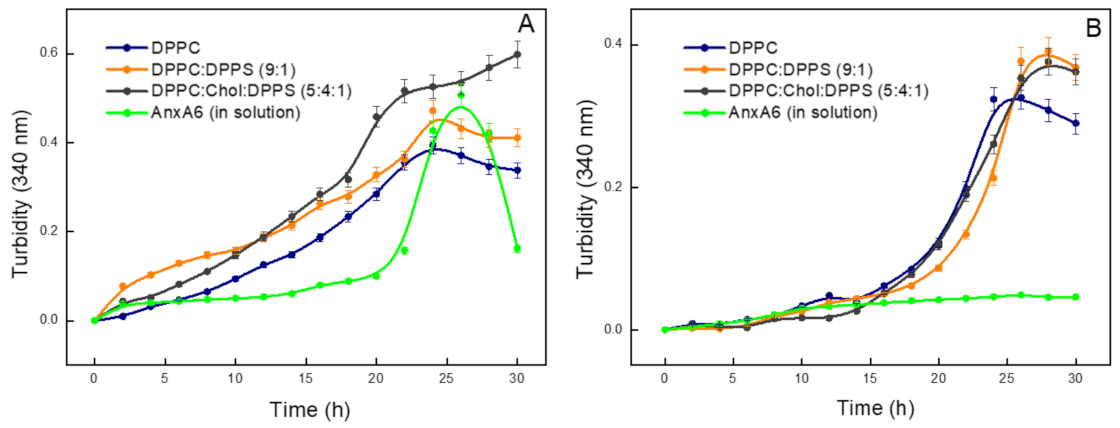
2.3. Biomineralization Mediated by AnxA6-Harbouring Proteoliposomes with Phosphatidylserine-Calcium Complex in the Absence of Type-I Collagen
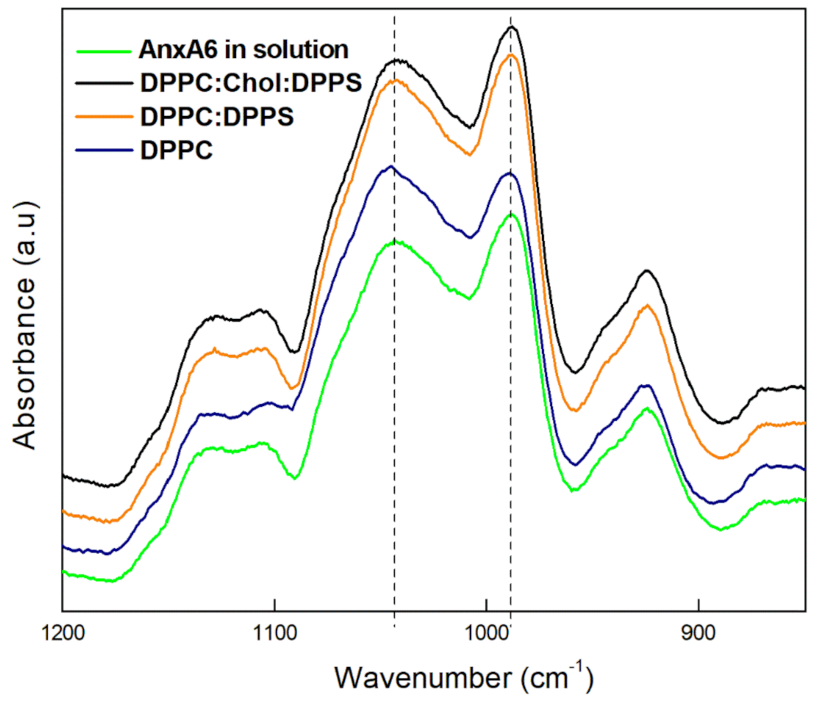
2.4. Spectroscopic Analysis of the Minerals Formed by Annexin A6-Harbouring Proteoliposomes
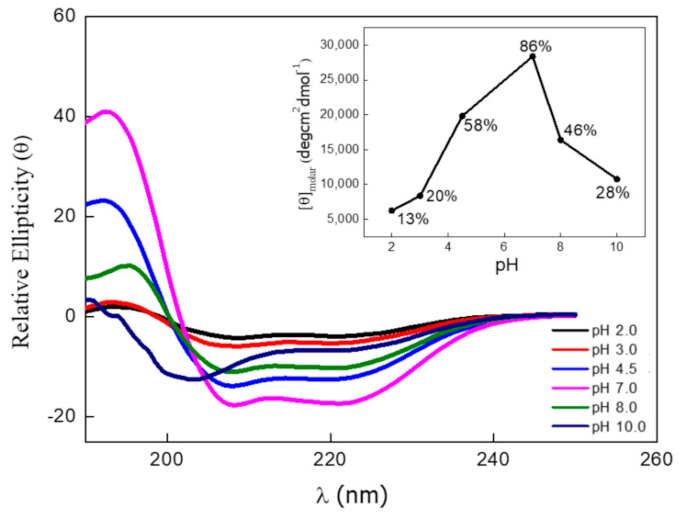
2.5. Circular Dichroism of Annexin A6 in Different pHs
3. Material and Methods
3.1. Materials
3.2. Expression of Annexin A6
3.3. Preparation of Liposomes
3.4. Preparation of Proteoliposomes
3.5. Synthesis of Amorphous Calcium Phosphate and of Phosphatidylserine Calcium Complex Nucleators
3.6. Mineralization Assays with Proteoliposomes and Characterization by Infrared Spectroscopy
3.7. Collagen Matrix Coating for Mineralization Assay
3.8. Circular Dichroism Spectroscopy
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Amorphous calcium carbonates |
| ACP | Amorphous calcium phosphate |
| Annexin A2, A4, A6 | AnxA2, AnxA4, AnxA6 |
| Chol | Cholesterol |
| DPPC | 1,2-dipalmitoylphosphatidylcholine |
| DPPS | 1,2-dipalmitoylphosphatidylserine |
| EDTA | Ethylenediaminetetraacetic acid |
| EGTA | Ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid |
| FTIR | Fourier transformed infrared spectroscopy |
| MVs | Matrix vesicles |
| NPP1 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 |
| Pi | Phosphate |
| PiT-1 | Sodium-dependent transporter |
| PMP | Potential of mineral propagation |
| PPi | Pyrophosphate |
| PS–CPLX | Phosphatidylserine-calcium phosphate complex |
| SCL | Synthetic cartilage lymph |
| SMPD3 | Sphingomyelin phosphodiesterase 3 gene |
| tf | Final time of mineral formation |
| ti | Initial time of mineral formation |
| tmax rate | Time at maximum rate of mineral formation |
| TNAP | Tissue-nonspecific alkaline phosphatase |
| Umax | Maximum value of turbidity |
References
- Moss, D.W.; Eaton, R.H.; Smith, J.K.; Whitby, L.G. Association of Inorganic-Pyrophosphatase Activity with Human Alkaline-Phosphatase Preparations. Biochem. J. 1967, 102, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Majeska, R.J.; Wuthier, R.E. Studies on Matrix Vesicles Isolated from Chick Epiphyseal Cartilage Association of Pyrophosphatase and ATPase Activities with Alkaline Phosphatase. Biochim. Biophys. Acta (BBA) Enzymol. 1975, 391, 51–60. [Google Scholar] [CrossRef]
- Johnson, K.A.; Hessle, L.; Vaingankar, S.; Wennberg, C.; Mauro, S.; Narisawa, S.; Goding, J.W.; Sano, K.; Millan, J.L.; Terkeltaub, R. Osteoblast Tissue-Nonspecific Alkaline Phosphatase Antagonizes and Regulates PC-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-Nonspecific Alkaline Phosphatase and Plasma Cell Membrane Glycoprotein-1 Are Central Antagonistic Regulators of Bone Mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M.; Harmey, D.; Millán, J.L.; McKee, M.D.; Karsenty, G. Unique Coexpression in Osteoblasts of Broadly Expressed Genes Accounts for the Spatial Restriction of ECM Mineralization to Bone. Genes Dev. 2005, 19, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Garimella, R.; Bi, X.; Anderson, H.C.; Camacho, N.P. Nature of Phosphate Substrate as a Major Determinant of Mineral Type Formed in Matrix Vesicle-Mediated in Vitro Mineralization: An FTIR Imaging Study. Bone 2006, 38, 811–817. [Google Scholar] [CrossRef]
- Anderson, H.C. Molecular Biology of Matrix Vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280. [Google Scholar] [CrossRef]
- Anderson, H.C. The Role of Matrix Vesicles in Growth Plate Development and Biomineralization. Front. Biosci. 2005, 10, 822–837. [Google Scholar] [CrossRef]
- Golub, E.E. Biomineralization and Matrix Vesicles in Biology and Pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Lipscomb, G.F. Matrix Vesicles: Structure, Composition, Formation and Function in Calcification. Front. Biosci. 2011, 16, 2812–2902. [Google Scholar] [CrossRef]
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef]
- Stewart, A.J.; Roberts, S.J.; Seawright, E.; Davey, M.G.; Fleming, R.H.; Farquharson, C. The Presence of PHOSPHO1 in Matrix Vesicles and Its Developmental Expression Prior to Skeletal Mineralization. Bone 2006, 39, 1000–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dillon, S.; Staines, K.A.; Millán, J.L.; Farquharson, C. How To Build a Bone: PHOSPHO1, Biomineralization, and Beyond. JBMR Plus 2019, 3, e10202. [Google Scholar] [CrossRef] [PubMed]
- Thouverey, C.; Malinowska, A.; Balcerzak, M.; Strzelecka-Kiliszek, A.; Buchet, R.; Dadlez, M.; Pikula, S. Proteomic Characterization of Biogenesis and Functions of Matrix Vesicles Released from Mineralizing Human Osteoblast-like Cells. J. Proteom. 2011, 74, 1123–1134. [Google Scholar] [CrossRef]
- Montessuit, C.; Bonjour, J.P.; Caverzasio, J. Expression and Regulation of Na-Dependent Pi Transport in Matrix Vesicles Produced by Osteoblast-like Cells. J. Bone Miner. Res. 1995, 10, 625–631. [Google Scholar] [CrossRef]
- Guicheux, J.; Palmer, G.; Shukunami, C.; Hiraki, Y.; Bonjour, J.P.; Caverzasio, J. A Novel in Vitro Culture System for Analysis of Functional Role of Phosphate Transport in Endochondral Ossification. Bone 2000, 27, 69–74. [Google Scholar] [CrossRef]
- Yadav, M.C.; Bottini, M.; Cory, E.; Bhattacharya, K.; Kuss, P.; Narisawa, S.; Sah, R.L.; Beck, L.; Fadeel, B.; Farquharson, C.; et al. Skeletal Mineralization Deficits and Impaired Biogenesis and Function of Chondrocyte-Derived Matrix Vesicles in Phospho1-/- and Phospho1/Pit1 Double-Knockout Mice. J. Bone Miner. Res. 2016, 31, 1275–1286. [Google Scholar] [CrossRef]
- Solomon, D.H.; Browning, J.A.; Wilkins, R.J. Inorganic Phosphate Transport in Matrix Vesicles from Bovine Articular Cartilage. Acta Physiol. 2007, 190, 119–125. [Google Scholar] [CrossRef]
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.D. The Roles of Annexins and Types II and X Collagen in Matrix Vesicle-Mediated Mineralization of Growth Plate Cartilage. J. Biol. Chem. 2000, 275, 35577–35583. [Google Scholar] [CrossRef] [PubMed]
- Blandford, N.R.; Sauer, G.R.; Genge, B.R.; Wu, L.N.Y.; Wuthier, R.E. Modeling of Matrix Vesicle Biomineralization Using Large Unilamellar Vesicles. J. Inorg. Biochem. 2003, 94, 14–27. [Google Scholar] [CrossRef]
- Genge, B.R.; Wu, L.N.Y.; Wuthier, R.E. In Vitro Modeling of Matrix Vesicle Nucleation: Synergistic Stimulation of Mineral Formation by Annexin A5 and Phosphatidylserine. J. Biol. Chem. 2007, 282, 26035–26045. [Google Scholar] [CrossRef]
- Veschi, E.A.; Bolean, M.; Strzelecka-kiliszek, A.; Bandorowicz-pikula, J.; Pikula, S.; Granjon, T.; Mebarek, S.; Magne, D.; Ramos, A.P.; Rosato, N.; et al. Localization of Annexin A6 in Matrix Vesicles during Physiological Mineralization. Int. J. Mol. Sci. 2020, 21, 1367. [Google Scholar] [CrossRef]
- Wang, Y.; Weremiejczyk, L.; Strzelecka-Kiliszek, A.; Maniti, O.; Amabile Veschi, E.; Bolean, M.; Ramos, A.P.; Ben Trad, L.; Magne, D.; Bandorowicz-Pikula, J.; et al. Fluorescence Evidence of Annexin A6 Translocation across Membrane in Model Matrix Vesicles during Apatite Formation. J. Extracell. Biol. 2022, 1, e38. [Google Scholar] [CrossRef]
- Genge, B.R.; Wu, L.N.Y.; Wuthier, R.E. Identification of Phospholipid-Dependent Calcium-Binding Proteins as Constituents of Matrix Vesicles. J. Biol. Chem. 1989, 264, 10917–10921. [Google Scholar] [CrossRef]
- Bolean, M.; Simão, A.M.S.; Barioni, M.B.; Favarin, B.Z.; Sebinelli, H.G.; Veschi, E.A.; Janku, T.A.B.; Bottini, M.; Hoylaerts, M.F.; Itri, R.; et al. Biophysical Aspects of Biomineralization. Biophys. Rev. 2017, 9, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.T. Octacalcium Phosphate Formation in Vitro: Implications for Bone Formation. Calcif. Tissue Int. 1985, 37, 91–94. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bomans, P.H.H.; Müller, F.A.; Will, J.; Frederik, P.M.; De With, G.; Sommerdijk, N.A.J.M. The Role of Prenucleation Clusters in Surface-Induced Calcium Phosphate Crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic Amorphous Calcium Phosphates (ACPs): Preparation, Structure, Properties, and Biomedical Applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef]
- Green, J. The Physicochemical Structure of Bone: Cellular and Noncellular Elements. Min. Electrolyte Metab. 1994, 20, 7–15. [Google Scholar]
- Cazalbou, S.; Combes, C.; Eichert, D.; Rey, C.; Glimcher, M.J. Poorly Crystalline Apatites: Evolution and Maturation in Vitro and in Vivo. J. Bone Miner. Metab. 2004, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. Biomed. Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Volkel, A.; Colfen, H. Stable Prenucleation Calcium Carbonate Clusters. Sciences 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Bone Structure, Composition, and Mineralization. Orthop. Clin. N. Am. 1984, 15, 597–612. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Rice, G.S.; Wallace, J.E.B.; Weaver, R.L.; Legeros, R.Z.; David Eanes, E. In Vitro Precipitation of Calcium Phosphate Under Intracellular Conditions: Formation of Brushite from an Amorphous Precursor in the Absence of ATP. Calcif. Tissue Int. 1985, 37, 401–410. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Genge, B.R.; Dunkelberger, D.G.; Legeros, R.Z.; Concannon, B.; Wuthier, R.E. Physicochemical Characterization of the Nucleational Core of Matrix Vesicles. J. Biol. Chem. 1997, 272, 4404–4411. [Google Scholar] [CrossRef]
- Bonucci, E. Bone Mineralization. Front Biosci 2012, 17, 100–128. [Google Scholar] [CrossRef]
- Zhang, L.; Balcerzak, M.; Radisson, J.; Thouverey, C.; Pikula, S.; Rard Azzar, G.; Buchet, R. Phosphodiesterase Activity of Alkaline Phosphatase in ATP-Initiated Ca2+ and Phosphate Deposition in Isolated Chicken Matrix Vesicles. J. Biol. Chem. 2005, 280, 37289–37296. [Google Scholar] [CrossRef]
- Farlay, D.; Panczer, G.; Rey, C.; Delmas, P.D.; Boivin, G. Mineral Maturity and Crystallinity Index Are Distinct Characteristics of Bone Mineral. J. Bone Miner. Metab. 2010, 28, 433–445. [Google Scholar] [CrossRef]
- Jiang, S.; Jin, W.; Wang, Y.N.; Pan, H.; Sun, Z.; Tang, R. Effect of the Aggregation State of Amorphous Calcium Phosphate on Hydroxyapatite Nucleation Kinetics. RSC Adv. 2017, 7, 25497–25503. [Google Scholar] [CrossRef]
- Fowler, B. Vibrational Assignments for Calcium, Strontium, and Barium Hydroxyapatites Utilizing Isotopic Substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Rey, C.; Shimizu, M.; Collins, B.; Glimcher, M.J. Resolution-Enhanced Fourier Transform Infrared Spectroscopy Study of the Environment of Phosphate Ion in the Early Deposits of a Solid Phase of Calcium Phosphate in Bone and Enamel and Their Evolution with Age: 2. Investigations in the v3 PO4 Domain. Calcif. Tissue Int. 1991, 49, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ślósarczyk, A.; Paluszkiewicz, C.; Gawlicki, M.; Paszkiewicz, Z. The FTIR Spectroscopy and QXRD Studies of Calcium Phosphate Based Materials Produced from the Powder Precursors with Different Ca/P Ratios. Ceram. Int. 1997, 23, 297–304. [Google Scholar] [CrossRef]
- Hamade, E.; Azzar, G.; Radisson, J.; Buchet, R.; Roux, B. Chick Embryo Anchored Alkaline Phosphatase and Mineralization Process in Vitro: Influence of Ca2+ and Nature of Substrates. Eur. J. Biochem. 2003, 270, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lin, K.; Chang, J. A Simple Way to Synthesize 3D Hierarchical HAp Porous Microspheres with Sustained Drug Release. Ceram. Int. 2015, 41, 11153–11160. [Google Scholar] [CrossRef]
- Sauer, G.R.; Wuthier, R.E. Fourier Transform Infrared Characterization of Mineral Phases Formed during Induction of Mineralization by Collagenase-Released Matrix Vesicles In Vitro. J. Biol. Chem. 1988, 263, 13718–13724. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Bolean, M.; Favarin, B.Z.; Veschi, E.A.; Tovani, C.B.; Ramos, A.P.; Bottini, M.; Buchet, R.; Millán, J.L.; Ciancaglini, P. Lipid Microenvironment Affects the Ability of Proteoliposomes Harboring TNAP to Induce Mineralization without Nucleators. J. Bone Miner. Metab. 2019, 37, 607–613. [Google Scholar] [CrossRef]
- Chou, Y.F.; Chiou, W.A.; Xu, Y.; Dunn, J.C.Y.; Wu, B.M. The Effect of PH on the Structural Evolution of Accelerated Biomimetic Apatite. Biomaterials 2004, 25, 5323–5331. [Google Scholar] [CrossRef]
- Golczak, M.; Kirilenko, A.; Bandorowicz-Pikula, J.; Pikula, S. Conformational States of Annexin VI in Solution Induced by Acidic PH. FEBS Lett. 2001, 496, 49–54. [Google Scholar] [CrossRef]
- Jäckle, S.; Beisiegel, U.; Rinninger, F.; Buck, F.; Grigoleit, A.; Block, A.; Gröger, I.; Greten, H.; Windler, E. Annexin VI, a Marker Protein of Hepatocytic Endosomes. J. Biol. Chem. 1994, 269, 1026–1032. [Google Scholar] [CrossRef]
- Kirilenko, A.; Golczak, M.; Pikula, S.; Buchet, R.; Bandorowicz-Pikula, J. GTP-Induced Membrane Binding and Ion Channel Activity of Annexin VI: Is Annexin VI a GTP Biosensor? Biophys. J. 2002, 82, 2737–2745. [Google Scholar] [CrossRef][Green Version]
- Bolean, M.; Borin, I.A.; Simão, A.M.S.; Bottini, M.; Bagatolli, L.A.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Topographic Analysis by Atomic Force Microscopy of Proteoliposomes Matrix Vesicle Mimetics Harboring TNAP and AnxA5. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E.F. Determination of Protein: A Modification of the Lowry Method That Gives a Linear Photometric Response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Bolean, M.; Simão, A.M.S.; Favarin, B.Z.; Millán, J.L.; Ciancaglini, P. Thermodynamic Properties and Characterization of Proteoliposomes Rich in Microdomains Carrying Alkaline Phosphatase. Biophys. Chem. 2011, 158, 111–118. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Bolean, M.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Effects of PH on the Production of Phosphate and Pyrophosphate by Matrix Vesicles’ Biomimetics. Calcif. Tissue Int. 2013, 93, 222–232. [Google Scholar] [CrossRef]
- Bolean, M.; Izzi, B.; Van Kerckhoven, S.; Bottini, M.; Ramos, A.P.; Millán, J.L.; Hoylaerts, M.F.; Ciancaglini, P. Matrix Vesicle Biomimetics Harboring Annexin A5 and Alkaline Phosphatase Bind to the Native Collagen Matrix Produced by Mineralizing Vascular Smooth Muscle Cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2020, 1864, 129629. [Google Scholar] [CrossRef]
- Adler, A.J.; Greenfield, N.J.; Fasman, G.D. Circular Dichroism and Optical Rotatory Dispersion of Proteins and Polypeptides. Methods Enzymol. 1973, 27, 675–735. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yang, J.T.; Martinez, H.M. Determination of the Secondary Structures of Proteins by Circular Dichroism and Optical Rotatory Dispersion. Biochemistry 1972, 11, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Cornely, R.; Rentero, C.; Enrich, C.; Grewal, T.; Gaus, K. Annexin A6 Is an Organizer of Membrane Microdomains to Regulate Receptor Localization and Signalling. IUBMB Life 2011, 63, 1009–1017. [Google Scholar] [CrossRef]
- Favarin, B.F.; Andrade, M.A.R.; Bolean, M.; Simão, A.M.S.; Ramos, A.P.; Hoylaerts, M.F.; Millán, J.L.; Ciancaglini, P. Effect of the Presence of Cholesterol in the Interfacial Microenvironment on the Modulation of the Alkaline Phosphatase Activity during in Vitro Mineralization. Colloids Surf. B Biointerfaces 2017, 155, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Favarin, B.Z.; Bolean, M.; Ramos, A.P.; Magrini, A.; Rosato, N.; Millán, J.L.; Bottini, M.; Costa-Filho, A.J.; Ciancaglini, P. Lipid Composition Modulates ATP Hydrolysis and Calcium Phosphate Mineral Propagation by TNAP-Harboring Proteoliposomes. Arch. Biochem. Biophys. 2020, 691, 108482. [Google Scholar] [CrossRef] [PubMed]
- Andrilli, L.H.S.; Sebinelli, H.G.; Favarin, B.Z.; Cruz, M.A.E.; Ramos, A.P.; Millán, J.L.; Bottini, M.; Ciancaglini, P. NPP1 and TNAP Hydrolyze ATP Synergistically during Biomineralization. Purinergic Signal. 2022, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
| Liposome/ Proteoliposome Lipid Composition | AnxA6 | Diameter (nm) | PI |
|---|---|---|---|
| DPPC | - | 132.2 ± 8.0 | 0.08 |
| + | 130.6 ± 1.7 | 0.04 | |
| DPPC:DPPS (9:1) | - | 114.4 ± 0.7 | 0.06 |
| + | 117.9 ± 0.9 | 0.07 | |
| DPPC:Chol:DPPS (5:4:1) | - | 199.1 ± 1.1 | 0.08 |
| + | 199.5 ± 1.4 | 0.09 |
| Proteoliposome | Parameters | |||||
|---|---|---|---|---|---|---|
| Lipid Composition | Monotone Slope (U/h) | ti (h) | tf (h) | tmax rate (h) | Umax (U) | PMP (h−1) |
| DPPC | 0.0084 | 9.5 ± 0.54 | 24.8 ± 0.56 | 18.5 ± 0.28 | 0.19 ± 0.05 | 0.010 |
| DPPC:DPPS (9:1) | 0.0022 | 14.9 ± 0.39 | 24.3 ± 0.18 | 22.9 ± 0.35 | 0.15 ± 0.05 | 0.007 |
| DPPC:Chol:DPPS (5:4:1) | 0.0013 | 10.7 ± 0.15 | 22.5 ± 0.34 | 19.5 ± 0.55 | 0.22 ± 0.09 | 0.011 |
| Control | - | 20.7 ± 0.41 | 24.5 ± 0.32 | 22.4 ± 0.21 | 0.42 ± 0.03 | 0.019 |
| Proteoliposome Composition | Parameters | ||||
|---|---|---|---|---|---|
| ti (h) | tf (h) | tmax rate (h) | Umax (U) | PMP (h−1) | |
| DPPC | 14.9 ± 0.40 | 27.5 ± 0.89 | 20.5 ± 0.63 | 0.33 ± 0.05 | 0.016 |
| DPPC:DPPS (9:1) | 15.2 ± 0.44 | 29.5 ± 0.57 | 23.2 ± 0.22 | 0.39 ± 0.07 | 0.017 |
| DPPC:Chol:DPPS(5:4:1) | 13.8 ± 0.28 | 29.9 ± 0.55 | 21.7 ± 0.39 | 0.38 ± 0.02 | 0.018 |
| Control | ND | ND | ND | ND | ND |
| Proteoliposome Composition | PO43−/HPO42− |
|---|---|
| DPPC | 1.2 |
| DPPC:DPPS (9:1) | 0.79 |
| DPPC:Chol:DPPS (5:4:1) | 0.64 |
| Control | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veschi, E.A.; Bolean, M.; da Silva Andrilli, L.H.; Sebinelli, H.G.; Strzelecka-Kiliszek, A.; Bandorowicz-Pikula, J.; Pikula, S.; Granjon, T.; Mebarek, S.; Magne, D.; et al. Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization. Int. J. Mol. Sci. 2022, 23, 8945. https://doi.org/10.3390/ijms23168945
Veschi EA, Bolean M, da Silva Andrilli LH, Sebinelli HG, Strzelecka-Kiliszek A, Bandorowicz-Pikula J, Pikula S, Granjon T, Mebarek S, Magne D, et al. Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization. International Journal of Molecular Sciences. 2022; 23(16):8945. https://doi.org/10.3390/ijms23168945
Chicago/Turabian StyleVeschi, Ekeveliny Amabile, Maytê Bolean, Luiz Henrique da Silva Andrilli, Heitor Gobbi Sebinelli, Agnieszka Strzelecka-Kiliszek, Joanna Bandorowicz-Pikula, Slawomir Pikula, Thierry Granjon, Saida Mebarek, David Magne, and et al. 2022. "Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization" International Journal of Molecular Sciences 23, no. 16: 8945. https://doi.org/10.3390/ijms23168945
APA StyleVeschi, E. A., Bolean, M., da Silva Andrilli, L. H., Sebinelli, H. G., Strzelecka-Kiliszek, A., Bandorowicz-Pikula, J., Pikula, S., Granjon, T., Mebarek, S., Magne, D., Millán, J. L., Ramos, A. P., Buchet, R., Bottini, M., & Ciancaglini, P. (2022). Mineralization Profile of Annexin A6-Harbouring Proteoliposomes: Shedding Light on the Role of Annexin A6 on Matrix Vesicle-Mediated Mineralization. International Journal of Molecular Sciences, 23(16), 8945. https://doi.org/10.3390/ijms23168945









