Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor–Arousal Cycles of the Daurian Ground Squirrel
Abstract
1. Introduction
2. Results
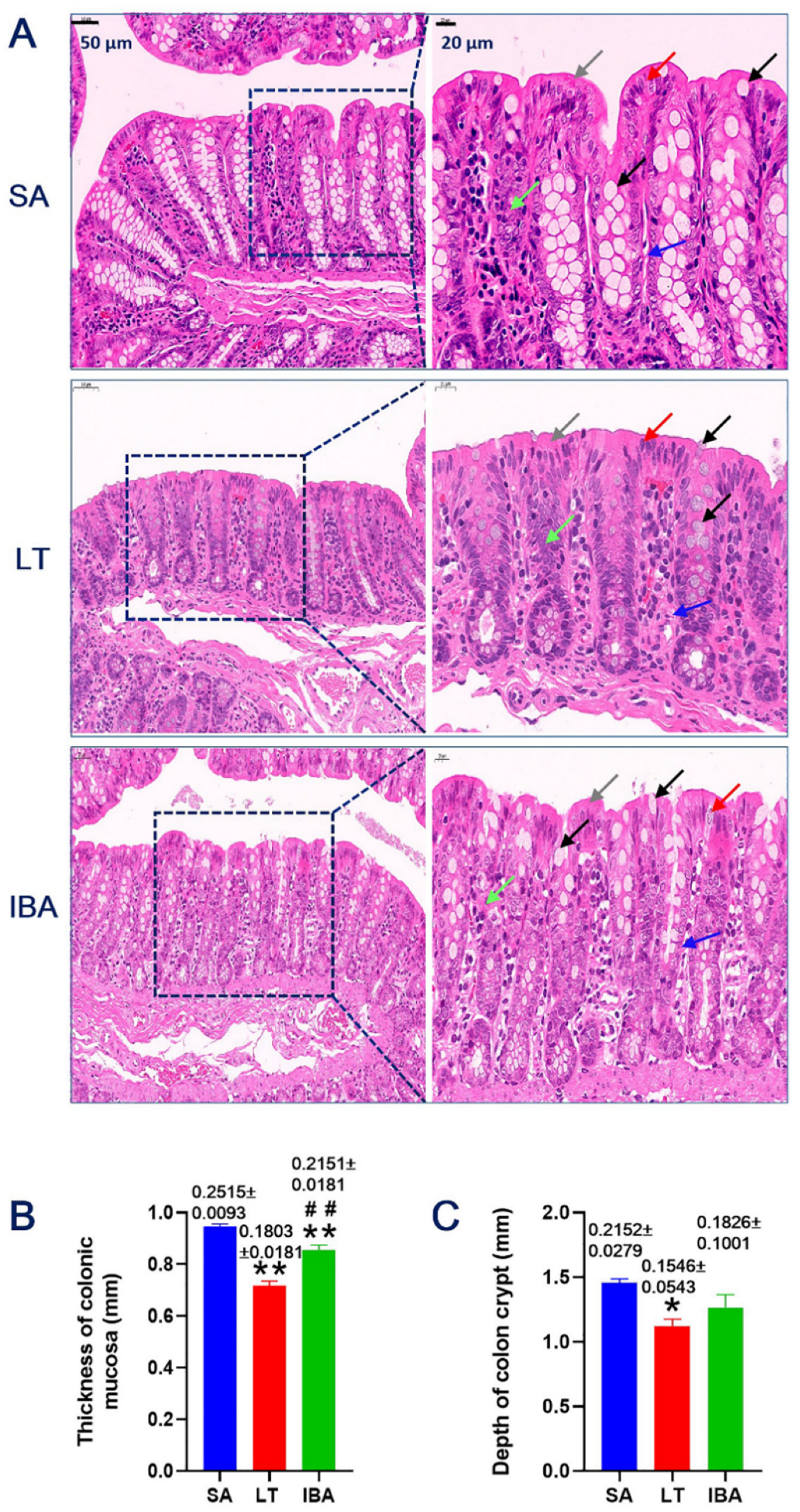
2.1. HE Staining of Colonic Mucosa
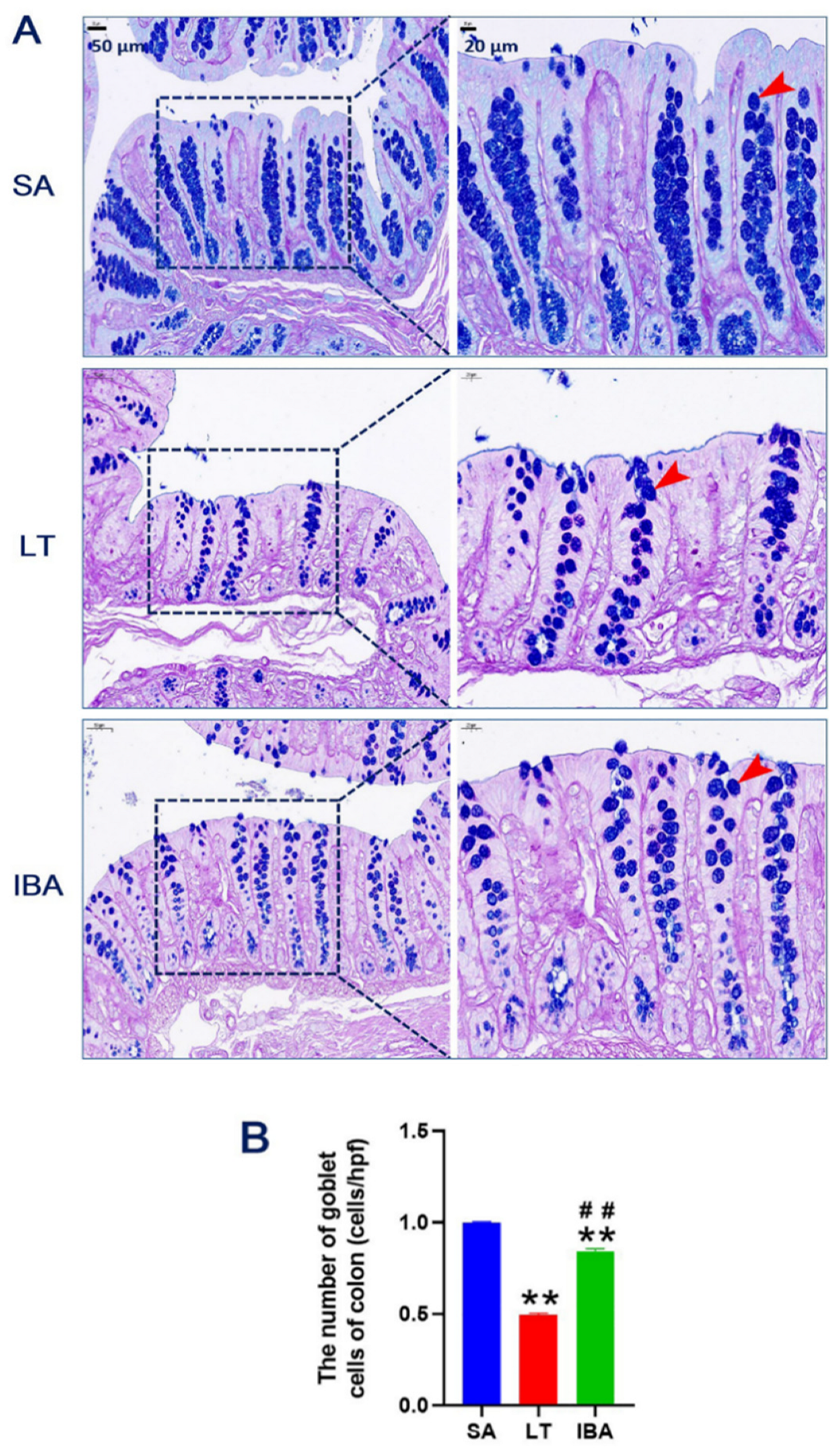
2.2. AB–PAS Staining of Colonic Mucosa
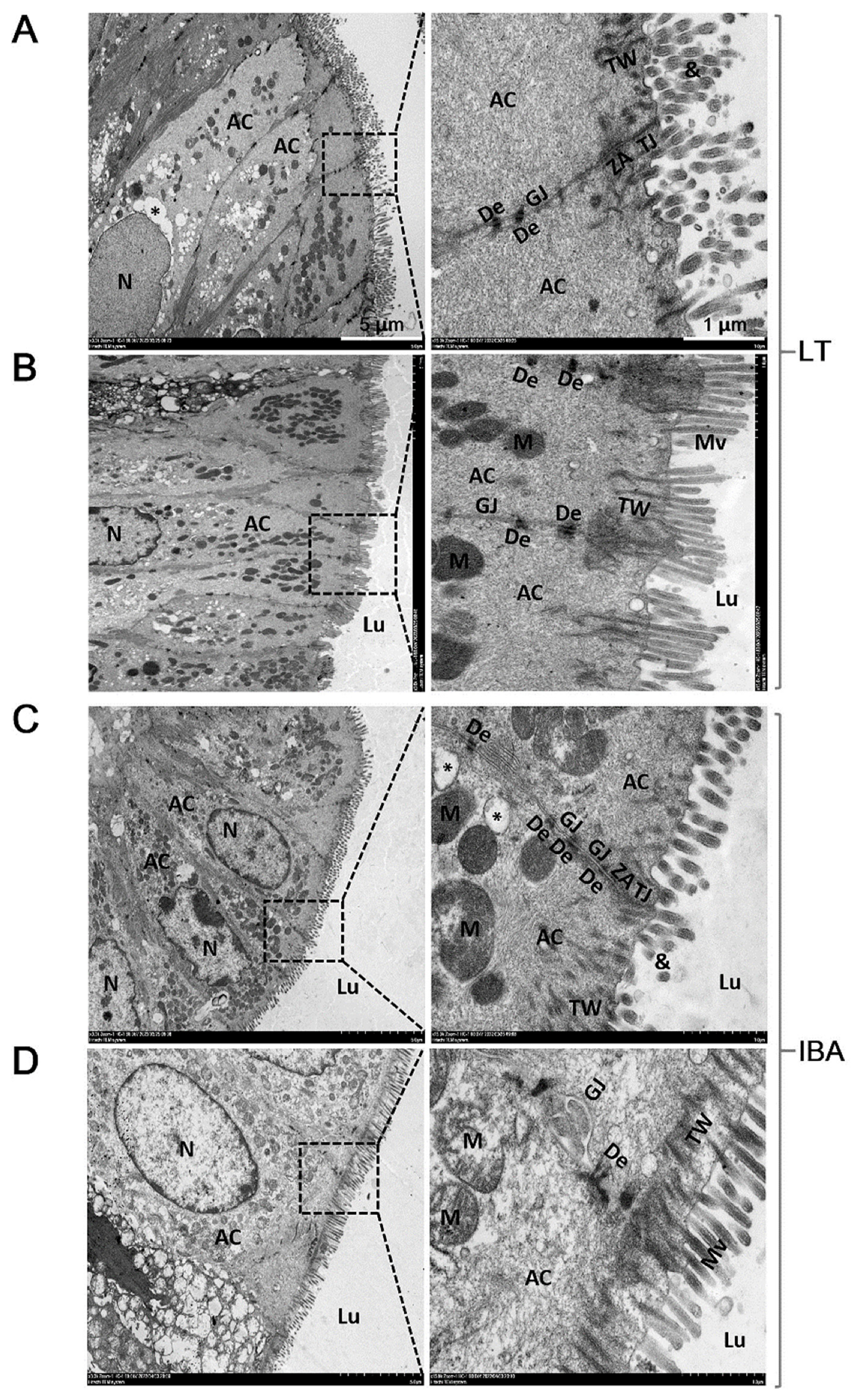
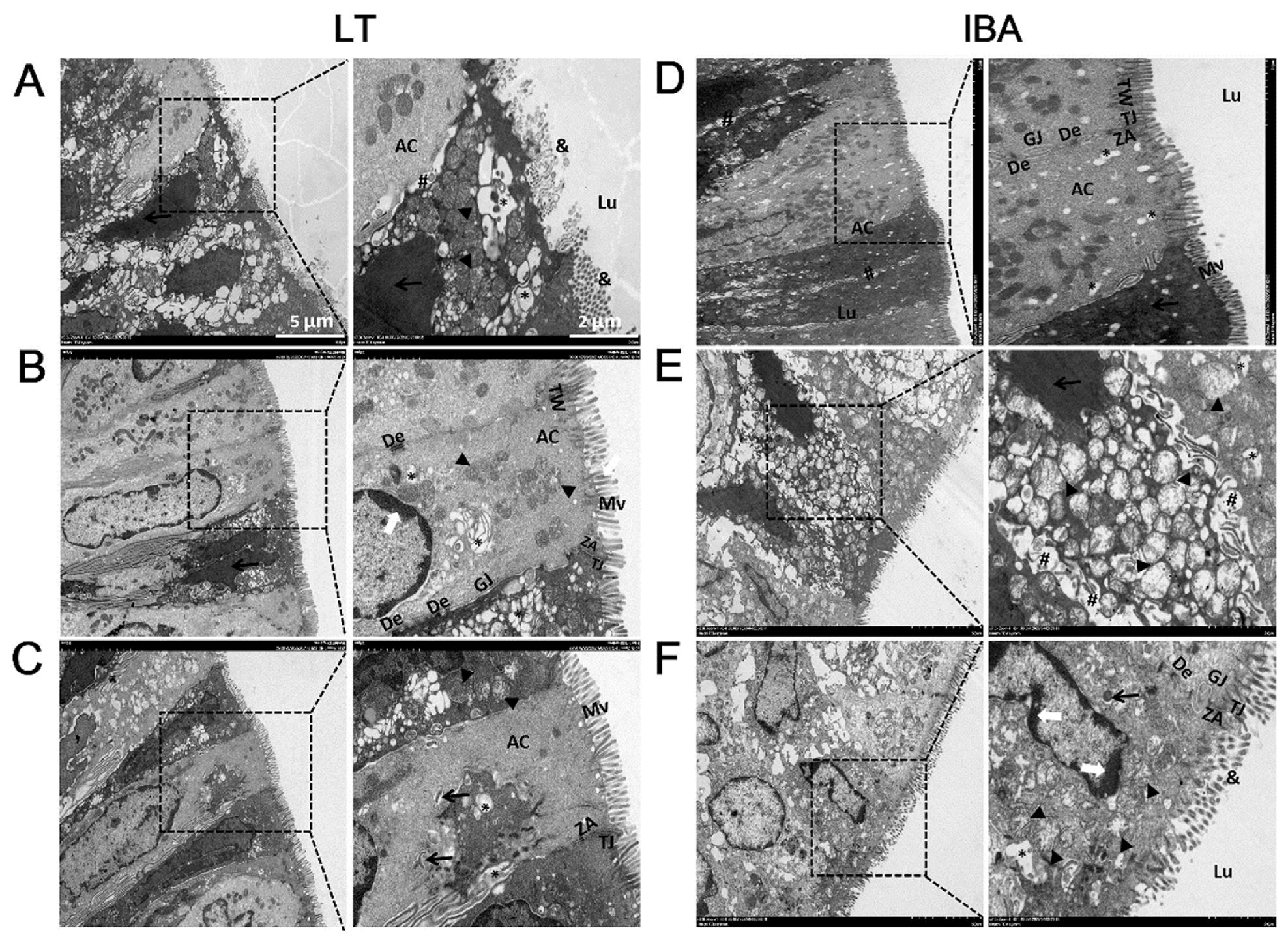
2.3. Ultrastructure of Colonic Mucosal Epithelial Cells
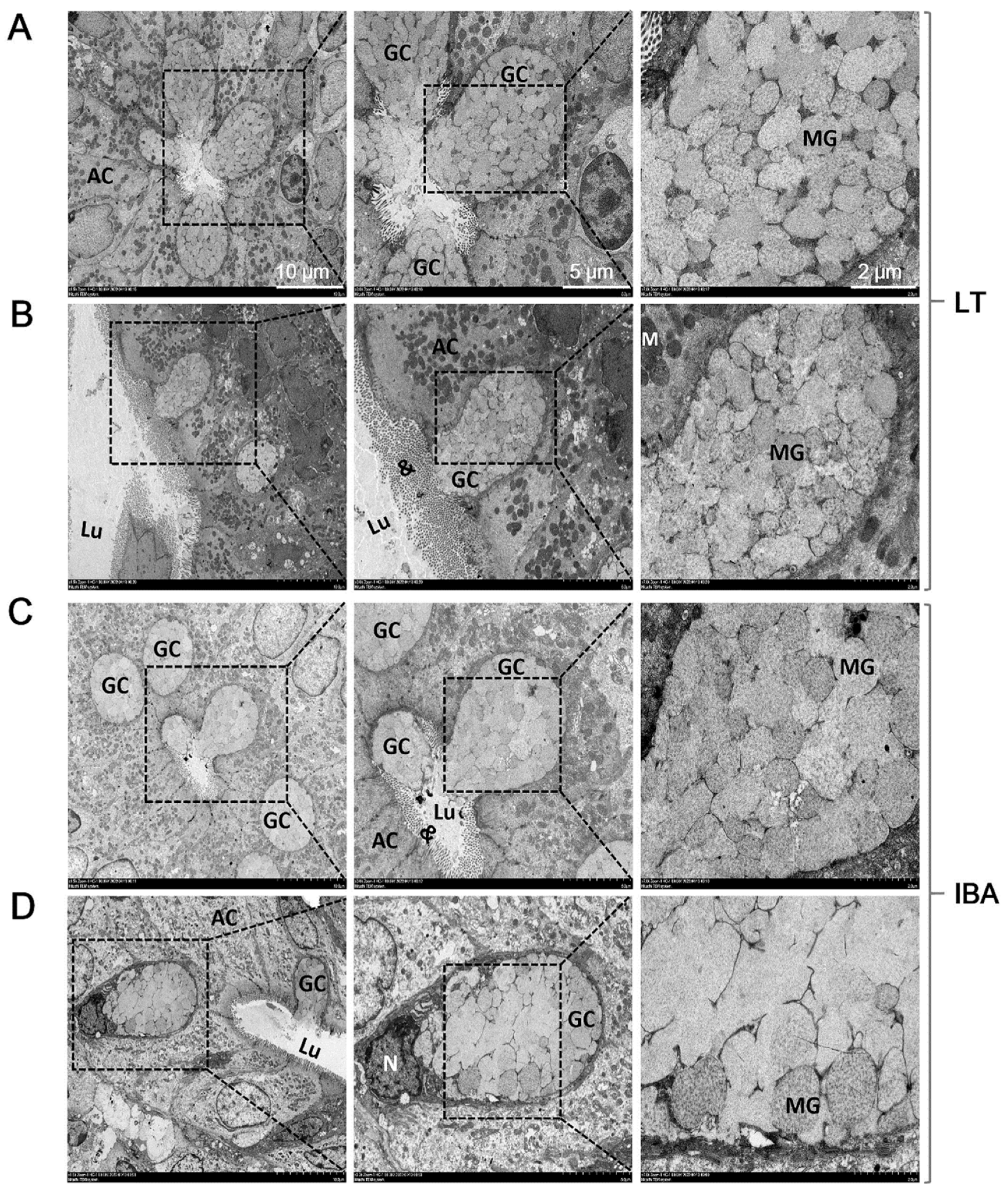
2.4. Ultrastructure of Goblet Cells in Colonic Mucosa
2.5. Ultrastructure of Apoptotic Cells in the Colonic Mucosa
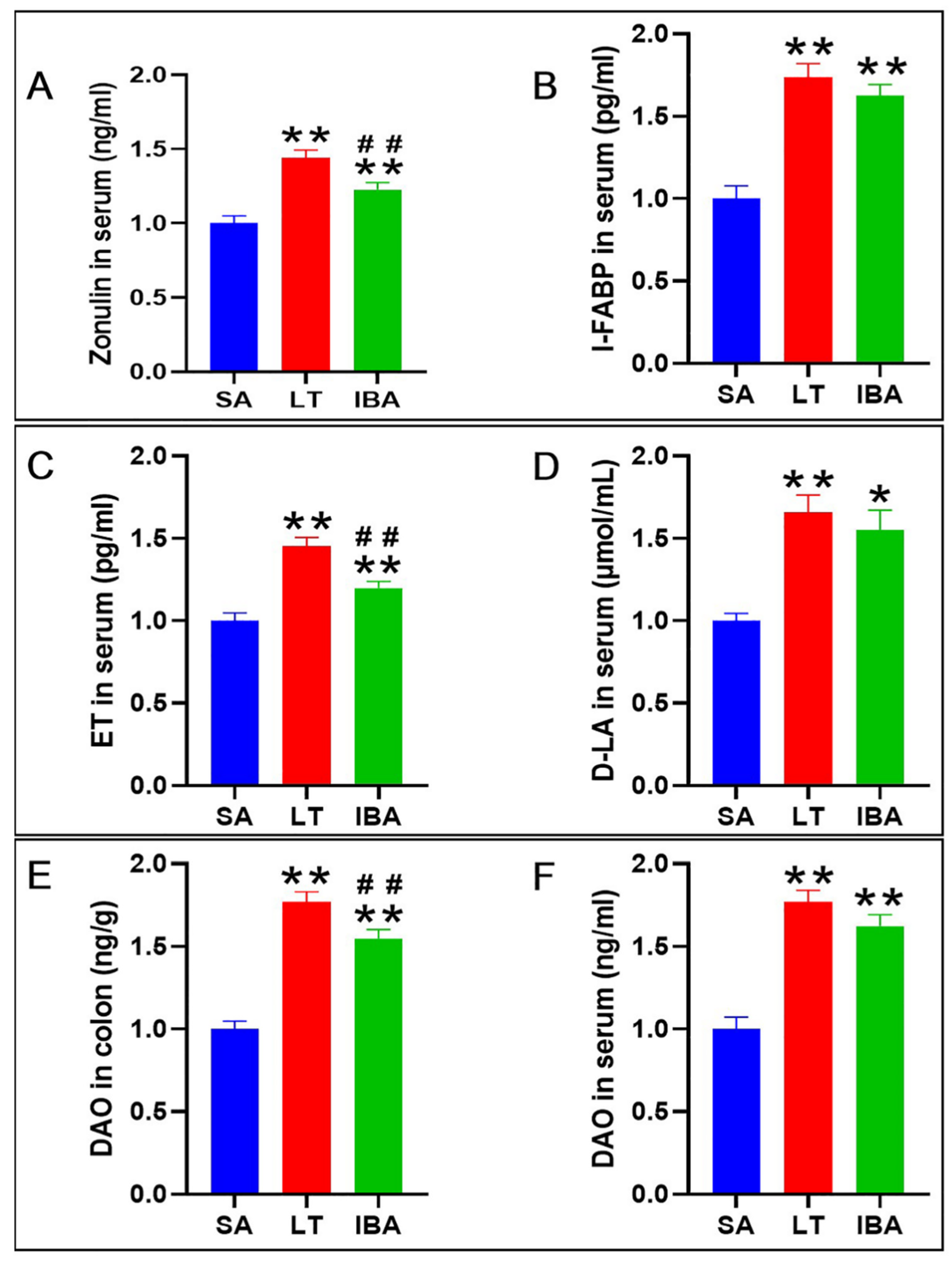
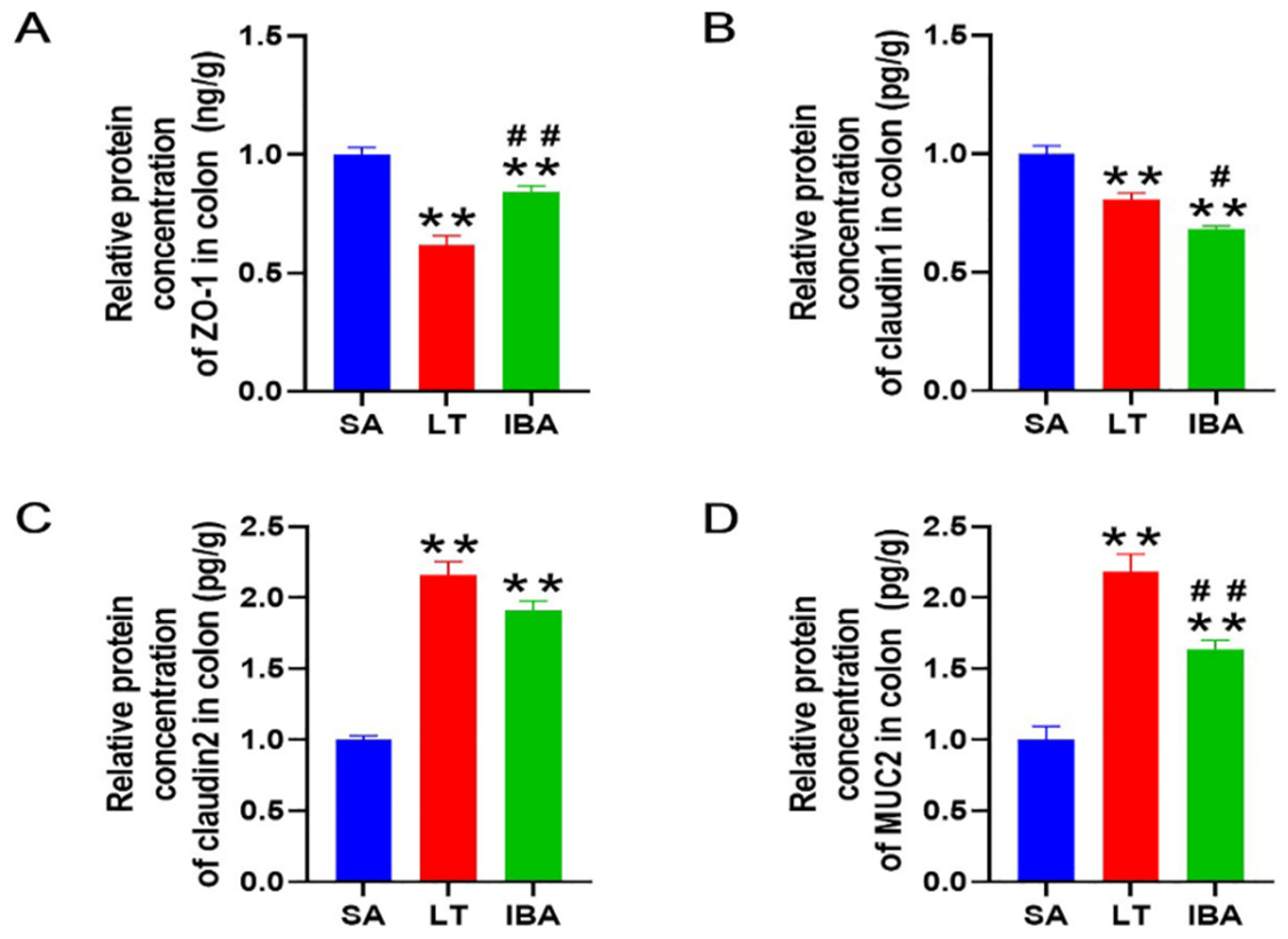
2.6. Detection of Intestinal Mucosal Barrier Permeability and Integrity Indicators
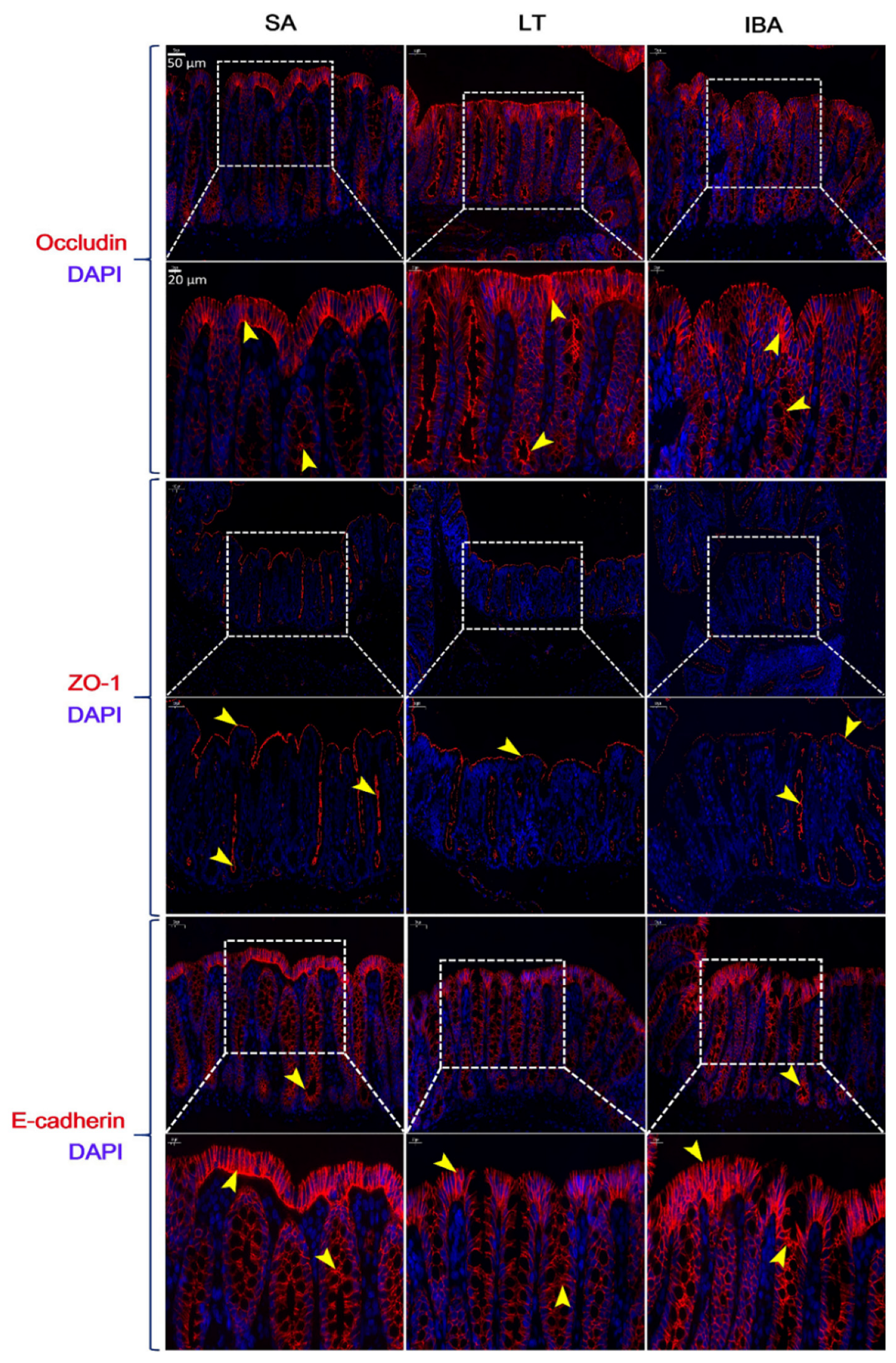
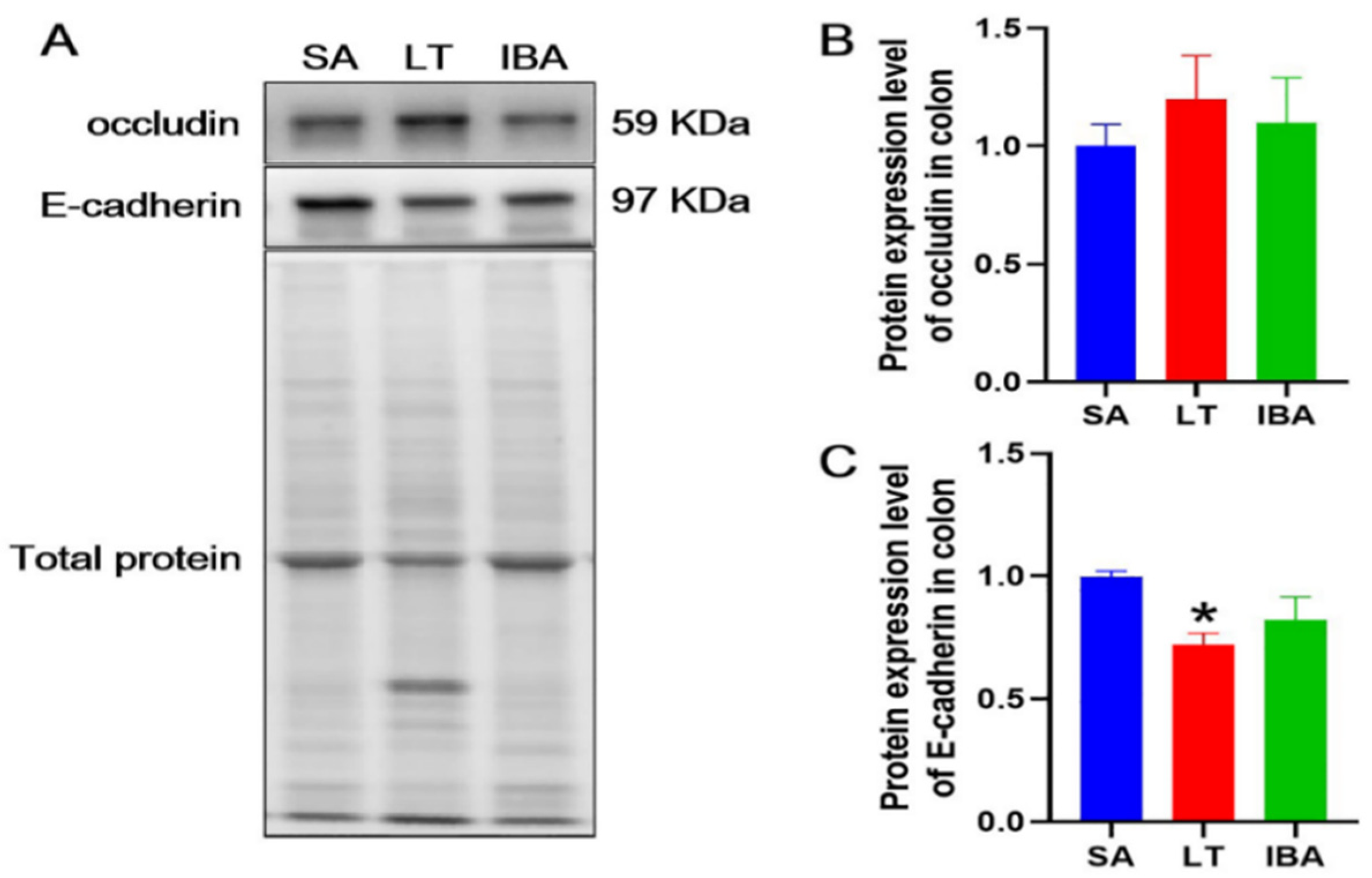
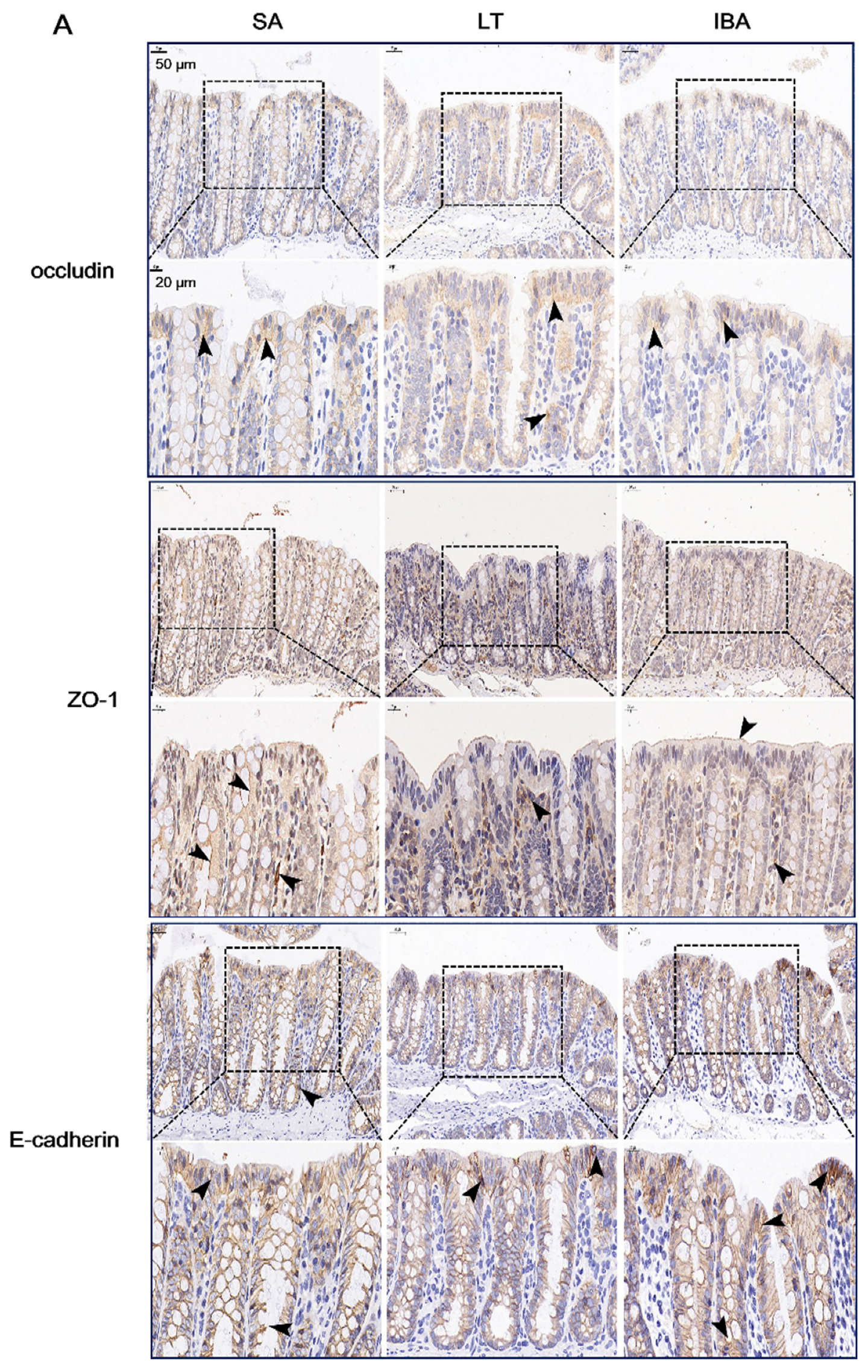
2.7. Distribution and Expression of Proteins Associated with Tight Junctions in the Colonic Mucosa
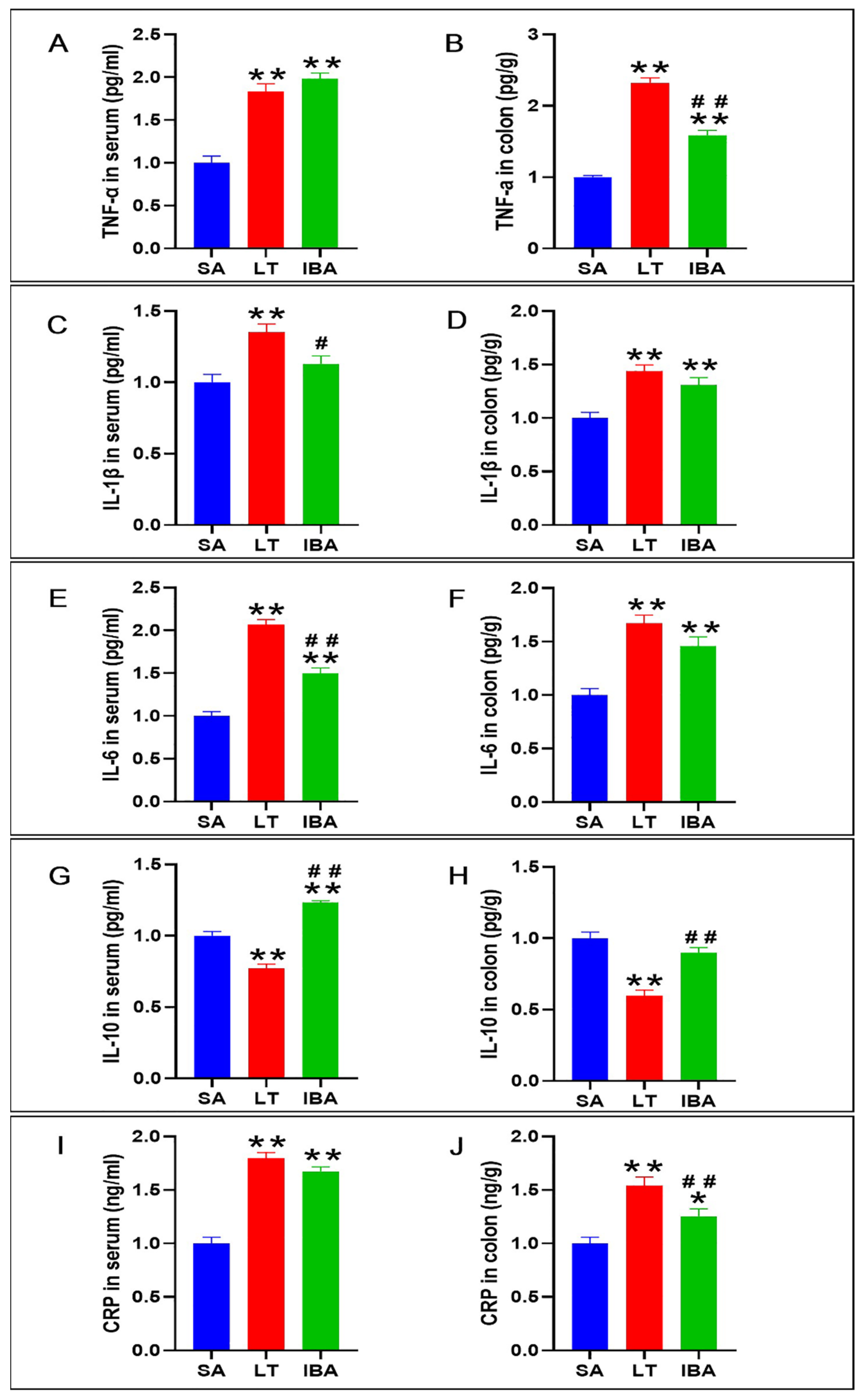
2.8. The Concentration of Inflammatory Factors in Serum and Colon
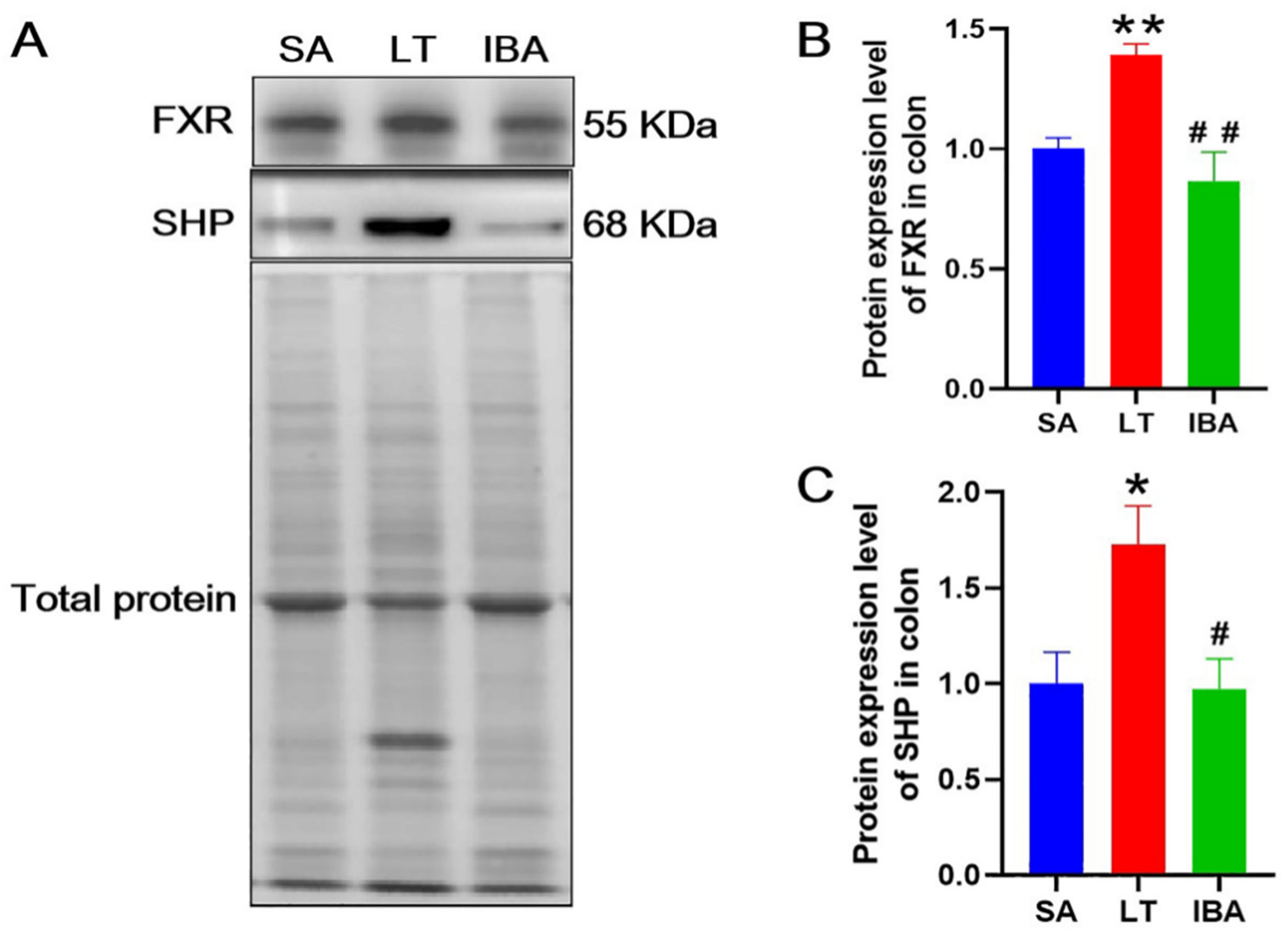
2.9. Protein Expression Levels of FXR and SHP in the Colon
2.10. Protein Expression Levels of Bcl-2 and Bax in the Colon
3. Discussion
4. Materials and Methods
4.1. Animals and Groups
4.2. Tissue Sample Collection and Preparation
4.2.1. Colonic Sample Collection and Preparation
4.2.2. Serum Sample Collection
4.3. Morphology of Colonic Mucosa
4.3.1. HE (Hematoxylin–Eosin) Staining
4.3.2. AB–PAS (Alcian Blue/Periodic Acid-Schiff) Staining
4.3.3. Transmission Electron Microscopy
4.4. Evaluation of Colonic Mucosal Barrier Function and Regulatory Mechanism
4.4.1. Immunofluorescence Analysis
4.4.2. Immunohistochemistry Analysis
4.4.3. Immunoblotting Analysis
4.4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4.5. Biochemical Testing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruf, T.; Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. Camb. Philos. Soc. 2015, 90, 891–926. [Google Scholar] [CrossRef] [PubMed]
- Galluser, M.; Raul, F.; Canguilhem, B. Adaptation of intestinal enzymes to seasonal and dietary changes in a hibernator: The European hamster (Cricetus cricetus). J. Comp. Physiol. B 1988, 158, 143–149. [Google Scholar] [CrossRef]
- Carey, H.V. Effects of fasting and hibernation on ion secretion in ground squirrel intestine. Am. J. Physiol. 1992, 263 Pt 2, R1203–R1208. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.V.; Bernick, S. Comparative histological studies of the stomach, small intestine, and colon of warm and active and hibernating arctic ground squirrels, Spermophilus undulatus. Anat. Rec. 1958, 130, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, J.; Xu, S.; Peng, X.; Yan, X.; Li, X.; Wang, H.; Chang, H.; Gao, Y. Controllable oxidative stress and tissue specificity in major tissues during the torpor-arousal cycle in hibernating Daurian ground squirrels. Open Biol. 2018, 8, 180068. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.; Howarth, G.S.; Nattrass, G.; Kitessa, S.M.; Barekatain, R.; Forder, R.E.; Tran, C.D.; Hughes, R.J. Gene expression and morphological changes in the intestinal mucosa associated with increased permeability induced by short-term fasting in chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, e653–e661. [Google Scholar] [CrossRef] [PubMed]
- Spreeuwenberg, M.A.; Verdonk, J.M.; Gaskins, H.R.; Verstegen, M.W. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef]
- Gilani, S.; Howarth, G.S.; Tran, C.D.; Barekatain, R.; Kitessa, S.M.; Forder, R.E.; Hughes, R.J. Reduced fasting periods increase intestinal permeability in chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, e486–e492. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, W.T.; Huang, C.Y.; Lee, T.C.; Chen, C.T.; Peng, W.H.; Lu, K.S.; Yang, C.Y.; Yu, L.C. Distinct cytoprotective roles of pyruvate and ATP by glucose metabolism on epithelial necroptosis and crypt proliferation in ischaemic gut. J. Physiol. 2017, 595, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, L.J.; Verbeke, L.; Decuypere, J.P.; Farré, R.; De Hertogh, G.; Lenaerts, K.; Jochmans, I.; Monbaliu, D.; Nevens, F.; Tack, J.; et al. Farnesoid X Receptor Activation Attenuates Intestinal Ischemia Reperfusion Injury in Rats. PLoS ONE 2017, 12, e0169331. [Google Scholar] [CrossRef]
- Horn, N.; Ruch, F.; Miller, G.; Ajuwon, K.M.; Adeola, O. Impact of acute water and feed deprivation events on growth performance, intestinal characteristics, and serum stress markers in weaned pigs. J. Anim. Sci. 2014, 92, 4407–4416. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, Y.; Wang, W.; Xiao, W.; Liang, H.; Zhang, C.; Yang, H.; Teitelbaum, D.H.; Sun, L.H.; Yang, H. Adenosine A2B receptor modulates intestinal barrier function under hypoxic and ischemia/reperfusion conditions. Int. J. Clin. Exp. Pathol. 2014, 7, 2006–2018. [Google Scholar] [PubMed]
- Bondow, B.J.; Faber, M.L.; Wojta, K.J.; Walker, E.M.; Battle, M.A. E-cadherin is required for intestinal morphogenesis in the mouse. Dev. Biol. 2012, 371, 1–12. [Google Scholar] [CrossRef]
- Forder, R.E.; Nattrass, G.S.; Geier, M.S.; Hughes, R.J.; Hynd, P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012, 91, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Wakita, D.; Franklin, M.K.; Carvalho, T.T.; Abolhesn, A.; Gomez, A.C.; Fishbein, M.C.; Chen, S.; Lehman, T.J.; Sato, K.; et al. Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation. Immunity 2019, 51, 508–521.e6. [Google Scholar] [CrossRef]
- Paksuz, E.P. The effect of hibernation on the morphology and histochemistry of the intestine of the greater mouse-eared bat, Myotis myotis. Acta Histochem. 2014, 116, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Dill-McFarland, K.A.; Neil, K.L.; Zeng, A.; Sprenger, R.J.; Kurtz, C.C.; Suen, G.; Carey, H.V. Hibernation alters the diversity and composition of mucosa-associated bacteria while enhancing antimicrobial defence in the gut of 13-lined ground squirrels. Mol. Ecol. 2014, 23, 4658–4669. [Google Scholar] [CrossRef] [PubMed]
- Carey, H.V. Seasonal changes in mucosal structure and function in ground squirrel intestine. Am. J. Physiol. 1990, 259 Pt 2, R385–R392. [Google Scholar] [CrossRef] [PubMed]
- Carey, H.V.; Pike, A.C.; Weber, C.R.; Turner, J.R.; Visser, A.; Beijer-Liefers, S.C.; Bouma, H.R.; Kroese, F.G. Impact of Hibernation on Gut Microbiota and Intestinal Barrier Function in Ground Squirrels; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kurtz, C.C.; Carey, H.V. Seasonal changes in the intestinal immune system of hibernating ground squirrels. Dev. Comp. Immunol. 2007, 31, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Renga, B.; Migliorati, M.; Mencarelli, A.; Fiorucci, S. Reciprocal regulation of the bile acid-activated receptor FXR and the interferon-gamma-STAT-1 pathway in macrophages. Biochim. Biophys. Acta 2009, 1792, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Schote, A.B.; Turner, J.D.; Schiltz, J.; Muller, C.P. Nuclear receptors in human immune cells: Expression and correlations. Mol. Immunol. 2007, 44, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Gores, G.J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am. J. Physiol. 1997, 273, G1174–G1188. [Google Scholar] [CrossRef] [PubMed]
- Dahly, E.M.; Guo, Z.; Ney, D.M. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J. Nutr. 2002, 132, 2010–2014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Groos, S.; Reale, E.; Hünefeld, G.; Luciano, L. Changes in epithelial cell turnover and extracellular matrix in human small intestine after TPN. J. Surg. Res. 2003, 109, 74–85. [Google Scholar] [CrossRef]
- Iwakiri, R.; Gotoh, Y.; Noda, T.; Sugihara, H.; Fujimoto, K.; Fuseler, J.; Aw, T.Y. Programmed cell death in rat intestine: Effect of feeding and fasting. Scand. J. Gastroenterol. 2001, 36, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Iwakiri, R.; Fujimoto, K.; Matsuo, S.; Aw, T.Y. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am. J. Physiol. 1998, 274, G270–G276. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Vistro, W.A.; Bai, X.; Wu, X.; Chen, C.; Huang, Y.; Fazlani, S.A.; Tarique, I.; Yang, P.; Chen, Q. Effect of seasonal variance on intestinal epithelial barriers and the associated innate immune response of the small intestine of the Chinese soft-shelled turtles. Fish Shellfish Immunol. 2020, 97, 173–181. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Barkla, D.H.; Gibson, P.R. The fate of epithelial cells in the human large intestine. Pathology 1999, 31, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Carey, H.V.; Walters, W.A.; Knight, R. Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R33–R42. [Google Scholar] [CrossRef] [PubMed]
- Carey, H.V.; Martin, S.L. Preservation of intestinal gene expression during hibernation. Am. J. Physiol. 1996, 271 Pt 1, G804–G813. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Bergstrom, K.S.; Guttman, J.A.; Rumi, M.; Ma, C.; Bouzari, S.; Khan, M.A.; Gibson, D.L.; Vogl, A.W.; Vallance, B.A. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect. Immun. 2008, 76, 796–811. [Google Scholar] [CrossRef]
- Talaei, F.; Hylkema, M.N.; Bouma, H.R.; Boerema, A.S.; Strijkstra, A.M.; Henning, R.H.; Schmidt, M. Reversible remodeling of lung tissue during hibernation in the Syrian hamster. J. Exp. Biol. 2011, 214 Pt 8, 1276–1282. [Google Scholar] [CrossRef][Green Version]
- Shea-Donohue, T.; Danquechin-Dorval, E.; Montcalm, E.; El-Bayar, H.; Durakovic, A.; Conklin, J.J.; Dubois, A. Alterations in gastric mucus secretion in rhesus monkeys after exposure to ionizing radiation. Gastroenterology 1985, 88, 685–690. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. olorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Yang, G.; Kolosov, V.P.; Perelman, J.M.; Zhou, X.D. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J. Allergy Clin. Immunol. 2011, 128, 626–634.e1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Zhang, W.X.; Qi, Q.Q.; Long, X.; Li, X.; Yu, Y.B.; Zuo, X.L. Brain-derived neurotrophic factor modulates intestinal barrier by inhibiting intestinal epithelial cells apoptosis in mice. Physiol. Res. 2018, 67, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.C.; Carey, H.V. Modulation of apoptotic pathways in intestinal mucosa during hibernation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R586–R595. [Google Scholar] [CrossRef]
- Wong, M.H.; Stappenbeck, T.S.; Gordon, J.I. Living and commuting in intestinal crypts. Gastroenterology 1999, 116, 208–210. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Schurink, M.; Kooi, E.M.; Hulzebos, C.V.; Kox, R.G.; Groen, H.; Heineman, E.; Bos, A.F.; Hulscher, J.B. Intestinal fatty acid-binding protein as a diagnostic marker for complicated and uncomplicated necrotizing enterocolitis: A prospective cohort study. PLoS ONE 2015, 10, e0121336. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, G.; Chen, Y.; Liu, D.; Qiu, J.; Zhou, J.; Zhu, P.; Wang, Z. ntra-hepatic expression of scavenger receptor and CD14 and their relationship with local inflammatory responses in endotoxemia in mice. Shock 2001, 16, 75–80. [Google Scholar] [CrossRef][Green Version]
- Murray, M.J.; Gonze, M.D.; Nowak, L.R.; Cobb, C.F. Serum D(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am. J. Surg. 1994, 167, 575–578. [Google Scholar] [CrossRef]
- D’Agostino, L.; D′Argenio, G.; Ciacci, C.; Daniele, B.; Macchia, V.; Mazzacca, G. Diamine oxidase in rat small bowel: Distribution in different segments and cellular location. Enzyme 1984, 31, 217–220. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef]
- Bohr, M.; Brooks, A.R.; Kurtz, C.C. Hibernation induces immune changes in the lung of 13-lined ground squirrels (Ictidomys tridecemlineatus). Dev. Comp. Immunol. 2014, 47, 178–184. [Google Scholar] [CrossRef]
- Kim, J.M.; Eckmann, L.; Savidge, T.C.; Lowe, D.C.; Witthöft, T.; Kagnoff, M.F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 1998, 102, 1815–1823. [Google Scholar] [CrossRef]
- Marini, M.; Bamias, G.; Rivera-Nieves, J.; Moskaluk, C.A.; Hoang, S.B.; Ross, W.G.; Pizarro, T.T.; Cominelli, F. TNF-alpha neutralization ameliorates the severity of murine Crohn′s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8366–8371. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi Porro, G. Biologic therapy for inflammatory bowel disease. Drugs 2005, 65, 2253–2286. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Laroux, F.S.; Coe, L.L.; Morise, Z.; Kawachi, S.; Bauer, P.; Grisham, M.B.; Specian, R.D.; Carter, P.; Jennings, S.; et al. Interfero.n-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc. Res. 2001, 61, 130–143. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O′Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Zhou, P.; Streutker, C.; Borojevic, R.; Wang, Y.; Croitoru, K. IL-10 modulates intestinal damage and epithelial cell apoptosis in T cell-mediated enteropathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G599–G604. [Google Scholar] [CrossRef]
- Lu, J.; Philpott, D.J.; Saunders, P.R.; Perdue, M.H.; Yang, P.C.; McKay, D.M. Epithelial ion transport and barrier abnormalities evoked by superantigen-activated immune cells are inhibited by interleukin-10 but not interleukin-4. J. Pharmacol. Exp. Ther. 1998, 287, 128–136. [Google Scholar] [PubMed]
- Denning, T.L.; Campbell, N.A.; Song, F.; Garofalo, R.P.; Klimpel, G.R.; Reyes, V.E.; Ernst, P.B. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. Int. Immunol. 2000, 12, 133–139. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Wang, Q.; Jiang, S.; Guo, S.; Liu, K. Laboratory feeding, breeding and hibernation bout of the Daurian ground squirrels. J. Vet. Sci. 2012, 32, 356–361. [Google Scholar]
- Lee, Y.S.; Huynh, T.V.; Lee, S.J. Paracrine and endocrine modes of myostatin action. J. Appl. Physiol. 2016, 120, 592–598. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, W.; Han, Y.; Yang, Y.; Hao, Z.; An, N.; Chen, J.; Zhang, Z.; Gao, X.; Storey, K.B.; Chang, H.; et al. Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor–Arousal Cycles of the Daurian Ground Squirrel. Int. J. Mol. Sci. 2022, 23, 9026. https://doi.org/10.3390/ijms23169026
Miao W, Han Y, Yang Y, Hao Z, An N, Chen J, Zhang Z, Gao X, Storey KB, Chang H, et al. Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor–Arousal Cycles of the Daurian Ground Squirrel. International Journal of Molecular Sciences. 2022; 23(16):9026. https://doi.org/10.3390/ijms23169026
Chicago/Turabian StyleMiao, Weilan, Yuting Han, Yingyu Yang, Ziwei Hao, Ning An, Jiayu Chen, Ziwen Zhang, Xuli Gao, Kenneth B. Storey, Hui Chang, and et al. 2022. "Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor–Arousal Cycles of the Daurian Ground Squirrel" International Journal of Molecular Sciences 23, no. 16: 9026. https://doi.org/10.3390/ijms23169026
APA StyleMiao, W., Han, Y., Yang, Y., Hao, Z., An, N., Chen, J., Zhang, Z., Gao, X., Storey, K. B., Chang, H., & Wang, S. (2022). Dynamic Changes in Colonic Structure and Protein Expression Suggest Regulatory Mechanisms of Colonic Barrier Function in Torpor–Arousal Cycles of the Daurian Ground Squirrel. International Journal of Molecular Sciences, 23(16), 9026. https://doi.org/10.3390/ijms23169026






