Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold
Abstract
1. Introduction
2. Results
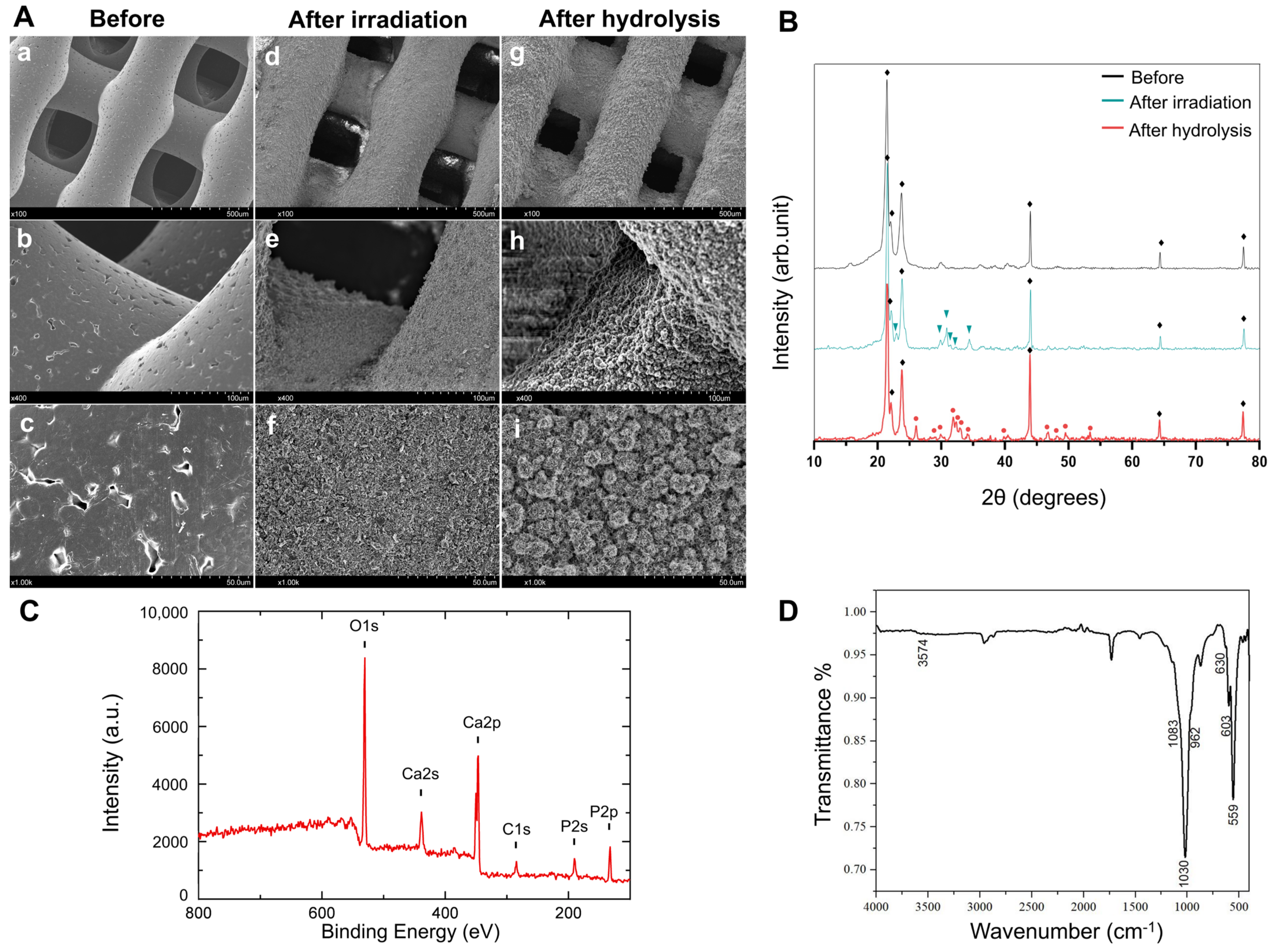
2.1. Hydroxyapatite Coating onto the PCL Porous Scaffold
2.2. Mechanical and Surface Properties of PCL-Based Discs/Scaffolds
2.3. Behavior of Stem Cells after Seeding on PCL-Based Discs/Scaffolds
2.4. Bone Formation by the Implantation of PCL-Based Scaffolds
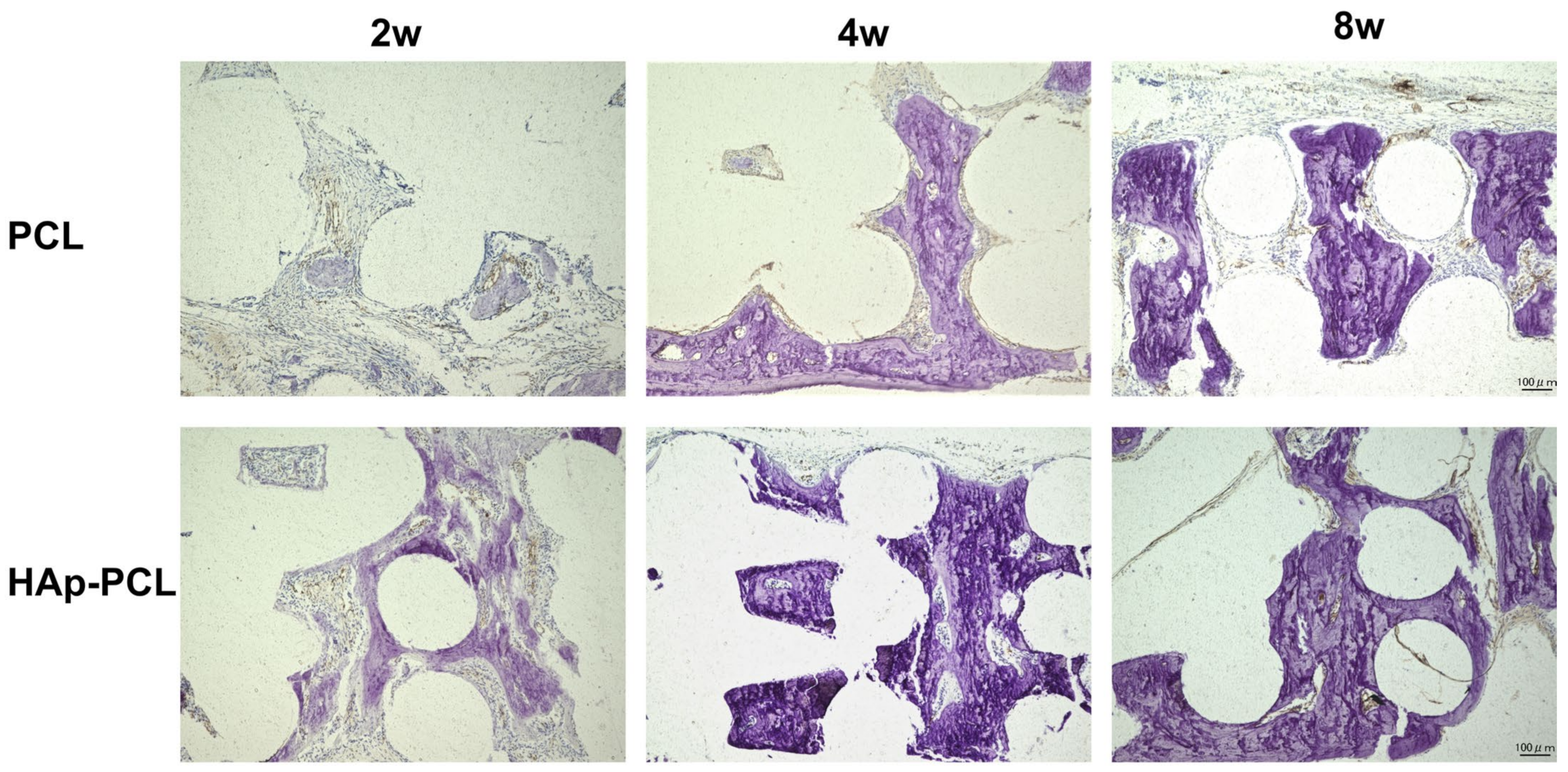
2.5. Histological Evaluation of Bone Formation by the Implantation of PCL-Based Scaffolds
3. Discussion
4. Materials and Methods
4.1. Hydroxyapatite Coating onto the PCL Porous Scaffold by the Er: YAG-PLD Method
4.2. Characterization of PCL Porous Scaffolds Treated by the Er: YAG-PLD Method
4.3. Evaluation of Mechanical and Physicochemical Properties of PCL-Based Discs/Scaffolds
4.4. In Vitro Cell-Based Studies
4.4.1. Initial Attachment and Viability of Cells on PCL-Based Discs
4.4.2. Adhesion and Proliferation of Cells on PCL-Based Scaffolds
4.5. Bone Formation by the Implantation of PCL-Based Scaffolds
4.5.1. Animal Experiments
4.5.2. Microcomputed Tomography (μCT)
4.5.3. Histological Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walia, A. Secondary alveolar bone grafting in cleft of the lip and palate patients. Contemp. Clin. Dent. 2011, 2, 146–154. [Google Scholar] [CrossRef]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofacial Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Mertens, C.; Decker, C.; Seeberger, R.; Hoffmann, J.; Sander, A.; Freier, K. Early bone resorption after vertical bone augmentation--a comparison of calvarial and iliac grafts. Clin. Oral Implants Res. 2013, 24, 820–825. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Weiss, L.E.; Sundine, M.J. New frontiers in bone tissue engineering. Clin. Plast. Surg. 2003, 30, 641–648. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically Inspired Smart Release System Based on 3D Bioprinted Perfused Scaffold for Vascularized Tissue Regeneration. Adv. Sci. 2016, 3, 1600058. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Nowicki, M.; Zhou, X.; Khademhosseini, A.; Zhang, L.G. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Adv. Healthc. Mater. 2016, 5, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Bulusu, K.; Plesniak, M.; Zhang, L.G. A synergistic approach to the design, fabrication and evaluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology 2016, 27, 064001. [Google Scholar] [CrossRef] [PubMed]
- Im, O.; Li, J.; Wang, M.; Zhang, L.G.; Keidar, M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int. J. Nanomed. 2012, 7, 2087–2099. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Nowicki, M.; Castro, N.J.; Rao, R.; Plesniak, M.; Zhang, L.G. Integrating three-dimensional printing and nanotechnology for musculoskeletal regeneration. Nanotechnology 2017, 28, 382001. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef]
- Huang, H.; Oizumi, S.; Kojima, N.; Niino, T.; Sakai, Y. Avidin-biotin binding-based cell seeding and perfusion culture of liver-derived cells in a porous scaffold with a three-dimensional interconnected flow-channel network. Biomaterials 2007, 28, 3815–3823. [Google Scholar] [CrossRef]
- Izquierdo, R.; Garcia-Giralt, N.; Rodriguez, M.T.; Cáceres, E.; García, S.J.; Gómez Ribelles, J.L.; Monleón, M.; Monllau, J.C.; Suay, J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J. Biomed. Mater. Res. A 2008, 85, 25–35. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Waleetorncheepsawat, S.; Suwantong, O.; Wutticharoenmongkol, P.; Weeranantanapan, O.; Chuenjitbuntaworn, B.; Cheepsunthorn, P.; Pavasant, P.; Supaphol, P. In vitro biocompatibility of schwann cells on surfaces of biocompatible polymeric electrospun fibrous and solution-cast film scaffolds. Biomacromolecules 2007, 8, 1587–1594. [Google Scholar] [CrossRef]
- Savarino, L.; Baldini, N.; Greco, M.; Capitani, O.; Pinna, S.; Valentini, S.; Lombardo, B.; Esposito, M.T.; Pastore, L.; Ambrosio, L.; et al. The performance of poly-epsilon-caprolactone scaffolds in a rabbit femur model with and without autologous stromal cells and BMP4. Biomaterials 2007, 28, 3101–3109. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-varepsilon-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Sharif, F.; Ur Rehman, I.; Muhammad, N.; MacNeil, S. Dental materials for cleft palate repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 1018–1028. [Google Scholar] [CrossRef]
- Li, X.; Ma, B.; Li, J.; Shang, L.; Liu, H.; Ge, S. A method to visually observe the degradation-diffusion-reconstruction behavior of hydroxyapatite in the bone repair process. Acta Biomater. 2020, 101, 554–564. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Endogan Tanir, T.; Buyuksungur, A.; Bektas, E.I.; Torun Kose, G.; Yucel, D.; Beyzadeoglu, T.; Cetinkaya, E.; Yenigun, C.; Tonuk, E.; et al. 3D printed poly(epsilon-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater. Sci. 2017, 5, 2144–2158. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Heimann, R.B. Plasma-Sprayed Hydroxylapatite-Based Coatings: Chemical, Mechanical, Microstructural, and Biomedical Properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Heimann, R.B. Osseoconductive and Corrosion-Inhibiting Plasma-Sprayed Calcium Phosphate Coatings for Metallic Medical Implants. Metals 2017, 7, 468. [Google Scholar] [CrossRef]
- Rajesh, P.; Muraleedharan, C.V.; Sureshbabu, S.; Komath, M.; Varma, H. Preparation and analysis of chemically gradient functional bioceramic coating formed by pulsed laser deposition. J. Mater. Sci. Mater. Med. 2012, 23, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Muraleedharan, C.V.; Komath, M.; Varma, H. Pulsed laser deposition of hydroxyapatite on titanium substrate with titania interlayer. J. Mater. Sci. Mater. Med. 2011, 22, 497–505. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, F.J.; Mayor, M.B.; Arias, J.L.; Pou, J.; León, B.; Pérez-Amor, M. Hydroxyapatite coatings: A comparative study between plasma-spray and pulsed laser deposition techniques. J. Mater. Sci. Mater. Med. 1997, 8, 861–865. [Google Scholar] [CrossRef]
- Ashfold, M.N.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2004, 33, 23–31. [Google Scholar] [CrossRef]
- Chen, L.; Hontsu, S.; Komasa, S.; Yamamoto, E.; Hashimoto, Y.; Matsumoto, N. Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials 2021, 14, 7475. [Google Scholar] [CrossRef]
- Ma, L.; Li, M.; Komasa, S.; Yan, S.; Yang, Y.; Nishizaki, M.; Chen, L.; Zeng, Y.; Wang, X.; Yamamoto, E.; et al. Characterization of Hydroxyapatite Film Obtained by Er:YAG Pulsed Laser Deposition on Sandblasted Titanium: An In Vitro Study. Materials 2022, 15, 2306. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Fernández, E.; Driessens, F.C.M.; Planell, J.A. Modeling of the Hydrolysis of α-Tricalcium Phosphate. J. Am. Ceram. Soc. 1999, 82, 2808–2812. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. alpha-Tricalcium phosphate hydrolysis to hydroxyapatite at and near physiological temperature. J. Mater. Sci. Mater. Med. 2000, 11, 365–371. [Google Scholar] [CrossRef]
- Boj, J.R.; Poirier, C.; Hernandez, M.; Espassa, E.; Espanya, A. Review: Laser soft tissue treatments for paediatric dental patients. Eur. Arch. Paediatr. Dent. 2011, 12, 100–105. [Google Scholar] [CrossRef]
- Suenaga, H.; Furukawa, K.S.; Suzuki, Y.; Takato, T.; Ushida, T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J. Mater. Sci. Mater. Med. 2015, 26, 254. [Google Scholar] [CrossRef]
- Tsumano, N.; Kubo, H.; Imataki, R.; Honda, Y.; Hashimoto, Y.; Nakajima, M. Bone Regeneration by Dedifferentiated Fat Cells Using Composite Sponge of Alfa-Tricalcium Phosphate and Gelatin in a Rat Calvarial Defect Model. Appl. Sci. 2021, 11, 11941. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Kinoshita, Y.; Maeda, H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci. World J. 2013, 2013, 863157. [Google Scholar] [CrossRef]
- Piard, C.; Luthcke, R.; Kamalitdinov, T.; Fisher, J. Sustained delivery of vascular endothelial growth factor from mesoporous calcium-deficient hydroxyapatite microparticles promotes in vitro angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2021, 109, 1080–1087. [Google Scholar] [CrossRef]
- Jang, C.H.; Kim, W.; Kim, G. Effects of fibrous collagen/CDHA/hUCS biocomposites on bone tissue regeneration. Int. J. Biol. Macromol. 2021, 176, 479–489. [Google Scholar] [CrossRef]
- Gopinath, V.K.; Soumya, S.; Chakrapani, V.Y.; Kumar, T.S.S. Odontogenic differentiation of inflamed dental pulp stem cells (IDPSCs) on polycaprolactone (PCL) nanofiber blended with hydroxyapatite. Dent. Mater. J. 2021, 40, 312–321. [Google Scholar] [CrossRef]
- Honda, M.; Watanabe, Y.; Tsuchiya, T.; Kanzawa, N.; Aizawa, M. Selective differentiation of bone marrow-derived mesenchymal stromal cells into osteocytes via endochondral ossification in an apatite-fiber scaffold. J. Ceram. Soc. Jpn. 2013, 121, 759–765. [Google Scholar] [CrossRef][Green Version]
- Honda, M.; Fujimi, T.J.; Izumi, S.; Izawa, K.; Aizawa, M.; Morisue, H.; Tsuchiya, T.; Kanzawa, N. Topographical analyses of proliferation and differentiation of osteoblasts in micro- and macropores of apatite-fiber scaffold. J. Biomed. Mater. Res. A 2010, 94, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis--a review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Han, J.; Zhang, S.; Liu, F.; Wang, S.; Duan, J.; Sang, Y.; Jiang, H.; Li, D.; Ge, S.; et al. Hydroxyapatite nanobelt/polylactic acid Janus membrane with osteoinduction/barrier dual functions for precise bone defect repair. Acta Biomater. 2018, 71, 108–117. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Sprayed Hydroxylapatite Coatings as Biocompatible Intermediaries Between Inorganic Implant Surfaces and Living Tissue. J. Therm. Spray Technol. 2018, 27, 1212–1237. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, J.; Lee, K.M.; Jin, Y.Z.; Yun, H.-S.; Kim, G.; Lee, J.H. Enhanced healing of rat calvarial defects with 3D printed calcium-deficient hydroxyapatite/collagen/bone morphogenetic protein 2 scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 108, 103782. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef]
- Lyu, J.; Hashimoto, Y.; Honda, Y.; Matsumoto, N. Comparison of Osteogenic Potentials of Dental Pulp and Bone Marrow Mesenchymal Stem Cells Using the New Cell Transplantation Platform, CellSaic, in a Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2021, 22, 9478. [Google Scholar] [CrossRef]
- Kawamoto, T.; Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot’s film method (2012). Methods Mol. Biol. 2014, 1130, 149–164. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jo, J.-I.; Chen, L.; Hontsu, S.; Hashimoto, Y. Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold. Int. J. Mol. Sci. 2022, 23, 9048. https://doi.org/10.3390/ijms23169048
Zhang Y, Jo J-I, Chen L, Hontsu S, Hashimoto Y. Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold. International Journal of Molecular Sciences. 2022; 23(16):9048. https://doi.org/10.3390/ijms23169048
Chicago/Turabian StyleZhang, Ye, Jun-Ichiro Jo, Liji Chen, Shigeki Hontsu, and Yoshiya Hashimoto. 2022. "Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold" International Journal of Molecular Sciences 23, no. 16: 9048. https://doi.org/10.3390/ijms23169048
APA StyleZhang, Y., Jo, J.-I., Chen, L., Hontsu, S., & Hashimoto, Y. (2022). Effect of Hydroxyapatite Coating by Er: YAG Pulsed Laser Deposition on the Bone Formation Efficacy by Polycaprolactone Porous Scaffold. International Journal of Molecular Sciences, 23(16), 9048. https://doi.org/10.3390/ijms23169048





