The Effect of Femtosecond Laser Irradiation and Plasmon Field on the Degree of Conversion of a UDMA-TEGDMA Copolymer Nanocomposite Doped with Gold Nanorods
Abstract
1. Introduction
2. Results
2.1. Nanocomposite Characterization
2.2. Irradiation Experiments and Raman Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials and Sample Preparation Procedures
4.2. Sample Characterization Methods
4.3. Laser Irradiation Experiments
4.4. Finite Element Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Pomes, B.; Sadoun, M.; Richaud, E. Curing of urethane dimetracrylate composites: A glass transition study. Polym. Test. 2019, 80, 106113. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, G. Monomer systems for dental composites and their future. J. Calif. Dent. Assoc. 2009, 37, 389–398. [Google Scholar]
- Par, M.; Gamulin, O.; Marovic, D.; Klaric, E.; Tarle, Z. Raman spectroscopic assessment of degree of conversion of bulk-fill resin composites—Changes at 24 hours post cure. Oper. Dent. 2015, 40, E92–E101. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, e213–e217. [Google Scholar] [CrossRef]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.B.; Samuel, S.M.W. Discrepancies in degree of conversion measurements by FTIR. Braz. Oral. Res. 2014, 28, 9–15. [Google Scholar]
- Stencel, R.; Pakieła, W.; Barszczewska-rybarek, I.; Z’mudzki, J.; Kasperski, J.; Chladek, G. Effects of different inorganic fillers on mechanical properties and degree of conversion of dental resin composites. Arch. Metall. Mater. 2018, 63, 1361–1369. [Google Scholar]
- BinMahfooz, A.M.; Qutub, O.A.; Marghalani, T.Y.; Ayad, M.F.; Maghrabi, A.A. Degree of conversion of resin cement with varying methacrylate compositions used to cement fiber dowels: A Raman spectroscopy study. J. Prosthet. Dent. 2018, 119, 1014–1020. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Fowler, B.O.; Weir, M.D.; Skrtic, D.; Stansbury, J.W. Effect of ethyl-alpha-hydroxymethylacrylate on selected properties of copolymers and ACP resin composites. J. Mater. Sci. Mater. Med. 2008, 19, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Nunes, T.; Kalachandra, S. Low viscosity dimethacrylate comonomer compositions [Bis-GMA and CH3Bis-GMA] for novel dental composites; analysis of the network by stray-field MRI, solid-state NMR and DSC & FTIR. Biomaterials 2002, 23, 3799–3806. [Google Scholar] [PubMed]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
- Bukovinszky, K.; Szalóki, M.; Csarnovics, I.; Bonyár, A.; Petrik, P.; Kalas, B.; Daróczi, L.; Kéki, S.; Kökényesi, S.; Hegedűs, C. Optimization of Plasmonic Gold Nanoparticle Concentration in Green LED Light Active Dental Photopolymer. Polymers 2021, 13, 275. [Google Scholar] [CrossRef]
- Gajewski, V.E.S.; Pfeifer, C.S.; Fróes-Salgado, N.R.G.; Boaro, L.C.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, S.V.; Arenas, G.F.; Vallo, C.I. Enhanced degree of polymerization of methacrylate and epoxy resins by plasmonic heating of embedded silver nanoparticles. Prog. Org. Coatings 2015, 88, 220–227. [Google Scholar] [CrossRef]
- Dong, J.; Firestone, G.E.; Bochinski, J.R.; Clarke, L.I.; Gorga, R.E. In situ curing of liquid epoxy via gold-nanoparticle mediated photothermal heating. Nanotechnology 2017, 28, 065601. [Google Scholar] [CrossRef]
- Baffou, G.; Rigneault, H. Femtosecond-pulsed optical heating of gold nanoparticles. Phys. Rev. B 2011, 84, 035415. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Laser Photothermal Melting and Fragmentation of Gold Nanorods: Energy and Laser Pulse-Width Dependence. J. Phys. Chem. A 1999, 103, 1165–1170. [Google Scholar] [CrossRef]
- Gordel, M.; Olesiak-Banska, J.; Matczyszyn, K.; Nogues, C.; Buckle, M.; Samoc, M. Post-synthesis reshaping of gold nanorods using a femtosecond laser. Phys. Chem. Chem. Phys. 2014, 16, 71–78. [Google Scholar] [CrossRef]
- Ekici, O.; Harrison, R.K.; Durr, N.J.; Eversole, D.S.; Lee, M.; Ben-Yakar, A. Thermal analysis of gold nanorods heated with femtosecond laser pulses. J. Phys. D: Appl. Phys. 2008, 41, 185501. [Google Scholar] [CrossRef] [PubMed]
- Csernai, L.P.; Csete, M.; Mishustin, I.N.; Motornenko, A.; Papp, I.; Satarov, L.M.; Stöcker, H.; Kroó, N. Radiation-Dominated Implosion with Flat Target, Phys. Wave Phenom. 2020, 28, 187–199. [Google Scholar] [CrossRef]
- Papp, I.; Bravina, L.; Csete, M.; Kumari, A.; Mishustin, I.N.; Molnár, D.; Motornenko, A.; Rácz, P.; Satarov, L.M.; Stöcker, H.; et al. Kinetic Model Evaluation of the Resilience of Plasmonic Nanoantennas for Laser-Induced Fusion. PRX ENERGY 2022, 1, 023001. [Google Scholar] [CrossRef]
- Zhang, G.; Huan, M.; Bonasera, A.; Ma, Y.G.; Shen, B.F.; Wang, H.W.; Xu, J.C.; Fan, G.T.; Fu, H.J.; Xue, H.; et al. Nuclear probes of an out-of-equilibrium, plasma at the highest compression. Phys. Lett. A. 2019, 383, 2285–2289. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.M.; Yang, X.H.; Wu, D.; Ma, Y.Y.; Jiao, J.L.; Zhang, Z.; Wu, F.Y.; Yuan, X.H.; Li, Y.T.; et al. Double-cone ignition scheme for inertial confinement fusion, Phil. Trans. R. Soc. A 2020, 378, 20200015. [Google Scholar]
- Burunkova, J.; Ohoueu, M.-J.; Csarnovics, I.; Veres, M.; Bonyár, A.; Kokenyesi, S. Peculiarities of interaction of gold nanoparticles with photoinitiators in polymer nanocomposites for holographic recording. J. Photochem. Photobiol. A: Chem. 2018, 359, 111–120. [Google Scholar] [CrossRef]
- Burunkova, J.; Kokenyesi, S.; Csarnovics, I.; Bonyár, A.; Veres, M.; Csik, A. Influence of gold nanoparticles on the photopolymerization processes and structure in acrylate nanocomposites. Eur. Polym. J. 2015, 64, 189–195. [Google Scholar] [CrossRef]
- Kerby, R.E.; Knobloch, L.A.; Schricker, S.; Gregg, B. Synthesis and evaluation of modified urethane dimethacrylate resins with reduced water sorption and solubility. Dent. Mater. 2009, 25, 302–313. [Google Scholar] [CrossRef]
- Moszner, N.; Völkel, T.; Fischer, U.K.; Klester, A.; Rheinberger, V. Synthesis and polymerisation of new multifunctional urethane methacrylates. Angew Makromol. Chem. 1999, 265, 31–35. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I. Study on the effects of urethane-dimethacrylates’ structures on the morphology and properties of polymers based on them. Polimery 2008, 53, 190–194. [Google Scholar] [CrossRef][Green Version]
- Sawada, H. Thermodynamics of Polymerization. I. J. Macromol. Sci. C 1969, 3, 313–338. [Google Scholar] [CrossRef]
- Soares, L.E.S.; Martin, A.A.; Pinheiro, A.L.B. Degree of Conversion of Composite Resin: A Raman Study. J. Clin. Laser Med. Surg. 2003, 21, 357–362. [Google Scholar] [CrossRef]
- Sulca, N.M.; Lungu, A.; Garea, S.A.; Iovu, H. Monitoring the synthesis of new polymer nanocomposites based on different polyhedral, oligomeric silsesquioxanes using Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 1634–1640. [Google Scholar] [CrossRef]
- Mahmood, M.H.; Himics, L.; Váczi, T.; Rigó, I.; Holomb, R.; Beiler, B.; Veres, M. Raman spectroscopic characterization of crater walls formed upon single-shot high energy femtosecond laser irradiation of dimethacrylate polymer doped with plasmonic gold. J. Raman Spectrosc. 2021, 52, 1735. [Google Scholar] [CrossRef]
- Drost, T.; Reimann, S.; Frentzen, M.; Meister, J. Effectiveness of photopolymerization in composite resins using a novel 445-nm diode laser in comparison to LED and halogen bulb technology. Lasers Med. Sci. 2019, 34, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kan, Z.; Shen, M. Study the plasmonic property of gold nanorods highly above damage threshold via single-pulse spectral hole-burning experiments. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Giannini, M.; Arrais, C.A.G.; Price, R.B.T. Light curing in dentistry and clinical implications: A literature review. Braz. Oral. Res. 2017, 31 (Suppl. S1), e61. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Yu, J.; Zhou, L.; Zhu, J. Plasmon-enhanced solar vapor generation. Nanophotonics 2019, 8, 771–778. [Google Scholar] [CrossRef]
- Ding, T.; Mertens, J.; Lombardi, A.; Scherman, O.A.; Baumberg, J.J. Light-Directed Tuning of Plasmon Resonances via Plasmon-Induced Polymerization Using Hot Electrons. ACS Photonics 2017, 4, 1453–1458. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-Induced Resonance Energy Transfer for Solar Energy Conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Minamimoto, H.; Toda, T.; Futashima, R.; Li, X.; Suzuki, K.; Yasuda, S.; Murakoshi, K. Visualization of Active Sites for Plasmon-Induced Electron Transfer Reactions Using Photoelectrochemical Polymerization of Pyrrole. J. Phys. Chem. C 2016, 120, 16051–16058. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient Hot-Electron Transfer by a Plasmon-Induced Interfacial Charge-Transfer Transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [PubMed]
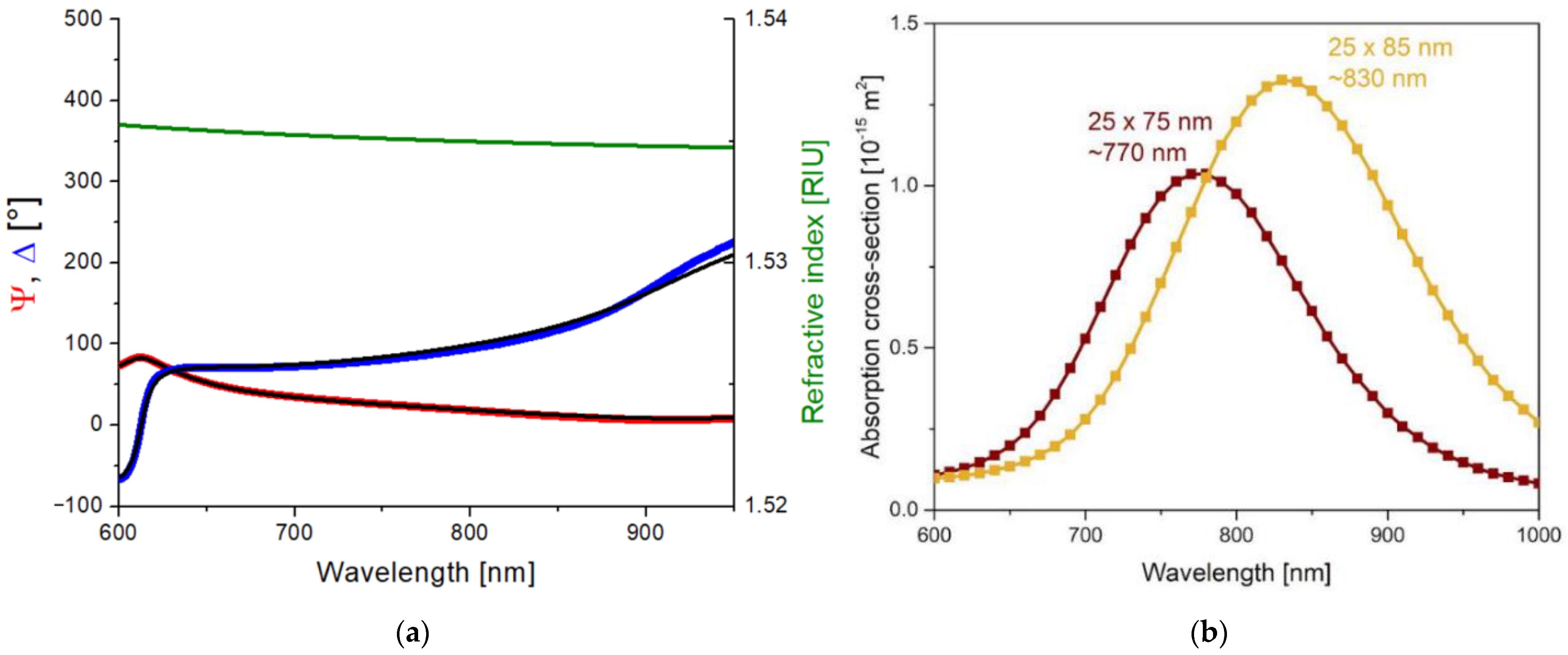
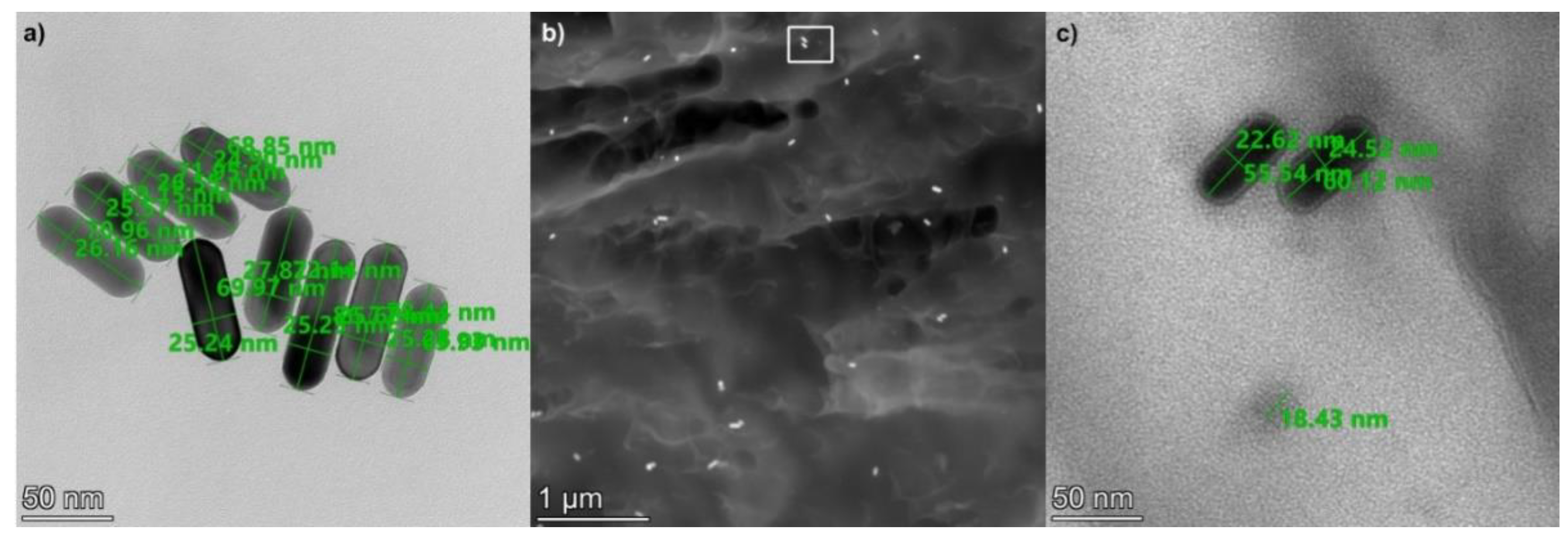


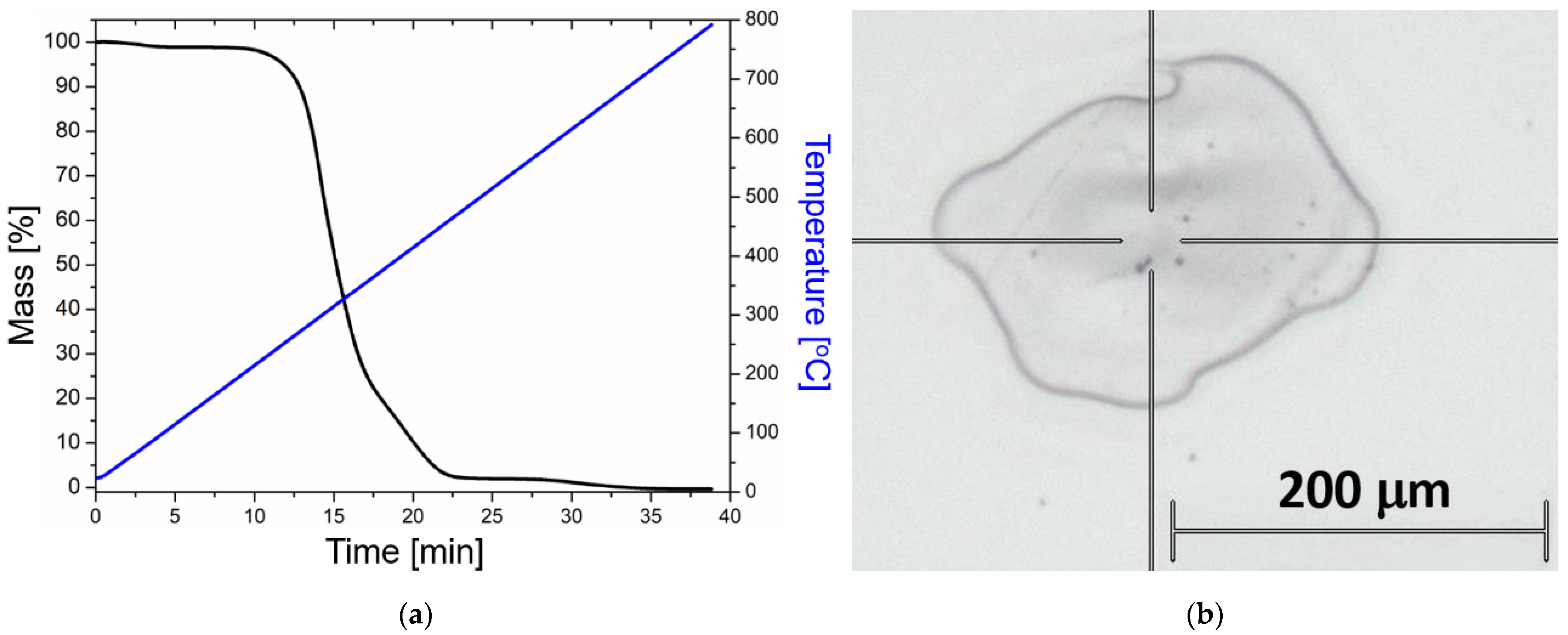
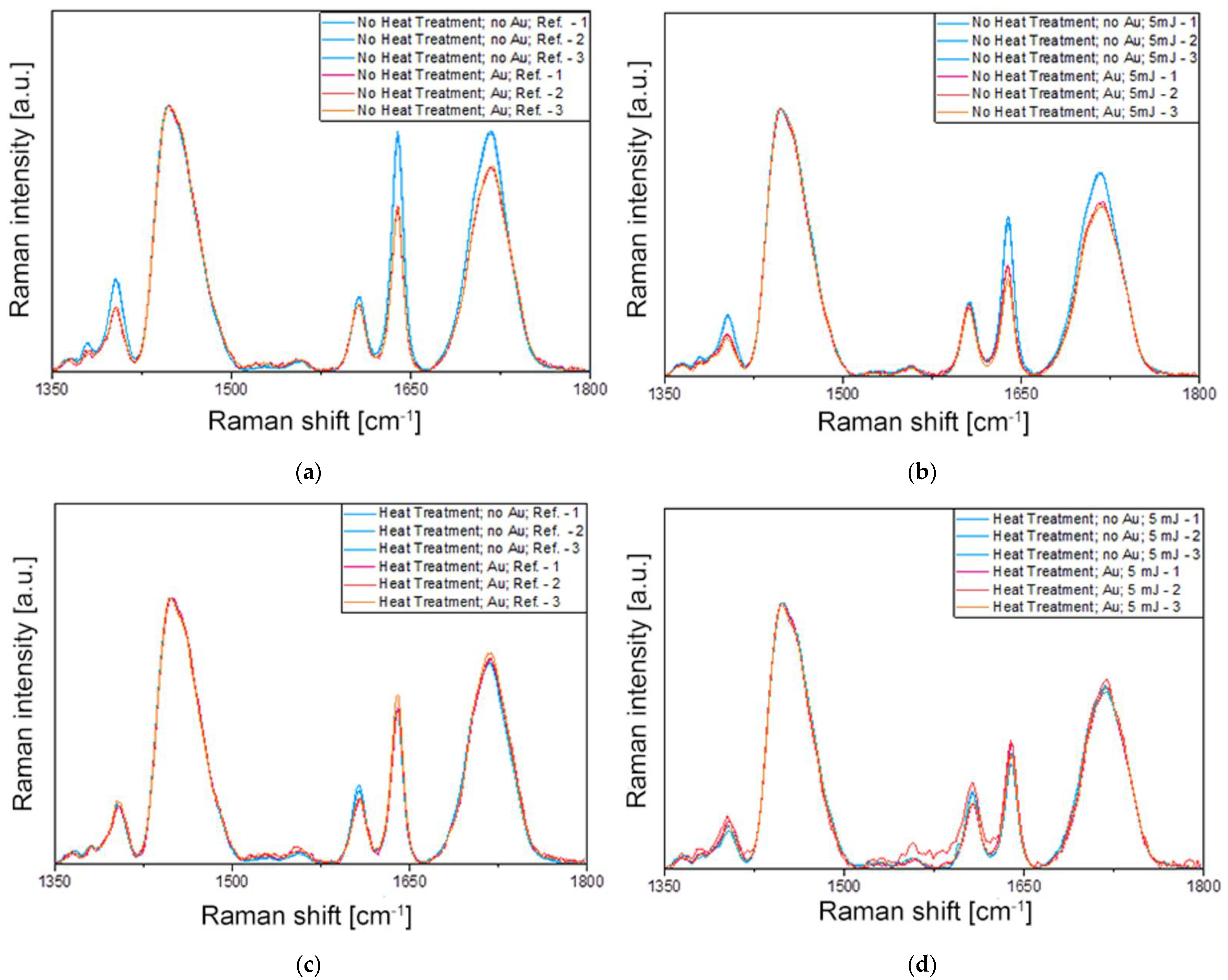

| ID | Heat Treatment | Laser Irradiation | Gold Nanorod Size, nm | Gold Nanorod Concentration, mL−1 |
|---|---|---|---|---|
| #1 | - | - | - | - |
| #2 | - | - | 25 × 75 | 1.9 × 1012 |
| #3 | - | + | - | - |
| #4 | - | + | 25 × 75 | 1.9 × 1012 |
| #5 | + | - | - | - |
| #6 | + | - | 25 × 75 | 1.9 × 1012 |
| #7 | + | + | - | - |
| #8 | + | + | 25 × 75 | 1.9 × 1012 |
| #9 | - | - | 25 × 85 | 9.5 × 1011 |
| #10 | - | + | 25 × 85 | 9.5 × 1011 |
| #11 | - | - | 25 × 85 | 1.9 × 1012 |
| #12 | - | + | 25 × 85 | 1.9 × 1012 |
| ID | Peak at 1447 cm−1 | Peak at 1640 cm−1 | 1640/1447 | DC [%] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity [a.u.] | FWHM [cm−1] | Area [a.u.] | Intensity [a.u.] | FWHM [cm−1] | Area [a.u.] | Intensity Ratio [-] | Area Ratio [-] | ||
| Ref. | 0.27 | 22.4 | 2.03 | 1.11 | 11.15 | 21.52 | 4.13 | 3.36 | - |
| #1 | 0.98 | 24.41 | 25.35 | 0.88 | 11.63 | 12.53 | 0.89 | 0.49 | 78.45 |
| #2 | 0.96 | 24.26 | 24.71 | 0.59 | 11.83 | 8.37 | 0.62 | 0.34 | 84.99 |
| #3 | 0.97 | 24.5 | 25.52 | 0.58 | 11.85 | 8.66 | 0.59 | 0.34 | 85.71 |
| #4 | 0.97 | 24.6 | 25.36 | 0.38 | 11.95 | 5.68 | 0.4 | 0.22 | 90.31 |
| #5 | 0.95 | 23.35 | 23.65 | 0.57 | 11.56 | 8.08 | 0.6 | 0.34 | 85.47 |
| #6 | 0.94 | 22.92 | 22.91 | 0.58 | 11.98 | 8.11 | 0.62 | 0.35 | 84.99 |
| #7 | 0.93 | 23.2 | 23.07 | 0.41 | 11.9 | 6.03 | 0.44 | 0.26 | 89.35 |
| #8 | 0.96 | 23.52 | 24.21 | 0.44 | 11.96 | 6.76 | 0.46 | 0.28 | 88.86 |
| #9 | 0.96 | 23.92 | 24.4 | 0.43 | 11.34 | 6.04 | 0.45 | 0.25 | 89.10 |
| #10 | 0.96 | 23.97 | 24.39 | 0.36 | 11.89 | 5.35 | 0.38 | 0.22 | 90.80 |
| #11 | 0.94 | 22.81 | 22.89 | 0.41 | 11.63 | 5.5 | 0.43 | 0.24 | 89.59 |
| #12 | 0.95 | 23.93 | 24.13 | 0.24 | 11.6 | 3.4 | 0.26 | 0.14 | 93.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonyár, A.; Szalóki, M.; Borók, A.; Rigó, I.; Kámán, J.; Zangana, S.; Veres, M.; Rácz, P.; Aladi, M.; Kedves, M.Á.; et al. The Effect of Femtosecond Laser Irradiation and Plasmon Field on the Degree of Conversion of a UDMA-TEGDMA Copolymer Nanocomposite Doped with Gold Nanorods. Int. J. Mol. Sci. 2022, 23, 13575. https://doi.org/10.3390/ijms232113575
Bonyár A, Szalóki M, Borók A, Rigó I, Kámán J, Zangana S, Veres M, Rácz P, Aladi M, Kedves MÁ, et al. The Effect of Femtosecond Laser Irradiation and Plasmon Field on the Degree of Conversion of a UDMA-TEGDMA Copolymer Nanocomposite Doped with Gold Nanorods. International Journal of Molecular Sciences. 2022; 23(21):13575. https://doi.org/10.3390/ijms232113575
Chicago/Turabian StyleBonyár, Attila, Melinda Szalóki, Alexandra Borók, István Rigó, Judit Kámán, Shereen Zangana, Miklós Veres, Péter Rácz, Márk Aladi, Miklós Ákos Kedves, and et al. 2022. "The Effect of Femtosecond Laser Irradiation and Plasmon Field on the Degree of Conversion of a UDMA-TEGDMA Copolymer Nanocomposite Doped with Gold Nanorods" International Journal of Molecular Sciences 23, no. 21: 13575. https://doi.org/10.3390/ijms232113575
APA StyleBonyár, A., Szalóki, M., Borók, A., Rigó, I., Kámán, J., Zangana, S., Veres, M., Rácz, P., Aladi, M., Kedves, M. Á., Szokol, Á., Petrik, P., Fogarassy, Z., Molnár, K., Csete, M., Szenes, A., Tóth, E., Vas, D., Papp, I., ... Collaboration, N. (2022). The Effect of Femtosecond Laser Irradiation and Plasmon Field on the Degree of Conversion of a UDMA-TEGDMA Copolymer Nanocomposite Doped with Gold Nanorods. International Journal of Molecular Sciences, 23(21), 13575. https://doi.org/10.3390/ijms232113575





