E4orf1 Prevents Progression of Fatty Liver Disease in Mice on High Fat Diet
Abstract
:1. Introduction
2. Results
2.1. Switching to Chow Diet Reduces Body Weight, but Inducing E4 Expression Attenuates Weight Gain despite the HFD
2.2. E4 Expression Reduced Endogenous Insulin Response to Glucose Load, and Improved Glycemic Control
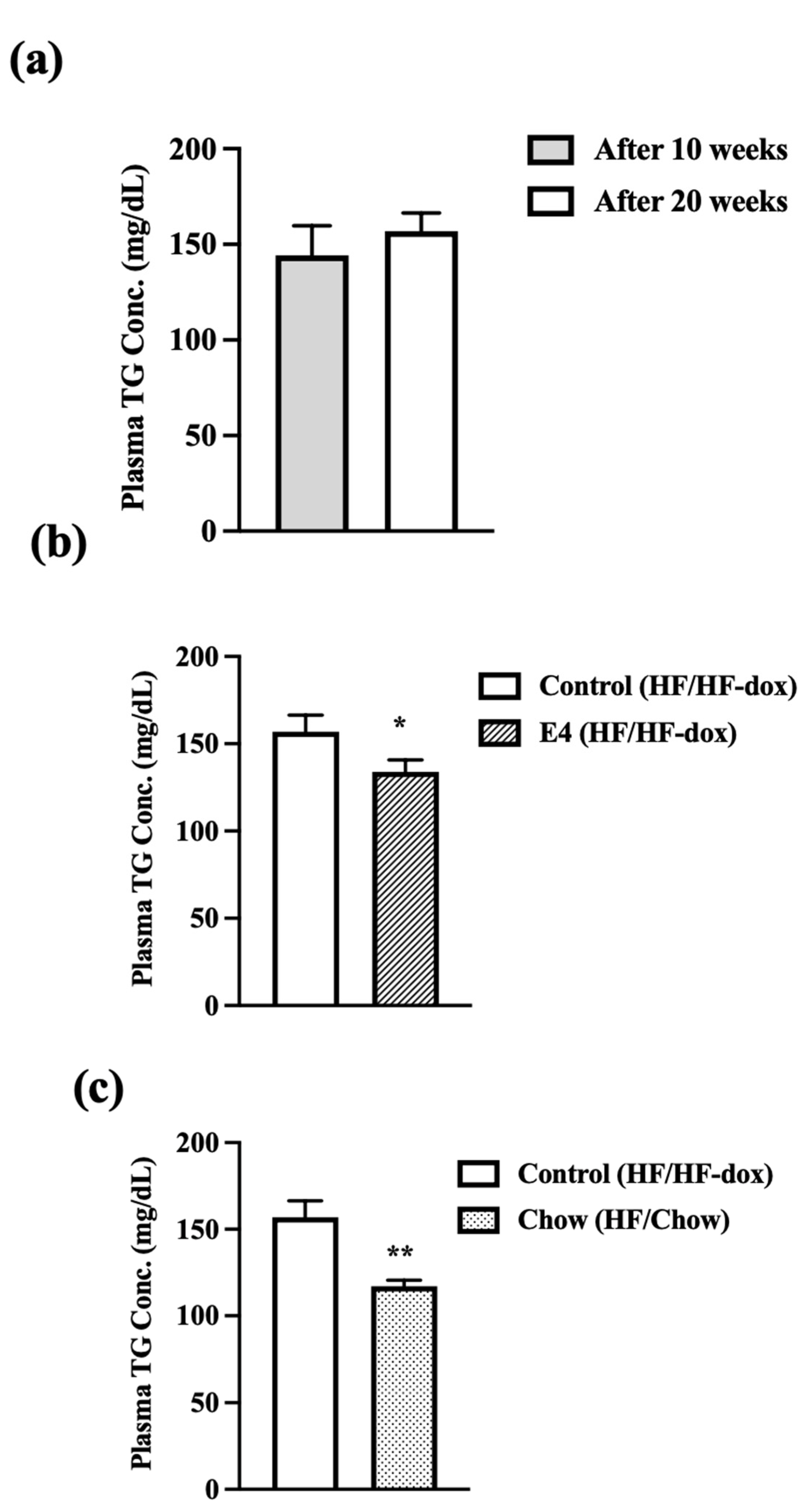
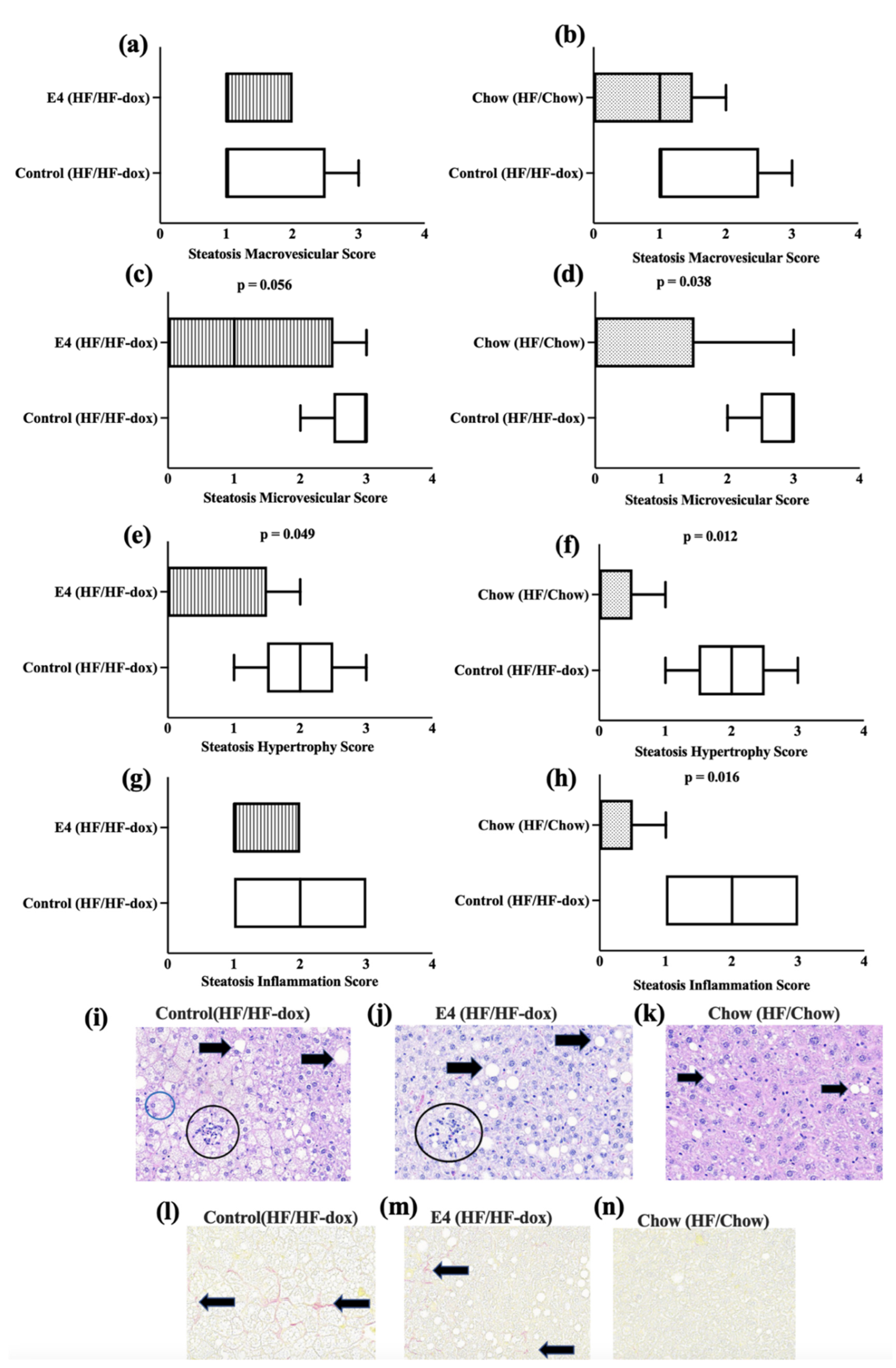
2.3. E4 and Chow Mice Display Significant Reduction in Serum Triglyceride and Improved Liver Histology
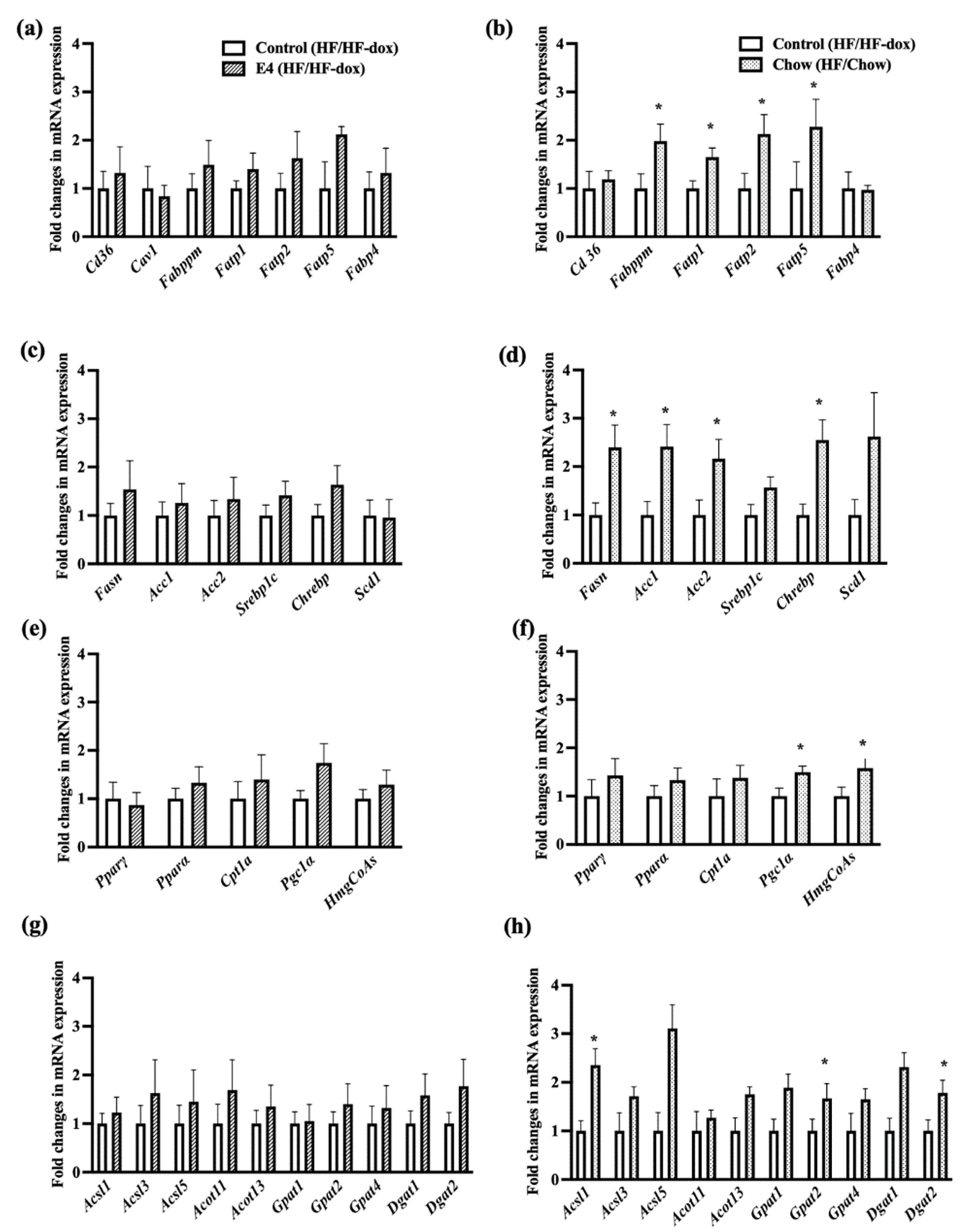
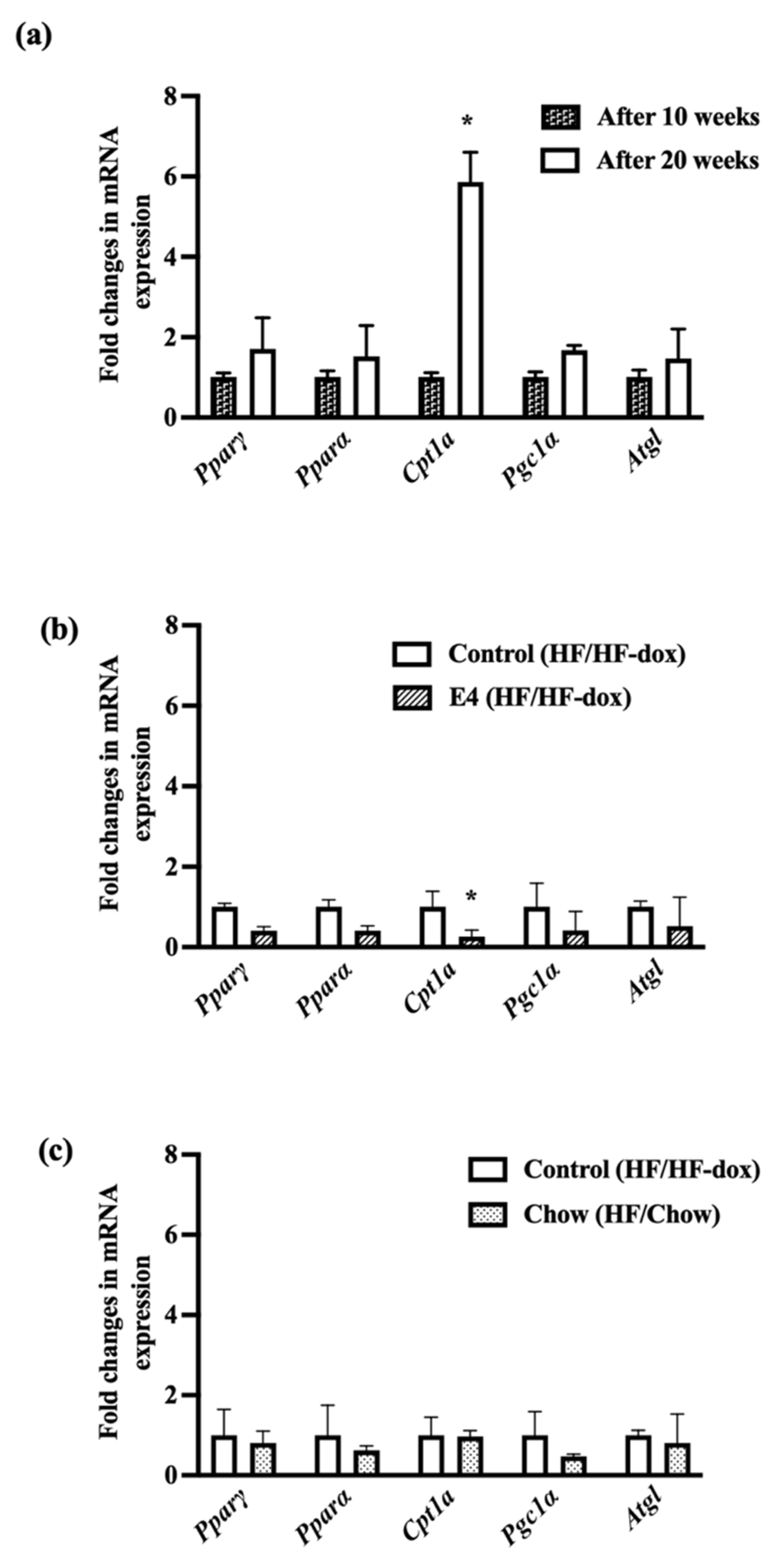
2.4. Dietary Fat Restriction Significantly Changes the Expression of Genes for Hepatic Lipid, Glucose, Insulin and Energy Metabolism
2.5. Significant Downregulation of Cpt1a in iWAT Suggests Less Fat Oxidation in E4 Mice
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Tissue Collection and Storage
4.3. RNA Extraction
4.4. cDNA Preparation and qRT-PCR
4.5. Serum Triglyceride Measurement
4.6. Hemoglobin A1c (HbA1c) Measurement in Blood
4.7. Glucose Tolerance Test
4.8. Serum Insulin Measurement
4.9. Liver Histology
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Li, Y.; Li, L.-Q.; Zheng, Y.; Lv, J.-H.; Huang, S.-C.; Zhang, W.; Liu, L.; Zhao, L.; Liu, Z. Risk factors and biomarkers of non-alcoholic fatty liver disease: An observational cross-sectional population survey. BMJ Open 2018, 8, e019974. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Hedges, C.P.; Masson, S.W.; Laube, B.; Pöhlmann, D.; Wueest, S.; Walsh, M.E.; Arnold, M.; Langhans, W.; Konrad, D. Partial impairment of insulin receptor expression mimics fasting to prevent diet-induced fatty liver disease. Nat. Commun. 2020, 11, 2080. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Wong, V.W.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Long-term mortality in nonalcoholic fatty liver disease: Is liver histology of any prognostic significance? Hepatology 2010, 51, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzen, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e6. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrabim, S.H.; Gores, G.J.; Malhi, H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to NASH pathogenesis. J. Lipid Res. 2016, 57, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V. Insulin sparing action of adenovirus 36 and its E4orf1 protein. J. Diabetes Complicat. 2013, 27, 191–199. [Google Scholar] [CrossRef]
- Dhurandhar, E.J.; Dubuisson, O.; Mashtalir, N.; Krishnapuram, R.; Hegde, V.; Dhurandhar, N.V. E4orf1: A novel ligand that improves glucose disposal in cell culture. PLoS ONE 2011, 6, e23394. [Google Scholar] [CrossRef]
- Akheruzzaman, M.; Hegde, V.; Shin, A.C.; Dhurandhar, N.V. Reducing endogenous insulin is linked with protection against hepatic steatosis in mice. Nutr. Diabetes 2020, 10, 11. [Google Scholar] [CrossRef]
- Hegde, V.; Na, H.N.; Dubuisson, O.; Burke, S.J.; Collier, J.J.; Burk, D.; Mendoza, T.; Dhurandhar, N.V. An adenovirus-derived protein: A novel candidate for anti-diabetic drug development. Biochimie 2016, 121, 140–150. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Gallardo-Montejano, V.I.; Wang, Z.V.; Hegde, V.; Bickel, P.E.; Dhurandhar, N.V.; Scherer, P.E. E4orf1 induction in adipose tissue promotes insulin-independent signaling in the adipocyte. Mol. Metab. 2015, 4, 653–664. [Google Scholar] [CrossRef]
- McMurphy, T.B.; Huang, W.; Xiao, R.; Liu, X.; Dhurandhar, N.V.; Cao, L. Hepatic expression of adenovirus 36 E4ORF1 improves glycemic control and promotes glucose metabolism through AKT activation. Diabetes 2017, 66, 358–371. [Google Scholar] [CrossRef]
- Na, H.N.; Hegde, V.; Dubuisson, O.; Dhurandhar, N.V. E4orf1 Enhances Glucose Uptake Independent of Proximal Insulin Signaling. PLoS ONE 2016, 11, e0161275. [Google Scholar] [CrossRef]
- Dhurandhar, E.J.; Krishnapuram, R.; Hegde, V.; Dubuisson, O.; Tao, R.; Dong, X.C.; Ye, J.; Dhurandhar, N.V. E4orf1 improves lipid and glucose metabolism in hepatocytes: A template to improve steatosis & hyperglycemia. PLoS ONE 2012, 7, e47813. [Google Scholar]
- Mostofinejad, Z.; Akheruzzaman, M.; Abu Bakkar Siddik, M.; Patkar, P.; Dhurandhar, N.V.; Hegde, V. Antidiabetic E4orf1 protein prevents hepatic steatosis and reduces markers of aging-related cellular damage in high fat fed older mice. BMJ Open Diabetes Res. Amp. Care 2021, 9, e002096. [Google Scholar] [CrossRef] [PubMed]
- Afruza, R.; Akheruzzaman, M.; Dhurandhar, N.V.; Hegde, V. E4orf1, an Adeno-viral protein, attenuates renal lipid accumulation in high fat fed mice: A novel approach to reduce a key risk factor for chronic kidney disease. Heliyon 2020, 6, e05261. [Google Scholar] [CrossRef] [PubMed]
- Afruza, R.; Akheruzzaman, M.; Dhurandhar, N.V.; Hegde, V. Role of E4orf1 in attenuation of renal fat accumulation and injury in mice exposed to high fat diet. FASEB J. 2020, 34 (Suppl. 1), 1. [Google Scholar] [CrossRef]
- Prabhakar, O.; Bhuvaneswari, M. Role of diet and lifestyle modification in the management of nonalcoholic fatty liver disease and type 2 diabetes. Tzu-Chi Med. J. 2021, 33, 135. [Google Scholar] [CrossRef]
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 37. [Google Scholar]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef]
- Elpek, G. Angiogenesis and liver fibrosis. World J. Hepatol. 2015, 7, 377–391. [Google Scholar] [CrossRef]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering, C. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Can. J. Kidney Health Dis. 2017, 4, 2054358117698669. [Google Scholar] [CrossRef] [PubMed]
- Vitola, B.E.; Deivanayagam, S.; Stein, R.I.; Mohammed, B.S.; Magkos, F.; Kirk, E.P.; Klein, S. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity 2009, 17, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Younossi, Z.M. Current treatment strategies for non-alcoholic fatty liver disease (NAFLD). Curr. Drug Discov. Technol. 2007, 4, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5, quiz e14-5. [Google Scholar] [CrossRef] [PubMed]
- Kucera, O.; Cervinkova, Z. Experimental models of non-alcoholic fatty liver disease in rats. World J. Gastroenterol. WJG 2014, 20, 8364. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Júnior, W.S.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 1–20. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1. [Google Scholar]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Wilding, J. The importance of weight management in type 2 diabetes mellitus. Int. J. Clin. Pract. 2014, 68, 682–691. [Google Scholar] [CrossRef]
- Albosta, M.; Bakke, J. Intermittent fasting: Is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin. Diabetes Endocrinol. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Akheruzzaman, M.; Hegde, V.; Dhurandhar, N.V. Twenty-five years of research about adipogenic adenoviruses: A systematic review. Obes. Rev. 2019, 20, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Krishnapuram, R.; Dhurandhar, E.J.; Dubuisson, O.; Hegde, V.; Dhurandhar, N.V. Doxycycline-regulated 3T3-L1 preadipocyte cell line with inducible, stable expression of adenoviral E4orf1 gene: A cell model to study insulin-independent glucose disposal. PLoS ONE 2013, 8, e60651. [Google Scholar] [CrossRef] [PubMed]
- Peddibhotla, S.; Hegde, V.; Akheruzzaman, M.; Dhurandhar, N.V. E4orf1 protein reduces the need for endogenous insulin. Nutr. Diabetes 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Akheruzzaman, M.; Hegde, V.; Siddik, M.A.B.; Feizy, Z.; Shin, A.C.; Dhurandhar, N.V. E4orf1-induced reduction in endogenous insulin level is independent of pancreas endocrine function. Int. J. Obes. 2022, 46, 918–925. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Araki, S.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Kubota, N.; Terauchi, Y.; Kadowaki, T.; et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 2007, 18, 2715–2723. [Google Scholar] [CrossRef]
- Siersbaek, M.; Varticovski, L.; Yang, S.; Baek, S.; Nielsen, R.; Mandrup, S.; Hager, G.L.; Chung, J.H.; Grontved, L. High fat diet-induced changes of mouse hepatic transcription and enhancer activity can be reversed by subsequent weight loss. Sci. Rep. 2017, 7, 40220. [Google Scholar] [CrossRef]
- Leung, A.; Trac, C.; Du, J.; Natarajan, R.; Schones, D.E. Persistent Chromatin Modifications Induced by High Fat Diet. J. Biol. Chem. 2016, 291, 10446–10455. [Google Scholar] [CrossRef]
- Drzewoski, J.; Kasznicki, J.; Trojanowski, Z. The role of "metabolic memory" in the natural history of diabetes mellitus. Pol. Arch. Med. Wewn. 2009, 119, 493–500. [Google Scholar] [CrossRef]
- Tanaka, M.; Yasuoka, A.; Shimizu, M.; Saito, Y.; Kumakura, K.; Asakura, T.; Nagai, T. Transcriptomic responses of the liver and adipose tissues to altered carbohydrate-fat ratio in diet: An isoenergetic study in young rats. Genes Nutr. 2017, 12, 10. [Google Scholar] [CrossRef]
- Nichols, R.G.; Zhang, J.; Cai, J.; Murray, I.A.; Koo, I.; Smith, P.B.; Perdew, G.H.; Patterson, A.D. Metatranscriptomic Analysis of the Mouse Gut Microbiome Response to the Persistent Organic Pollutant 2,3,7,8-Tetrachlorodibenzofuran. Metabolites 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Okawa, S.; Takahashi, M. The effects on weight loss and gene expression in adipose and hepatic tissues of very-low carbohydrate and low-fat isoenergetic diets in diet-induced obese mice. Nutr. Metab. 2016, 13, 78. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy Balance and Obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Randle, P.J. Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes/Metab. Rev. 1998, 14, 263–283. [Google Scholar] [CrossRef]
- Muoio, D.M.; Newgard, C.B. Fatty acid oxidation and insulin action: When less is more. Diabetes 2008, 57, 1455–1456. [Google Scholar] [CrossRef]
- Warfel, J.D.; Vandanmagsar, B.; Dubuisson, O.S.; Hodgeson, S.M.; Elks, C.M.; Ravussin, E.; Mynatt, R.L. Examination of carnitine palmitoyltransferase 1 abundance in white adipose tissue: Implications in obesity research. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R816–R820. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Henkel, J.; Buchheim-Dieckow, K.; Castro, J.P.; Laeger, T.; Wardelmann, K.; Kleinridders, A.; Johrens, K.; Puschel, G.P. Reduced Oxidative Stress and Enhanced FGF21 Formation in Livers of Endurance-Exercised Rats with Diet-Induced NASH. Nutrients 2019, 11, 2709. [Google Scholar] [CrossRef]



| Control | |||||||
|---|---|---|---|---|---|---|---|
| Sex | F | M | M | M | F | ||
| N | 5 | ||||||
| Sample ID | L6 | L7 | L8 | L9 | L10 | # Abnormal | Average |
| Steatosis: macrovesicular | 2 | 1 | 1 | 1 | 3 | 5 | 1.60 |
| Steatosis: microvesicular | 2 | 3 | 3 | 3 | 3 | 5 | 2.80 |
| Steatosis: hypertrophy | 1 | 3 | 2 | 2 | 2 | 5 | 2.00 |
| Inflammation | 2 | 3 | 1 | 1 | 3 | 5 | 2.00 |
| Sum-Scores: | 7 | 10 | 7 | 7 | 11 | 8.40 | |
| Fibrosis | 0 | 1 | 0 | 1 | 0 | 2 | 0.40 |
| E4 | |||||||
| Sex | M | F | F | M | F | ||
| N | 5 | ||||||
| Sample ID | L1 | L2 | L3 | L4 | L5 | # Abnormal | Average |
| Steatosis: macrovesicular | 2 | 1 | 2 | 1 | 1 | 5 | 1.40 |
| Steatosis: microvesicular | 3 | 1 | 2 | 0 | 0 | 3 | 1.20 |
| Steatosis: hypertrophy | 2 | 0 | 1 | 0 | 0 | 2 | 0.60 |
| Inflammation | 1 | 2 | 2 | 1 | 1 | 5 | 1.40 |
| Sum-Scores: | 8 | 4 | 7 | 2 | 2 | 4.60 | |
| Fibrosis | 1 | 1 | 1 | 1 | 0 | 4 | 0.80 |
| Chow | |||||||
| Sex | M | M | M | F | F | ||
| N | 5 | ||||||
| Sample ID | L11 | L12 | L13 | L14 | L15 | # Abnormal | Average |
| Steatosis: macrovesicular | 1 | 2 | 1 | 0 | 0 | 3 | 0.80 |
| Steatosis: microvesicular | 0 | 3 | 0 | 0 | 0 | 1 | 0.60 |
| Steatosis: hypertrophy | 0 | 1 | 0 | 0 | 0 | 0 | 0.20 |
| Inflammation | 0 | 1 | 0 | 0 | 0 | 1 | 0.20 |
| Sum-Scores: | 1 | 7 | 1 | 0 | 0 | 1.80 | |
| Fibrosis | 1 | 1 | 0 | 0 | 0 | 0 | 0.40 |
| Gene Name | Primer Sequence (5′-3′) |
|---|---|
| ACC1-Fw | GCAGCAGTTACACCACATAC |
| ACC1-Rev | TCCGCCATCTTCCACAATA |
| ACC2-Fw | TACGGCGGCATCAAGTAT |
| ACC2-Rev | ACTGTCAACCTCTTCCTTCAT |
| ACOT11-Fw | CTGACTCTTGGCTCTACTTGT |
| ACOT11-Rev | CTCTGAACCTCCGCTCTC |
| ACOT13-Fw | ACGAGAAGTAATGAAGGTTATGTT |
| ACOT13-Rev | AGATGCTGTCCACTAAGGT |
| ACSL1-Fw | CAACACTGAAGGCGAAGAG |
| ACSL1-Rev | CGAGGAGGATTGTGGAGAT |
| ACSL3-Fw | AGGAAGATGTGTATATTGGCTACT |
| ACSL3-Rev | CTGCTAATGTCTGTGGTGAAG |
| ACSL5-Fw | CCATCTCCACTCCAGTCTT |
| ACSL5-Rev | TGTCAGCCACATCTTCCA |
| ATG5-Fw | TCCATCCAAGGATGCGGTTG |
| ATG5-Rev | TCTGCATTTCGTTGATCACTTGAC |
| ATGL Fw | CGCTATGATGGCAATGTGTAT |
| ATGL Rev | TAGTAAGATTCGTGGACCTCTG |
| Beclin1-Fw | ACCAGCGGGAGTATAGTGAGT |
| Beclin1-Rev | CAGCTGGATCTGGGCGTAG |
| beta-actin Fw | AATCTTCCGCCTTAATACTTCATT |
| beta-actin Rev | CTGCCTCAACACCTCAAC |
| BniP3i-Fw | GCACGTTCCTTCCTCGTCT |
| BniP3i-Rev | GCTCTGTCCCGACTCATGC |
| CD36-Fw | AGGTCTATCTACGCTGTGTTC |
| CD36-Rev | AGGCATTGGCTGGAAGAA |
| ChREBP1-Fw | TTCCACAAGCATCCTGACT |
| ChREBP1-Rev | AGAAGCGTGTTCACAAGTTG |
| Collagen type IV Fw | CCAGAGGAGGTGTATAGATAGC |
| Collagen type IV Rev | GCAGAGCAGAAGCAAGAAG |
| Collagen14a-Fw | ATCCTCTATGCTCCTCTC |
| Collagen14a-Rev | CCACTCAGTTCAATGTCT |
| Collagen1a-Fw | AGGTATGCTTGATCTGTAT |
| Collagen1a-Rev | CAGTCCAGTTCTTCATTG |
| Collagen3a-Fw | CAACGGTCATACTCATTC |
| Collagen3a-Rev | TATAGTCTTCAGGTCTCAG |
| CPT1a-Fw | CAAGCCAGACGAAGAACATC |
| CPT1a-Rev | TGACCATAGCCATCCAGATT |
| DGAT1-Fw | GATTGGTGGAATGCTGAGTC |
| DGAT1-Rev | GGCTTGTAGAAGTGTCTGATG |
| DGAT2-Fw | TCCAGAAGAAGTTCCAGAAGTAT |
| DGAT2-Rev | CAGGTGTCAGAGGAGAAGAG |
| FABP4-Fw | TGGACTTCAGAGGCTCATAG |
| FABP4-Rev | CCACAAAGGCATCACACAT |
| FABPpm-Fw | CGAGCAGTGGAAGGAGAT |
| FABPpm-Rev | GCAGAGGCAGACATTGATG |
| Fasn-Fw | GTCGTCTATACCACTGCTTACT |
| Fasn-Rev | ACACCACCTGAACCTGAG |
| FATP1-Fw | AGACTCAGGAAGGTTGTTGT |
| FATP1-Rev | AGATGAAGGCAGGCAGAG |
| FATP2-Rev | CCATACACATTCACTTCTTCAACA |
| FATP2-Fw | AGGCGACATCTACTTCAACA |
| FATP5-Fw | AGCCAGCCATCTTATCACAT |
| FATP5-Rev | AAGCAGCCAAGGAATCCA |
| FGF21-Fw | CCAAGACCAAGCAGGATTC |
| FGF21-Rev | AGAGTCAGGACGCATAGC |
| Fibronectin-Fw | GGTTGATGATACTTCCATTGTTGT |
| Fibronectin-Rev | GTGCTACTGCCTTCTACTGA |
| Foxo1-Fw | GCTCTGTCCTGAAGAATCCT |
| Foxo1-Rev | CTAATCCTGCCACTGTCTGTA |
| G6Pase-Fw | GGAAGGATGGAGGAAGGAAT |
| G6Pase-Rev | TCAGGTCAGCAATCACAGA |
| GPAT1-Fw | CTATCCAGTAACGAGTCCAGAA |
| GPAT1-Rev | GGCGGTGAAGAGAATGTG |
| GPAT2-Fw | GTCTTCCTACTGCTACTGTCA |
| GPAT2-Rev | TGCTGTCTTCCTGTGTCA |
| GPAT4-Fw | GGTGGAGAACAGCGAGTA |
| GPAT4-Rev | TCAGAAGGAAGGACAGAAGG |
| HMGCS2-Fw | CTTGAACGAGTGGATGAGATG |
| HMGCS2-Rev | CTATGAGGCTGCTGTGTCTA |
| IL-18 Fw | CCAAGTTCTCTTCGTTGACAA |
| IL-18 Rev | TCACAGCCAGTCCTCTTAC |
| IL-1β-Fw | TTCAGGCAGGCAGTATCA |
| IL-1β-Rev | CCAGCAGGTTATCATCATCATC |
| IL-6-Fw | ACAGAAGGAGTGGCTAAG |
| IL-6-Rev | AGAGAACAACATAAGTCAGATAC |
| Lc3a-Fw | CCCATCGCTGACATCTATGAAC |
| Lc3a-Rev | AAGGTTTCTTGGGAGGCGTA |
| Lc3b-Fw | TCCACTCCCATCTCCGAAGT |
| Lc3b-Rev | TTGCTGTCCCGAATGTCTCC |
| Lipin1-Fw | GCCGTGTCATATCAGCAAT |
| Lipin1-Rev | ATCGCCAGAAGTAGAGGAG |
| MCP1-Fw | GCCCCACTCACCTGCTGCTACT |
| MCP1-Rev | CCTGCTGCTGGTGATCCTCTTGT |
| mFn1-Fw | GCAGACAGCACATGGAGAGA |
| mFn1-Rev | GATCCGATTCCGAGCTTCCG |
| mFn2-Fw | TGCACCGCCATATAGAGGAAG |
| mFn2-Rev | TCTGCAGTGAACTGGCAATG |
| PEPCK-Fw | GACATTGCCTGGATGAAGTT |
| PEPCK-Rev | CGTTGGTGAAGATGGTGTT |
| PGC1a-Fw | ACAATAACAACAACAACCATACCA |
| PGC1a-Rev | ATTCTGTCTCTTGCCTCTTCA |
| Pink1-Fw | CCATCGGGATCTCAAGTCCG |
| Pink1-Rev | GATCACTAGCCAGGGACAGC |
| PLIN2-Fw | AGAAGCCGAGCAACTATGA |
| PLIN2-Rev | TGAGAGCCTGGTGATAAGC |
| PLIN3-Fw | CGACAGGAGCAGAACTACT |
| PLIN3-Rev | CCGAGCACACTTGTTAGC |
| PLIN5-Fw | CACAGTGGAGGAGCAGAG |
| PLIN5-Rev | AAGAGTGTTCATAGGCGAGAT |
| PPARα-Fw | TCGCTATCCAGGCAGAAG |
| PPARα-Rev | ACAACAACAACAATAACCACAGA |
| PPARγ-Fw | CCACCAACTTCGGAATCAG |
| PPARγ-Rev | GCTCTTGTGAATGGAATGTCT |
| SCD1-Fw | TGCCTCTTAGCCACTGAAT |
| SCD1-Rev | ACTGTTGAGATGTGAGACTGT |
| Srebp1c-Fw | GCTTCTCTTCTGCTTCTCTG |
| Srebp1c-Rev | GGCTGTAGGATGGTGAGT |
| TGFβ-Rev | AAGGTAACGCCAGGAATTG |
| TGFβ-Fw | GCAACAACGCCATCTATGA |
| VEGF A Fw | CTCTTCTCGCTCCGTAGTAG |
| VEGF A Rev | CCTCTCCTCTTCCTTCTCTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afruza, R.; Dhurandhar, N.V.; Hegde, V. E4orf1 Prevents Progression of Fatty Liver Disease in Mice on High Fat Diet. Int. J. Mol. Sci. 2022, 23, 9286. https://doi.org/10.3390/ijms23169286
Afruza R, Dhurandhar NV, Hegde V. E4orf1 Prevents Progression of Fatty Liver Disease in Mice on High Fat Diet. International Journal of Molecular Sciences. 2022; 23(16):9286. https://doi.org/10.3390/ijms23169286
Chicago/Turabian StyleAfruza, Rownock, Nikhil V. Dhurandhar, and Vijay Hegde. 2022. "E4orf1 Prevents Progression of Fatty Liver Disease in Mice on High Fat Diet" International Journal of Molecular Sciences 23, no. 16: 9286. https://doi.org/10.3390/ijms23169286
APA StyleAfruza, R., Dhurandhar, N. V., & Hegde, V. (2022). E4orf1 Prevents Progression of Fatty Liver Disease in Mice on High Fat Diet. International Journal of Molecular Sciences, 23(16), 9286. https://doi.org/10.3390/ijms23169286






