The Synergistic Effect of Thiamethoxam and Synapsin dsRNA Targets Neurotransmission to Induce Mortality in Aphis gossypii
Abstract
1. Introduction
2. Results
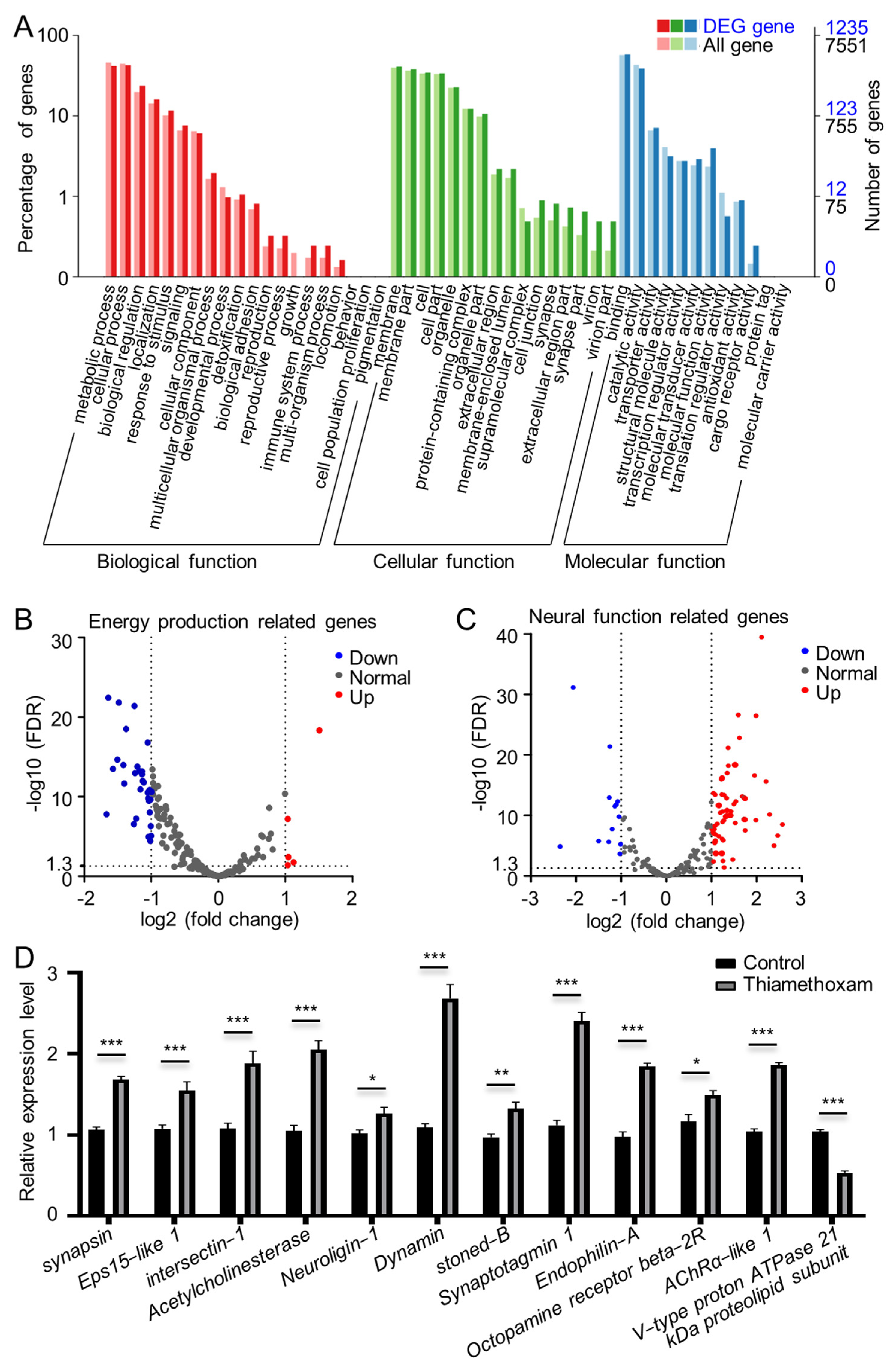
2.1. Genes Related to Metabolism and Neural Function Were Differentially Expressed
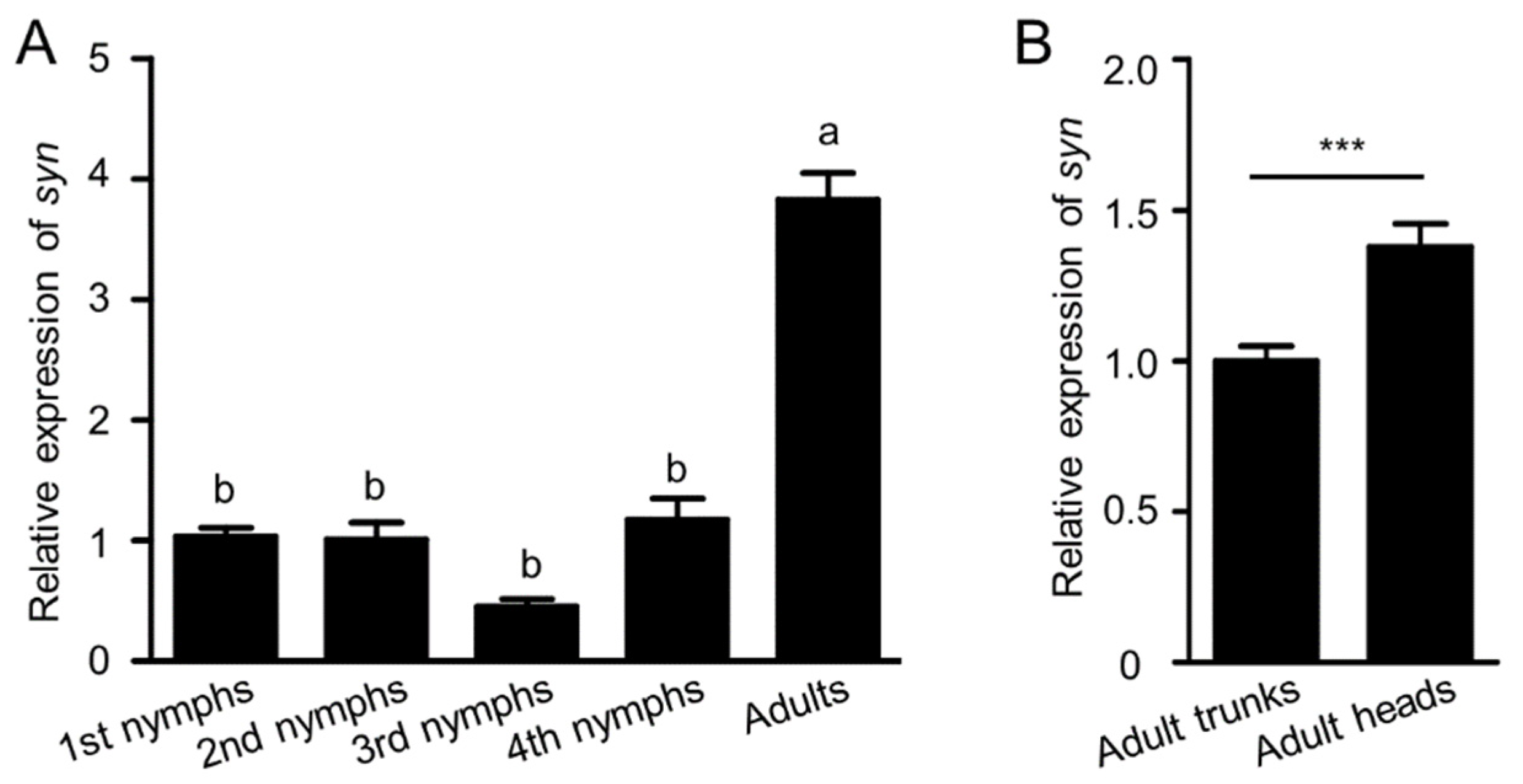
2.2. Expression Profiles of Synapsin at Different Stages and Tissues
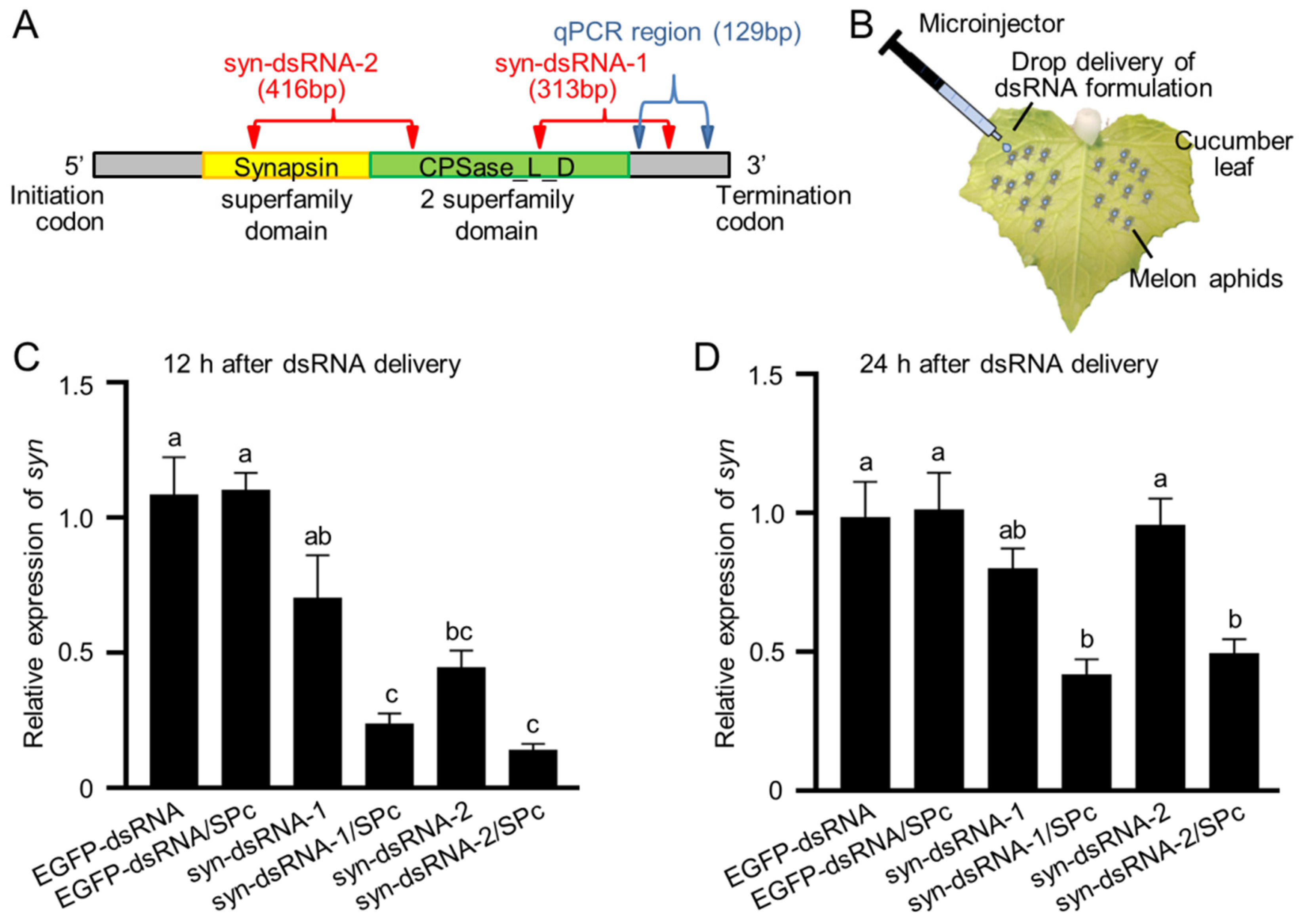
2.3. Knockdown of Synapsin Led to Death of the Melon Aphid
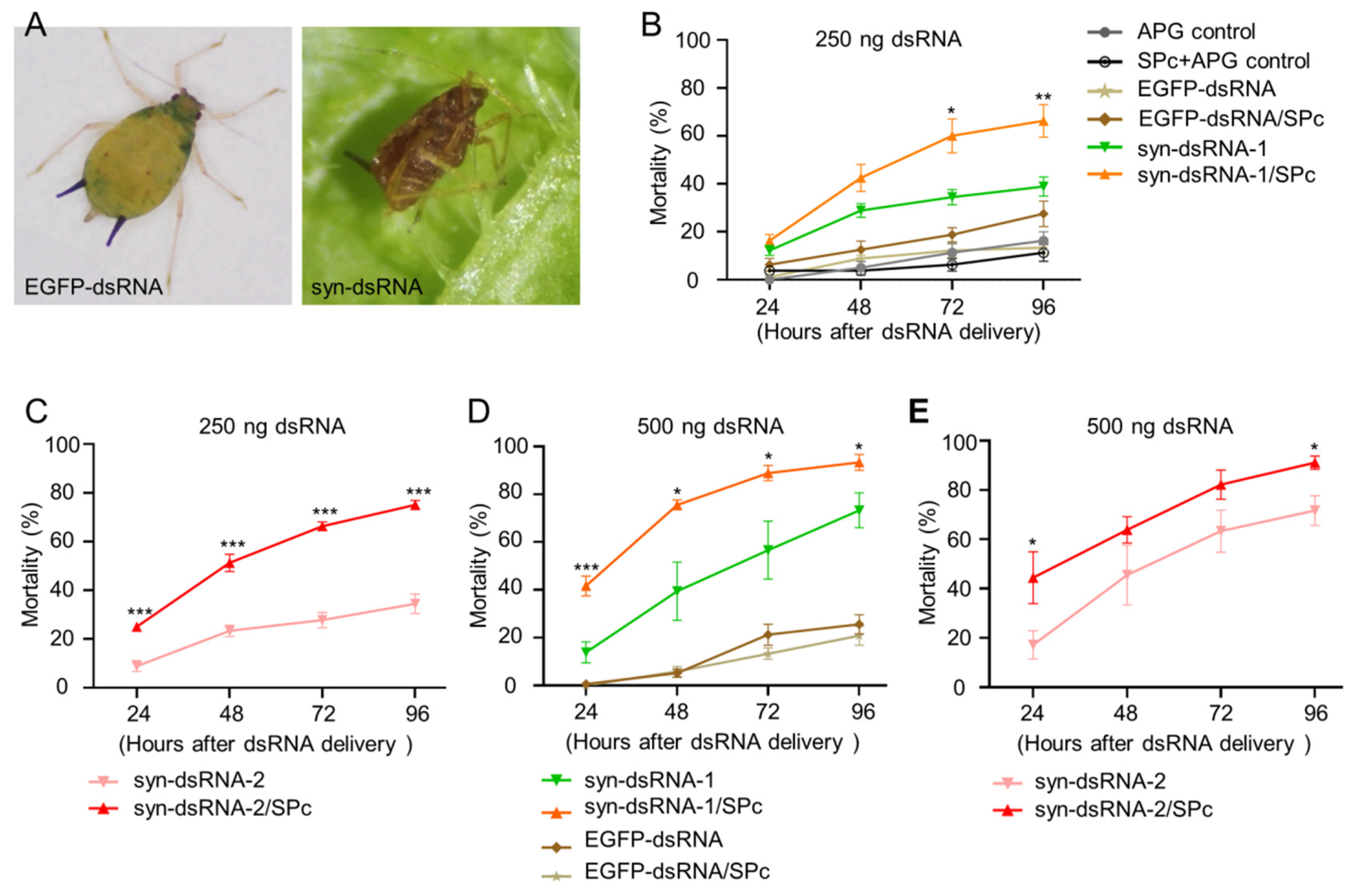
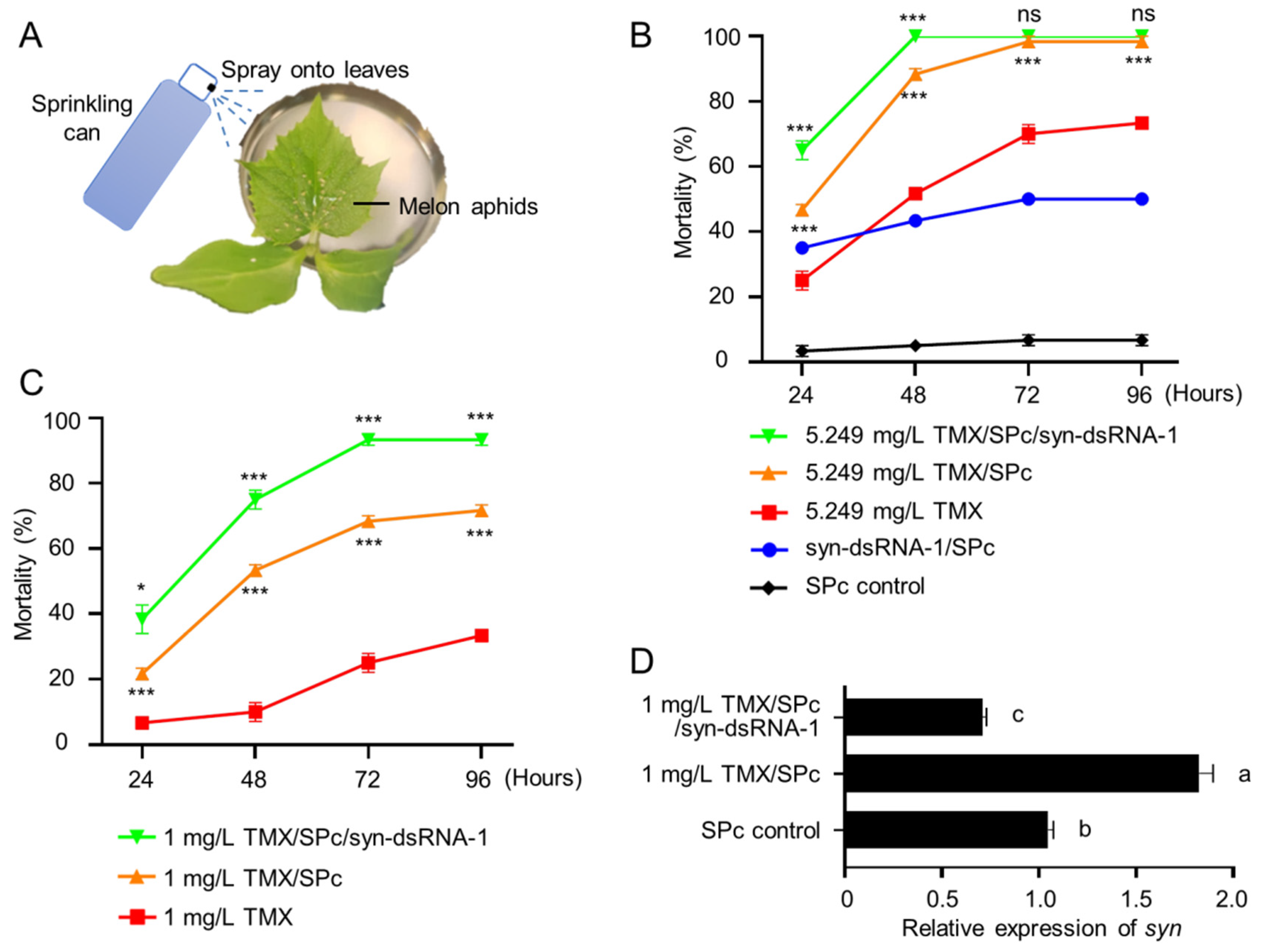
2.4. Co-Delivery of syn-dsRNA and Thiamethoxam Caused Efficient Lethality to Melon Aphids
2.5. The Upregulation of Synapsin as an Acute Response to Thiamethoxam
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. RNA Sequencing (RNA-Seq)
4.3. Real-Time Quantitative PCR (qPCR)
4.4. Phylogenetic Analysis and Sequence Alignment
4.5. dsRNA Synthesis
4.6. Bioassay
4.7. Stability Test of Nanocarrier-Delivered dsRNA in Aphid Hemolymph
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomizawa, M.; Casida, J.E. Molecular recognition of neonicotinoid insecticides: The determinants of life or death. Acc. Chem. Res. 2009, 42, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.A.; Goven, D.; Raymond, V. Exposure to sublethal doses of insecticide and their effects on insects at cellular and physiological levels. Curr. Opin. Insect Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Guedes, R.N.C. Occurrence and Significance of Insecticide-Induced Hormesis in Insects. In Pesticide Dose: Effects on the Environment and Target and Non-Target Organisms; Duke, S.O., Kudsk, P., Solomon, K., Eds.; American Chemical Society: Washington, DC, USA, 2017; pp. 101–119. [Google Scholar] [CrossRef]
- Christopher Cutler, G.; Ramanaidu, K.; Astatkie, T.; Isman, M.B. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag. Sci. 2009, 65, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Rix, R.R.; Ayyanath, M.M.; Cutler, G.C. Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. J. Pest Sci. 2016, 89, 581–589. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Jiang, Y.; Ma, C.; Yang, G. Sublethal effects of imidacloprid on fecundity, apoptosis and virus transmission in the small brown planthopper Laodelphax striatellus. Insects 2021, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, K.H.; Huang, J.C.; Lai, H.T.; Uapipatanakul, B.; Roldan, M.J.M.; Macabeo, A.P.G.; Ger, T.R.; Hsiao, C.D. Physiological effects of neonicotinoid insecticides on non-target aquatic animals-an updated review. Int. J. Mol. Sci. 2021, 22, 9591. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, L.; Wang, H.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Zhang, A.; Zhu, L.; Shi, Z.; Liu, T.; Han, L.; Zhao, K. Effects of imidacloprid and thiamethoxam on the development and reproduction of the soybean aphid Aphis glycines. PLoS ONE 2021, 16, e0250311. [Google Scholar] [CrossRef] [PubMed]
- Lamsa, J.; Kuusela, E.; Tuomi, J.; Juntunen, S.; Watts, P.C. Low dose of neonicotinoid insecticide reduces foraging motivation of bumblebees. Proc. Biol. Sci. 2018, 285, 20180506. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Tackenberg, M.C.; Giannoni-Guzman, M.A.; Sanchez-Perez, E.; Doll, C.A.; Agosto-Rivera, J.L.; Broadie, K.; Moore, D.; McMahon, D.G. Neonicotinoids disrupt circadian rhythms and sleep in honey bees. Sci. Rep. 2020, 10, 17929. [Google Scholar] [CrossRef]
- Tasman, K.; Hidalgo, S.; Zhu, B.F.; Rands, S.A.; Hodge, J.J.L. Neonicotinoids disrupt memory, circadian behaviour and sleep. Sci. Rep. 2021, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Tasman, K.; Rands, S.A.; Hodge, J.J.L. The neonicotinoid insecticide imidacloprid disrupts bumblebee foraging rhythms and sleep. Iscience 2020, 23, 101827. [Google Scholar] [CrossRef]
- Palmer, M.J.; Moffat, C.; Saranzewa, N.; Harvey, J.; Wright, G.A.; Connolly, C.N. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 2013, 4, 1634. [Google Scholar] [CrossRef]
- Peng, Y.C.; Yang, E.C. Sublethal dosage of imidacloprid reduces the microglomerular density of honey bee mushroom bodies. Sci. Rep. 2016, 6, 19298. [Google Scholar] [CrossRef]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J.; et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, H.; Shan, T.; Shi, X.; Gao, X. The overexpression of three cytochrome P450 genes CYP6CY14, CYP6CY22 and CYP6UN1 contributed to metabolic resistance to dinotefuran in melon/cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2020, 167, 104601. [Google Scholar] [CrossRef]
- Enders, L.S.; Rault, L.C.; Heng-Moss, T.M.; Siegfried, B.D.; Miller, N.J. Transcriptional responses of soybean aphids to sublethal insecticide exposure. Insect Biochem. Mol. Biol. 2020, 118, 103285. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Xu, H.F.; Pan, Y.O.; Gao, X.W.; Xi, J.H.; Zhang, J.H.; Shang, Q.L. Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 149, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Yang, Y.X.; Sun, H.H.; Liu, Z.W. Metabolic imidacloprid resistance in the brown planthopper, Nilaparvata lugens, relies on multiple P450 enzymes. Insect Biochem. Mol. Biol. 2016, 79, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Williamson, M.S.; Lansdell, S.J.; Denholm, I.; Han, Z.; Millar, N.S. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc. Natl. Acad. Sci. USA 2005, 102, 8420–8425. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Andrews, M.; Cutler, P.; Daniels, M.; Elias, J.; Paul, V.L.; Crossthwaite, A.J.; Denholm, I.; Field, L.M.; et al. Mutation of a nicotinic acetylcholine receptor beta subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci. 2011, 12, 51. [Google Scholar] [CrossRef]
- Colgan, T.J.; Fletcher, I.K.; Arce, A.N.; Gill, R.J.; Ramos Rodrigues, A.; Stolle, E.; Chittka, L.; Wurm, Y. Caste- and pesticide-specific effects of neonicotinoid pesticide exposure on gene expression in bumblebees. Mol. Ecol. 2019, 28, 1964–1974. [Google Scholar] [CrossRef]
- Lv, N.; Ma, K.; Li, R.; Liang, P.; Gao, X. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol. Environ. Saf. 2021, 212, 111969. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Hunt, G.J.; Guzman-Novoa, E. Effects of sublethal doses of clothianidin and/or V. destructor on honey bee (Apis mellifera) self-grooming behavior and associated gene expression. Sci. Rep. 2019, 9, 5196. [Google Scholar] [CrossRef]
- Chen, Y.R.; Tzeng, D.T.W.; Yang, E.C. Chronic effects of imidacloprid on honey bee worker development-molecular pathway perspectives. Int. J. Mol. Sci. 2021, 22, 11835. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Liu, Y.; Fan, J.Y.; Ma, Y.J.; Yuan, C.Y.; Lou, B.H.; Wang, J.J. Odorant binding protein 2 reduces imidacloprid susceptibility of Diaphorina citri. Pestic. Biochem. Physiol. 2020, 168, 104642. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Gokce, A.; Aksoy, E.; Bakhsh, A. Downregulation of imidacloprid resistant genes alters the biological parameters in Colorado potato beetle, Leptinotarsa decemlineata Say (chrysomelidae: Coleoptera). Chemosphere 2020, 240, 124857. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Ma, K.S.; Liu, J.J.; Lu, L.Y.; Chen, X.L.; Zhang, S.P.; Gao, X.W. Differential expression of genes in greenbug (Schizaphis graminum Rondani) treated by imidacloprid and RNA interference. Pest Manag. Sci. 2019, 75, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.W.; Pellino, J.L.; Lee, Y.S.; Carthew, R.W.; Sontheimer, E.J. A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell 2004, 117, 83–94. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.Y.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. Biotechniques 2020, 68, 283–290. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Chen, A.; Ma, K.; Liang, P.; Liu, Y.; Song, D.; Gao, X. Both point mutations and low expression levels of the nicotinic acetylcholine receptor beta1 subunit are associated with imidacloprid resistance in an Aphis gossypii (Glover) population from a Bt cotton field in China. Pestic. Biochem. Physiol. 2017, 141, 1–8. [Google Scholar] [CrossRef]
- Gore, J.; Cook, D.; Catchot, A.; Leonard, B.R.; Stewart, S.D.; Lorenz, G.; Kerns, D. Cotton aphid (Heteroptera: Aphididae) susceptibility to commercial and experimental insecticides in the southern United States. J. Econ. Entomol. 2013, 106, 1430–1439. [Google Scholar] [CrossRef]
- Wei, X.; Pan, Y.; Xin, X.; Zheng, C.; Gao, X.; Xi, J.; Shang, Q. Cross-resistance pattern and basis of resistance in a thiamethoxam-resistant strain of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2017, 138, 91–96. [Google Scholar] [CrossRef]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.J.; Zahid, S. The role of synapsins in neurological disorders. Neurosci. Bull. 2018, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Adden, A.; Wibrand, S.; Pfeiffer, K.; Warrant, E.; Heinze, S. The brain of a nocturnal migratory insect, the Australian Bogong moth. J. Comp. Neurol. 2020, 528, 1942–1963. [Google Scholar] [CrossRef] [PubMed]
- Cabirol, A.; Haase, A. Automated quantification of synaptic boutons reveals their 3D distribution in the honey bee mushroom body. Sci Rep. 2019, 9, 19322. [Google Scholar] [CrossRef]
- Von Hadeln, J.; Althaus, V.; Hager, L.; Homberg, U. Anatomical organization of the cerebrum of the desert locust Schistocerca gregaria. Cell Tissue Res. 2018, 374, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, M.; Minoli, S.; Bonhomme, J.; Homberg, U.; Schachtner, J.; Tagu, D.; Anton, S. Revisiting the anatomy of the central nervous system of a hemimetabolous model insect species: The pea aphid Acyrthosiphon pisum. Cell Tissue Res. 2011, 343, 343–355. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, W.Y.; Han, Z.H.; Wang, D.; Yin, M.Z.; Du, X.G.; Shen, J. Nanometerization of thiamethoxam by a cationic star polymer nanocarrier efficiently enhances the contact and plant-uptake dependent stomach toxicity against green peach aphids. Pest Manag. Sci. 2021, 77, 1954–1962. [Google Scholar] [CrossRef]
- Fahrbach, S.E.; Van Nest, B.N. Synapsin-based approaches to brain plasticity in adult social insects. Curr. Opin. Insect Sci. 2016, 18, 27–34. [Google Scholar] [CrossRef][Green Version]
- Klagges, B.R.E.; Heimbeck, G.; Godenschwege, T.A.; Hofbauer, A.; Pflugfelder, G.O.; Reifegerste, R.; Reisch, D.; Schaupp, M.; Buchner, S.; Buchner, E. Invertebrate synapsins: A single gene codes for several isoforms in Drosophila. J. Neurosci. 1996, 16, 3154–3165. [Google Scholar] [CrossRef]
- Godenschwege, T.A.; Reisch, D.; Diegelmann, S.; Eberle, K.; Funk, N.; Heisenberg, M.; Hoppe, V.; Hoppe, J.; Klagges, B.R.E.; Martin, J.R.; et al. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur. J. Neurosci. 2004, 20, 611–622. [Google Scholar] [CrossRef]
- Michels, B.; Diegelmann, S.; Tanimoto, H.; Schwenkert, I.; Buchner, E.; Gerber, B. A role for Synapsin in associative learning: The Drosophila larva as a study case. Learn Mem. 2005, 12, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Vasin, A.; Zueva, L.; Torrez, C.; Volfson, D.; Littleton, J.T.; Bykhovskaia, M. Synapsin regulates activity-dependent outgrowth of synaptic boutons at the Drosophila neuromuscular junction. J. Neurosci. 2014, 34, 10554–10563. [Google Scholar] [CrossRef] [PubMed]
- Gitler, D.; Takagishi, Y.; Feng, J.; Ren, Y.; Rodriguiz, R.M.; Wetsel, W.C.; Greengard, P.; Augustine, G.J. Different presynaptic roles of Synapsins at excitatory and inhibitory synapses. J. Neurosci. 2004, 24, 11368–11380. [Google Scholar] [CrossRef] [PubMed]
- Rosahl, T.W.; Spillane, D.; Missler, M.; Herz, J.; Selig, D.K.; Wolff, J.R.; Hammer, R.E.; Malenka, R.C.; Sudhof, T.C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 1995, 375, 488–493. [Google Scholar] [CrossRef]
- Akbergenova, Y.; Bykhovskaia, M. Synapsin regulates vesicle organization and activity-dependent recycling at Drosophila motor boutons. Neuroscience 2010, 170, 441–452. [Google Scholar] [CrossRef]
- Winther, A.M.; Vorontsova, O.; Rees, K.A.; Nareoja, T.; Sopova, E.; Jiao, W.; Shupliakov, O. An endocytic scaffolding protein together with synapsin regulates synaptic vesicle clustering in the Drosophila neuromuscular junction. J. Neurosci. 2015, 35, 14756–14770. [Google Scholar] [CrossRef]
- Koh, T.W.; Korolchuk, V.I.; Wairkar, Y.P.; Jiao, W.; Evergren, E.; Pan, H.; Zhou, Y.; Venken, K.J.; Shupliakov, O.; Robinson, I.M.; et al. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J. Cell Biol. 2007, 178, 309–322. [Google Scholar] [CrossRef]
- Koh, T.W.; Verstreken, P.; Bellen, H.J. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron 2004, 43, 193–205. [Google Scholar] [CrossRef]
- Marie, B.; Sweeney, S.T.; Poskanzer, K.E.; Roos, J.; Kelly, R.B.; Davis, G.W. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron 2004, 43, 207–219. [Google Scholar] [CrossRef]
- Wang, S.W.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I Is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when eeleased via glia-derived exosomes. J. Neurosci. 2011, 31, 7275–7290. [Google Scholar] [CrossRef]
- Barbieri, R.; Contestabile, A.; Ciardo, M.G.; Forte, N.; Marte, A.; Baldelli, P.; Benfenati, F.; Onofri, F. Synapsin I and Synapsin II regulate neurogenesis in the dentate gyrus of adult mice. Oncotarget 2018, 9, 18760–18774. [Google Scholar] [CrossRef] [PubMed]
- Corradi, A.; Zanardi, A.; Giacomini, C.; Onofri, F.; Valtorta, F.; Zoli, M.; Benfenati, F. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J. Cell Sci. 2008, 121, 3042–3051. [Google Scholar] [CrossRef][Green Version]
- Cartereau, A.; Taillebois, E.; Le Questel, J.Y.; Thany, S.H. Mode of action of neonicotinoid insecticides imidacloprid and thiacloprid to the cockroach Pamealpha7 nicotinic acetylcholine receptor. Int. J. Mol. Sci. 2021, 22, 9880. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.; Lapied, B.; Corronc, H.; Sattelle, F. Imidacloprid actions on insect neuronal acetylcholine receptors. J. Exp. Biol. 1997, 200, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Thany, S.H. Agonist actions of clothianidin on synaptic and extrasynaptic nicotinic acetylcholine receptors expressed on cockroach sixth abdominal ganglion. Neurotoxicology 2009, 30, 1045–1052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavares, D.A.; Roat, T.C.; Silva-Zacarin, E.C.M.; Nocelli, R.C.F.; Malaspina, O. Exposure to thiamethoxam during the larval phase affects synapsin levels in the brain of the honey bee. Ecotoxicol. Environ. Saf. 2019, 169, 523–528. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Q.; Li, J.H.; Chao, Z.J.; Cai, C.; Yin, M.Z.; Du, X.G.; Shen, J. A star polycation acts as a drug nanocarrier to improve the toxicity and persistence of botanical pesticides. ACS Sustain. Chem. Eng. 2019, 7, 17406–17413. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. J. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.; Zhou, J.; Song, W.; Xiao, Y.; Cui, C.; Gao, W.; Ke, F.; Zhu, J.; Gu, Z.; et al. Delivery of acetamiprid to tea leaves enabled by porous silica nanoparticles: Efficiency, distribution and metabolism of acetamiprid in tea plants. BMC Plant Biol. 2021, 21, 337. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, M.; Chao, Z.; Du, X.; Yan, S.; Shen, J. A first greenhouse application of bacteria-expressed and nanocarrier-delivered RNA pesticide for Myzus persicae control. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Li, J.H.; Qian, J.; Xu, Y.Y.; Yan, S.; Shen, J.; Yin, M.Z. A Facile-Synthesized Star Polycation Constructed as a Highly Efficient Gene Vector in Pest Management. ACS Sustain. Chem. Eng. 2019, 7, 6316. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.Z.; Li, J.H.; Yin, M.Z.; Ren, B.Y.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, Z.; Wang, Z.; Yan, S.; Wang, D.; Shen, J. Hedgehog signaling regulates regenerative patterning and growth in Harmonia axyridis leg. Cell. Mol. Life Sci. 2021, 78, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qi, H.; Yang, D.; Yuan, H.; Rui, C. Cycloxaprid: A novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic. Biochem. Physiol. 2016, 132, 96–101. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Wang, S.; Lin, G.; Li, M.; Shen, J.; Wang, D. The Synergistic Effect of Thiamethoxam and Synapsin dsRNA Targets Neurotransmission to Induce Mortality in Aphis gossypii. Int. J. Mol. Sci. 2022, 23, 9388. https://doi.org/10.3390/ijms23169388
Qu X, Wang S, Lin G, Li M, Shen J, Wang D. The Synergistic Effect of Thiamethoxam and Synapsin dsRNA Targets Neurotransmission to Induce Mortality in Aphis gossypii. International Journal of Molecular Sciences. 2022; 23(16):9388. https://doi.org/10.3390/ijms23169388
Chicago/Turabian StyleQu, Xueting, Sijia Wang, Guangze Lin, Mingshan Li, Jie Shen, and Dan Wang. 2022. "The Synergistic Effect of Thiamethoxam and Synapsin dsRNA Targets Neurotransmission to Induce Mortality in Aphis gossypii" International Journal of Molecular Sciences 23, no. 16: 9388. https://doi.org/10.3390/ijms23169388
APA StyleQu, X., Wang, S., Lin, G., Li, M., Shen, J., & Wang, D. (2022). The Synergistic Effect of Thiamethoxam and Synapsin dsRNA Targets Neurotransmission to Induce Mortality in Aphis gossypii. International Journal of Molecular Sciences, 23(16), 9388. https://doi.org/10.3390/ijms23169388





