Involvement of Porcine β-Defensin 129 in Sperm Capacitation and Rescue of Poor Sperm in Genital Tract Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Experimental Design
2.2.1. Experiment 1: Expression and Purification of Recombinant pBD129 Protein, and Preparation of Anti-pBD129 Antibody
2.2.2. Experiment 2: pBD129 in the Sperm and Reproductive System
2.2.3. Experiment 3: Effect of pBD129 on Sperm Function
2.2.4. Experiment 4: Effect of pBD129 on Sperm Intracellular Ca2+
2.2.5. Experiment 5: Effect of pBD129 on In Vitro Fertilization
2.2.6. Experiment 6: Effect of Recombinant pBD129 on Sperm Motility in E. coli
2.3. Preparation of pBD129 Protein and Antibody
2.4. Antimicrobial and Hemolytic Activity Assays
2.5. De-N-Glycosylation of Recombinant pBD129 and Specificity Analysis of Antibodies
2.6. Western Blotting
2.7. Immunohistochemistry
2.8. Indirect Immunofluorescence
2.9. Sperm Motility Assay
2.10. Evaluation of Sperm AR
2.11. Measurement of Sperm Intracellular Ca2+
2.12. In Vitro Fertilization (IVF) Assay
2.13. Statistical Analysis
3. Results
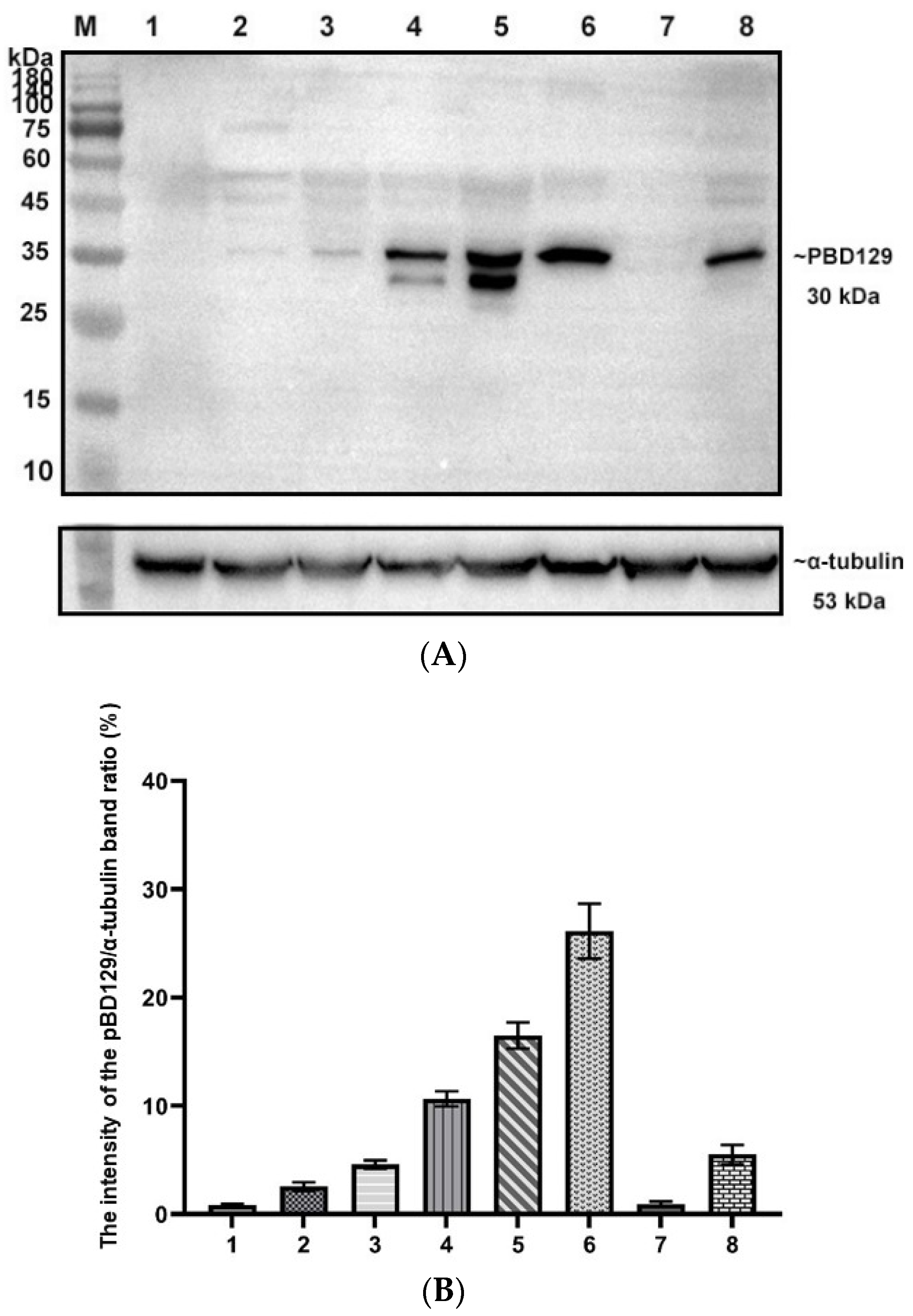
3.1. Expression, Purification, and Characterization of Recombinant pBD129 Protein
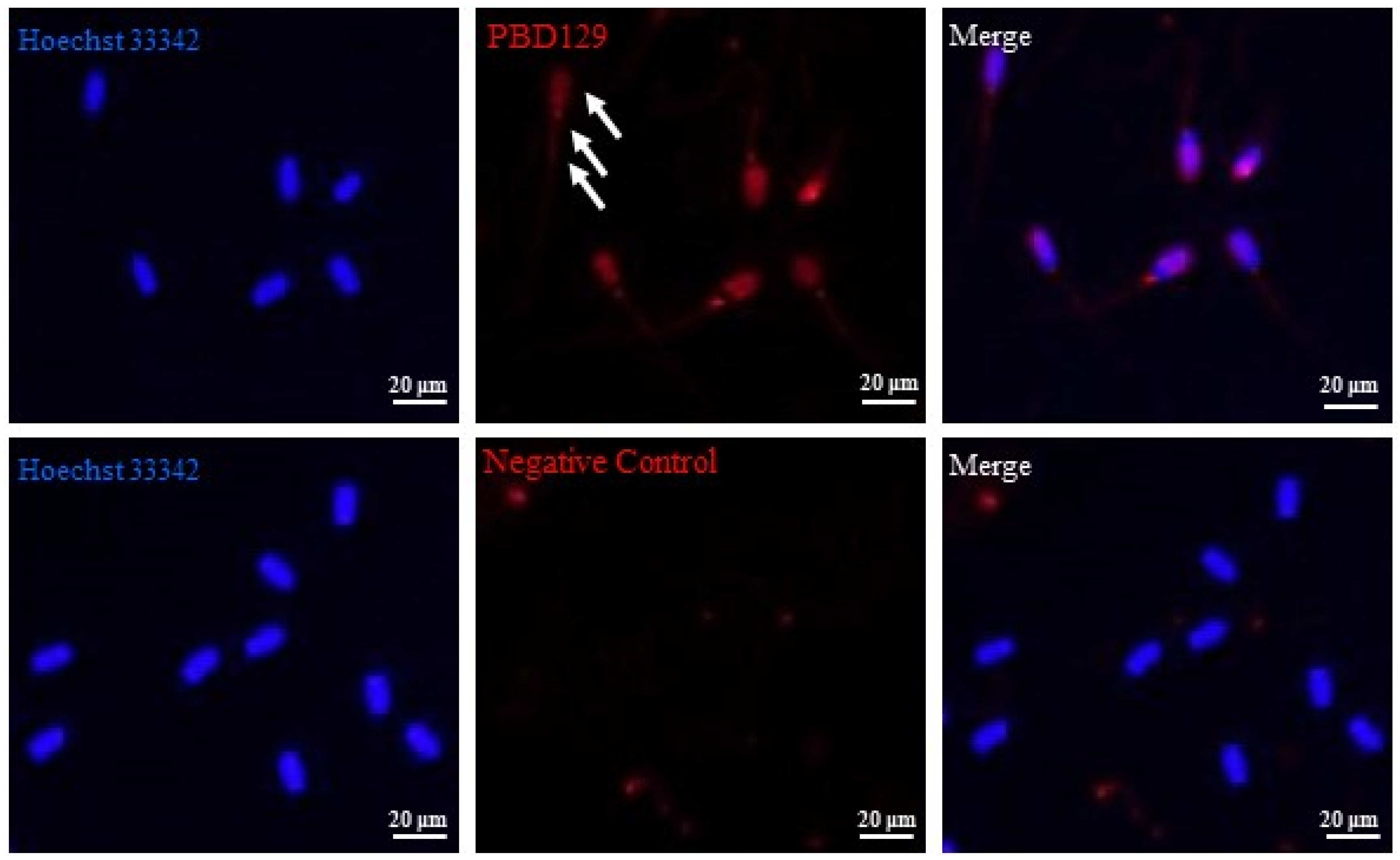
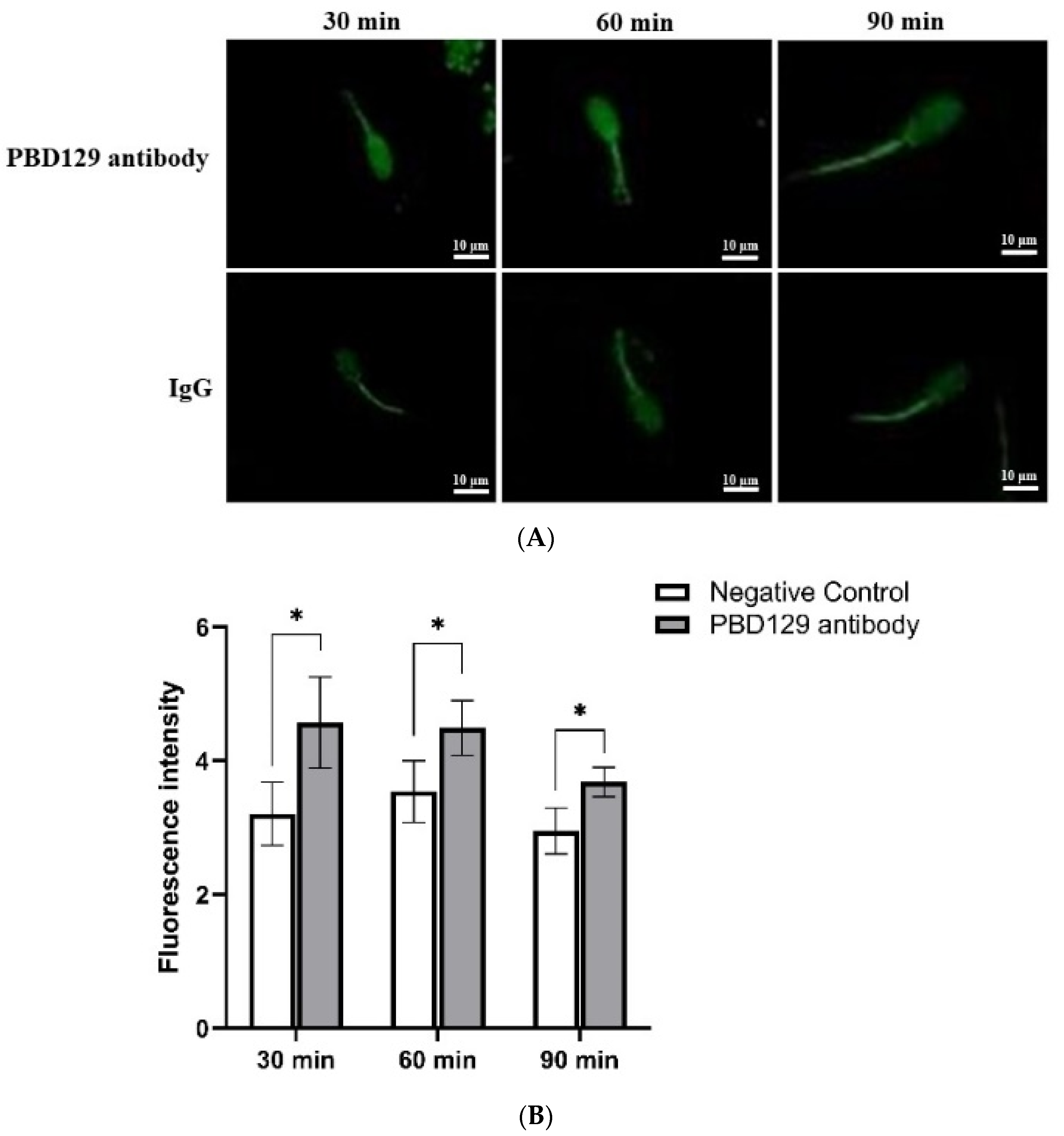
3.2. Localization of pBD129 in Sperm and Reproductive System
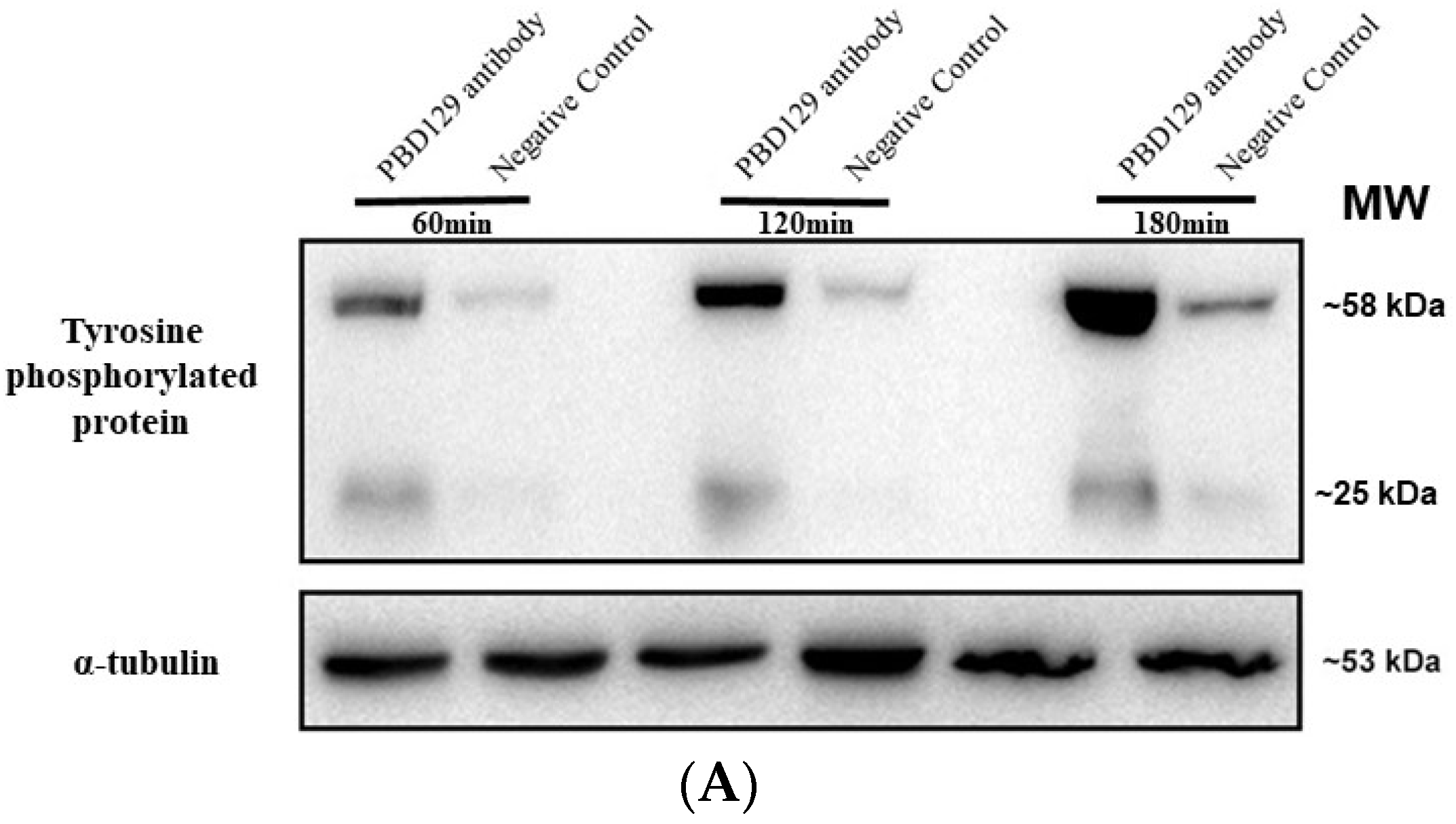
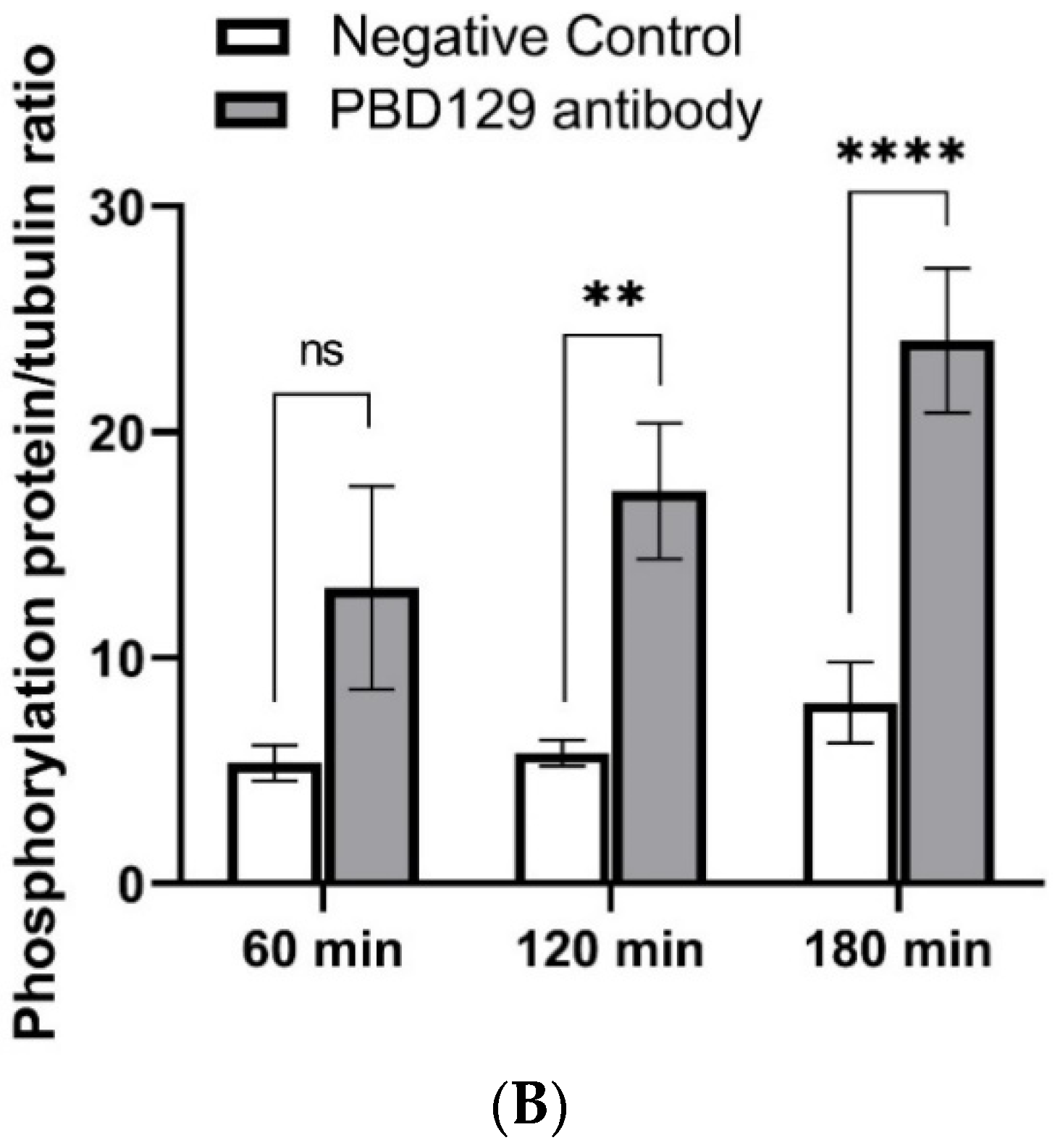
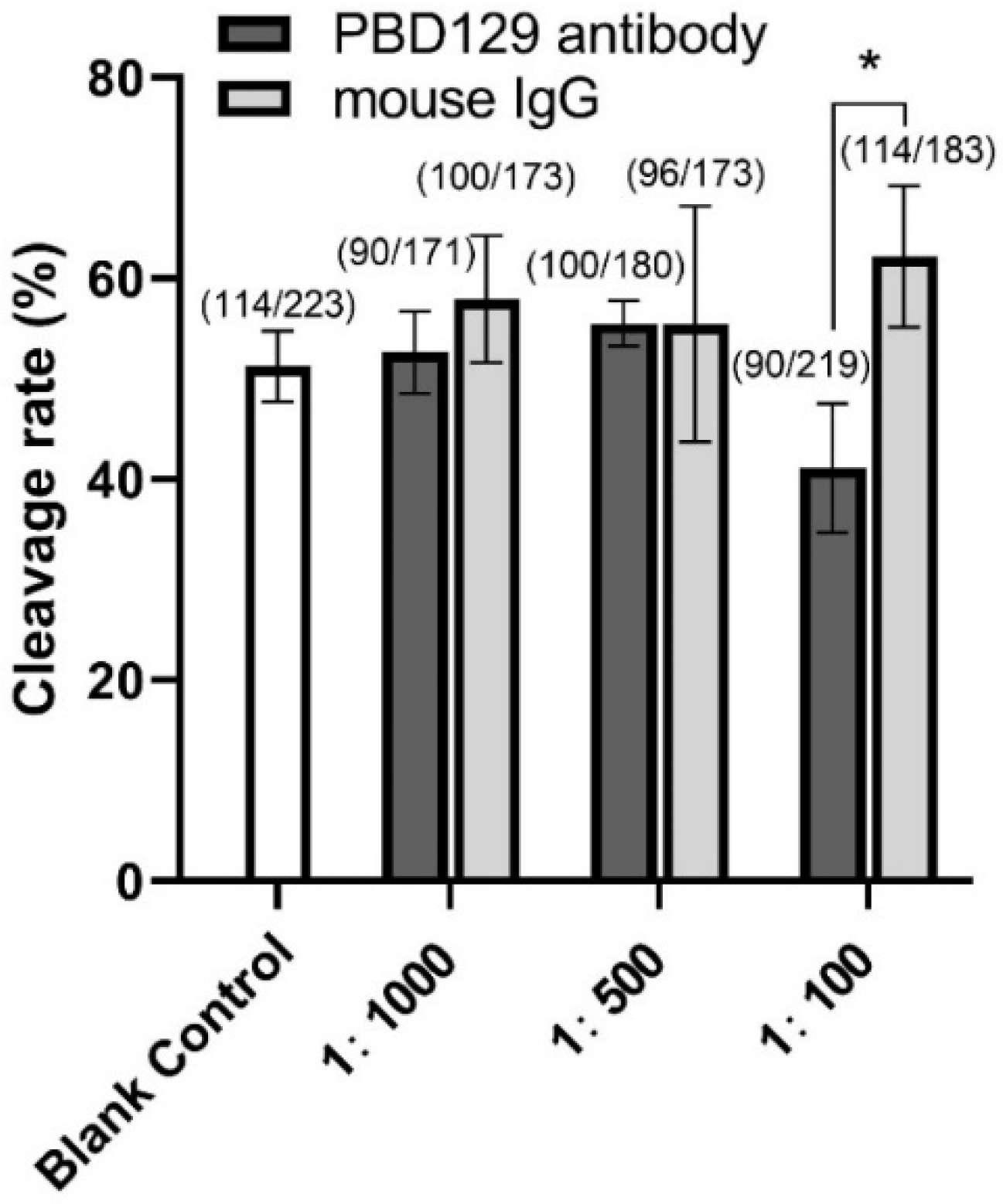
3.3. Blocking pBD129 on Sperm Surface Promotes AR and Tyrosine Phosphorylation but Hinders Cleavage
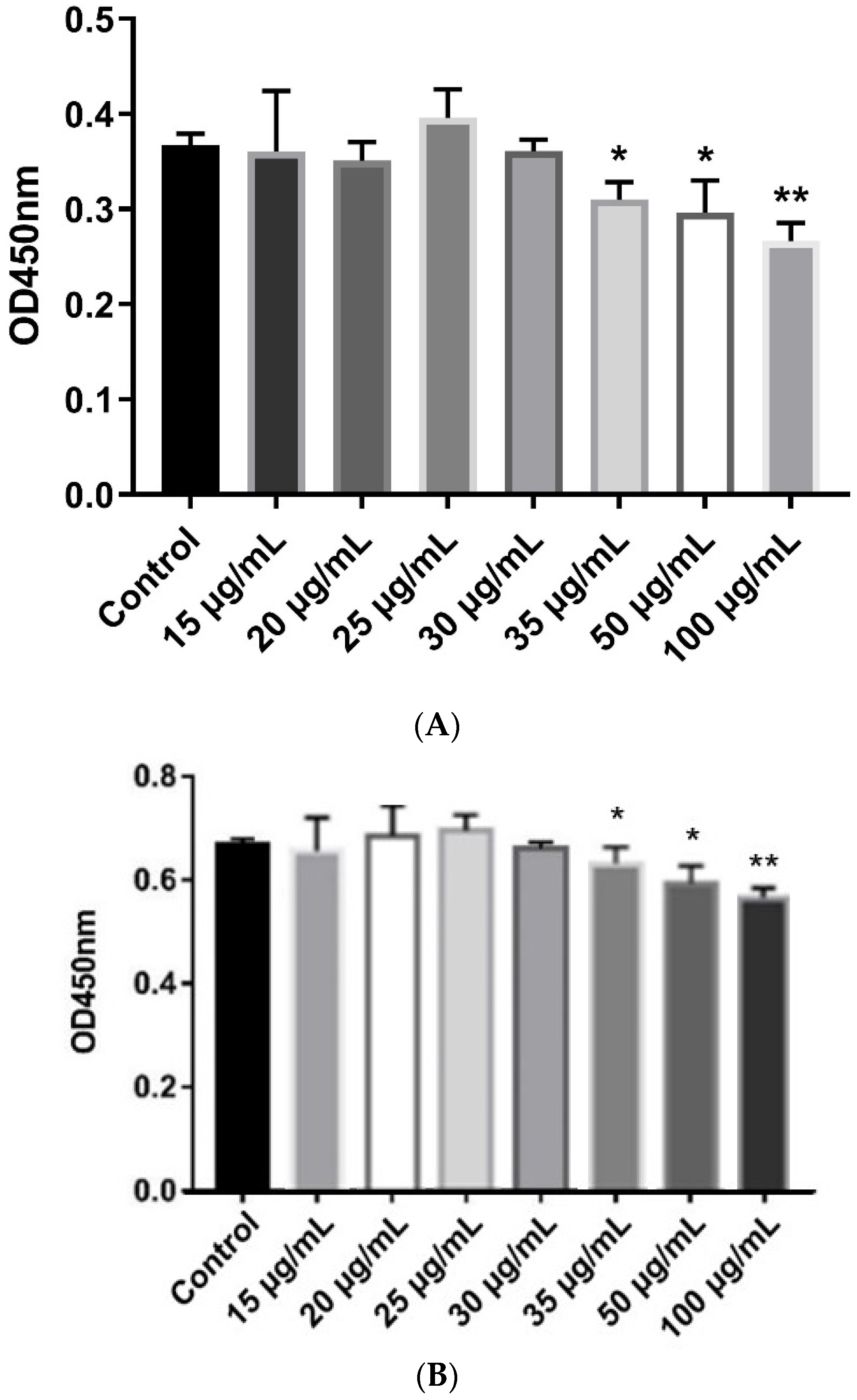
3.4. PBD129 Protein Contributes to Motility and Antibactericidal Activity in the Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef] [Green Version]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Keck, C.; Gerber-Schäfer, C.; Clad, A.; Wilhelm, C.; Breckwoldt, M. Seminal tract infections: Impact on male fertility and treatment options. Hum. Reprod. Update 1998, 4, 891–903. [Google Scholar] [CrossRef]
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, K.; Zhang, D.; Zhao, Z.; Huang, J.; Zhou, L.; Feng, M.; Shi, J.; Wei, H.; Li, L.; et al. GPx6 is involved in the in vitro induced capacitation and acrosome reaction in porcine sperm. Theriogenology 2020, 156, 107–115. [Google Scholar] [CrossRef]
- Baskaran, S.; Panner Selvam, M.K.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhou, Y.; Chen, W.Y.; Zheng, M.; Yu, J.; Li, C.; Zhang, Y.; Shi, Q.X. HongrES1, a cauda epididymis-specific protein, is involved in capacitation of guinea pig sperm. Mol. Reprod. Dev. 2009, 76, 984–993. [Google Scholar] [CrossRef]
- Skerget, S.; Rosenow, M.A.; Petritis, K.; Karr, T.L. Sperm Proteome Maturation in the Mouse Epididymis. PLoS ONE 2015, 10, e0140650. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. Plasma membrane structure and remodelling during sperm maturation in the epididymis. J. Reprod. Fertil. Suppl. 1998, 53, 73–84. [Google Scholar] [PubMed]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Holly, M.K.; Diaz, K.; Smith, J.G. Defensins in Viral Infection and Pathogenesis. Annu. Rev. Virol. 2017, 4, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human beta-defensins. Cell. Mol. Life Sci. 2006, 63, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef] [PubMed]
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional modulators of infection, inflammation and more? J. Innate. Immun. 2012, 4, 337–348. [Google Scholar] [CrossRef]
- Dorin, J.R.; Barratt, C.L. Importance of β-defensins in sperm function. Mol. Hum. Reprod. 2014, 20, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Avellar, M.C.W.; Ribeiro, C.M.; Dias-da-Silva, M.R.; Silva, E.J.R. In search of new paradigms for epididymal health and disease: Innate immunity, inflammatory mediators, and steroid hormones. Andrology 2019, 7, 690–702. [Google Scholar] [CrossRef] [Green Version]
- Diao, R.; Fok, K.L.; Chen, H.; Yu, M.K.; Duan, Y.; Chung, C.M.; Li, Z.; Wu, H.; Li, Z.; Zhang, H.; et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci. Transl. Med. 2014, 6, 249ra108. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Webb, S.; Lettice, L.; Tardif, S.; Kilanowski, F.; Tyrrell, C.; Macpherson, H.; Semple, F.; Tennant, P.; Baker, T.; et al. Partial deletion of chromosome 8 β-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013, 9, e1003826. [Google Scholar] [CrossRef] [PubMed]
- Tollner, T.L.; Bevins, C.L.; Cherr, G.N. Multifunctional glycoprotein DEFB126--a curious story of defensin-clad spermatozoa. Nat. Rev. Urol. 2012, 9, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Patil, A.A.; Zhang, G.; Ross, C.R.; Blecha, F. Bioinformatic and expression analysis of novel porcine β-defensins. Mamm. Genome 2006, 17, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xie, H.; Su, G.; Chen, D.; Yu, B.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; et al. β-Defensin 129 Attenuates Bacterial Endotoxin-Induced Inflammation and Intestinal Epithelial Cell Apoptosis. Front. Immunol. 2019, 10, 2333. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Su, G.; Chen, D.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Luo, Y.; Yan, H.; Li, H.; et al. The immunomodulatory function of the porcine β-defensin 129: Alleviate inflammatory response induced by LPS in IPEC-J2 cells. Int. J. Biol. Macromol. 2021, 188, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Hermo, L.; Chan, P.T.; Cyr, D.G. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol. Reprod. 2008, 78, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Mengal, K.; Yuan, M.; Quansah, E.; Li, P.; Wu, S.; Xu, C.; Yi, C.; Cai, X. Comparative RNA-Seq Analysis of Differentially Expressed Genes in the Epididymides of Yak and Cattleyak. Curr. Genom. 2019, 20, 293–305. [Google Scholar] [CrossRef]
- Batra, V.; Bhushan, V.; Ali, S.A.; Sarwalia, P.; Pal, A.; Karanwal, S.; Solanki, S.; Kumaresan, A.; Kumar, R.; Datta, T.K. Buffalo sperm surface proteome profiling reveals an intricate relationship between innate immunity and reproduction. BMC Genom. 2021, 22, 480. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G.; et al. Expression Pattern of Seminal Plasma Extracellular Vesicle Small RNAs in Boar Semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef]
- Ren, M.; Cai, S.; Zhou, T.; Zhang, S.; Li, S.; Jin, E.; Che, C.; Zeng, X.; Zhang, T.; Qiao, S. Isoleucine attenuates infection induced by E. coli challenge through the modulation of intestinal endogenous antimicrobial peptide expression and the inhibition of the increase in plasma endotoxin and IL-6 in weaned pigs. Food Funct. 2019, 10, 3535–3542. [Google Scholar] [CrossRef]
- Diao, H.; Yu, H.G.; Sun, F.; Zhang, Y.L.; Tanphaichitr, N. Rat recombinant β-defensin 22 is a heparin-binding protein with antimicrobial activity. Asian J. Androl. 2011, 13, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.X.; Guo, J.Y.; Shen, L.; Chen, Y.; Zhang, Z.C.; Zhang, Y.L. Get effective polyclonal antisera in one month. Cell Res. 2002, 12, 157–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Stephenson, C.G.; Iadarola, M.J. Recombinant proteins attached to a nickel-NTA column: Use in affinity purification of antibodies. Biotechniques 1994, 17, 257–262. [Google Scholar] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Yenugu, S.; Hamil, K.G.; Birse, C.E.; Ruben, S.M.; French, F.S.; Hall, S.H. Antibacterial properties of the sperm-binding proteins and peptides of human epididymis 2 (HE2) family; salt sensitivity, structural dependence and their interaction with outer and cytoplasmic membranes of Escherichia coli. Biochem. J. 2003, 372 Pt 2, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Kawano, N.; Araki, N.; Yoshida, K.; Hibino, T.; Ohnami, N.; Makino, M.; Kanai, S.; Hasuwa, H.; Yoshida, M.; Miyado, K.; et al. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc. Natl. Acad. Sci. USA 2014, 111, 4145–4150. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Su, Y.Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Mathialagan, N.; Walters, E.; Mao, J.; Lai, L.; Becker, D.; Li, W.; Critser, J.; Prather, R.S. Osteopontin reduces polyspermy during in vitro fertilization of porcine oocytes. Biol. Reprod. 2006, 75, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Xie, S.; Yin, Q.; Tang, C.; Ni, Z.; Fei, J.; Zhang, Y. CRISPR/Cas9-mediated genome editing reveals the synergistic effects of β-defensin family members on sperm maturation in rat epididymis. FASEB J. 2018, 32, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Nagase, T.; Makita, R.; Fukuhara, S.; Tomita, T.; Tominaga, T.; Kurihara, H.; Ouchi, Y. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J. Immunol. 2002, 169, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chan, H.C.; He, B.; So, S.C.; Chung, Y.W.; Shang, Q.; Zhang, Y.D.; Zhang, Y.L. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001, 291, 1783–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Dong, J.; Gu, Y.; Liu, H.; Xin, A.; Shi, H.; Sun, F.; Zhang, Y.; Lin, D.; Diao, H. The novel human β-defensin 114 regulates lipopolysaccharide (LPS)-mediated inflammation and protects sperm from motility loss. J. Biol. Chem. 2013, 288, 12270–12282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Diao, H.; Ni, Z.; Hu, S.; Yu, H.; Zhang, Y. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell. Mol. Life Sci. 2011, 68, 697–708. [Google Scholar] [CrossRef]




| Motility Parameters | Negative Control | Anti-pBD129 Antibody |
|---|---|---|
| TM (%) | 83.30 ± 2.51 A | 80.07 ± 3.89 A |
| PM (%) | 75.43 ± 5.24 A | 77.17 ± 2.87 A |
| VSL (μm/s) | 52.70 ± 1.21 A | 52.53 ± 2.45 A |
| VCL(μm/s) | 37.17 ± 1.87 A | 36.63 ± 0.98 A |
| VAP (μm/s) | 76.29 ± 3.21 A | 75.90 ± 2.58 A |
| ALH (μm) | 20.89 ± 1.67 A | 21.73 ± 0.53 A |
| WOB (%) | 87.5 ± 0.5 A | 80.5 ± 5.5 A |
| BCF (Hz) | 0.78 ± 0 A | 0.82 ± 0.015 A |
| LIN (%) | 61 ± 1 A | 61.5 ± 0.5 A |
| MAD (°) | 85.09 ± 16.48 A | 68.77 ± 4.56 A |
| STR (%) | 86 ± 1 A | 87 ± 0 A |
| Parameters | PBS | 6.25 μg/mL | 12.5 μg/mL | 25 μg/mL | 50 μg/mL | 100 μg/mL |
|---|---|---|---|---|---|---|
| TM(%) | 69.05 ± 4.28 A | 76.49 ± 3.74 A | 85.18 ± 2.35 B | 79.18 ± 2.93 B | 68.31 ± 2.93 A | 58.76 ± 4.78 B |
| PM (%) | 34.76 ± 2.84 A | 49.3 ± 7.28 B | 56.90 ± 4.28 B | 46.48 ± 2.74 B | 37.60 ± 2.40 A | 36.26 ± 6.68 A |
| VSL (um/s) | 17.41 ± 1.38 A | 18.76 ± 0.92 A | 21.52 ± 2.46 A | 23.29 ± 4.27 A | 29.24 ± 1.85 B | 23.26 ± 0.49 B |
| VCL (um/s) | 33.81 ± 2.83 A | 36.80 ± 4.40 A | 46.57 v 4.78 B | 54.76 ± 7.44 B | 71.62 ± 5.14 B | 58.68 ± 6.77 B |
| VAP (um/s) | 23.90 ± 2.00 A | 26.02 ± 3.10 A | 32.93 ± 3.39 B | 38.74 ± 5.26 B | 50.64 ± 3.64 B | 41.50 ± 4.80 B |
| ALH (um) | 9.90 ± 0.80 A | 10.78 ± 1.28 A | 13.64 ± 1.40 B | 16.04 ± 2.18 B | 20.98 ± 1.50 B | 17.19 ± 1.98 B |
| WOB (%) | 78.00 ± 6.00 A | 84.00 ± 2.00 A | 90.00 ± 2.00 B | 93.00 ± 2.00 B | 93.00 ± 4.00 B | 90.00 ± 5.00 B |
| BCF (Hz) | 1.19 ± 0.06 A | 1.00 ± 0.02 | 0.97 ± 0.12 B | 0.92 ± 0.06 B | 0.67 ± 0.06 B | B 0.66 ± 0.04 B |
| LIN (%) | 52.00 ± 6.00 A | 51.00 ± 4.00 A | 46.00 ± 2.00 A | 42.00 ± 2.00 A | 41.00 ± 5.00 A | 40.00 ± 4.00 B |
| MAD (°) | 70.04 ± 9.77 A | 84.52 ± 2.12 A | 86.70 ± 3.60 A | 110.69 ± 5.41 B | 35.66 ± 2.25 B | 39.37 ± 2.25 B |
| STR (%) | 73.00 ± 9.00 A | 72.00 ± 5.00 A | 65.00 ± 3.00 A | 60.00 ± 4.00 A | 58.00 ± 7.00 A | 57.00 ± 5.00 B |
| OD450 nm | |
|---|---|
| Sperm + LB | 0.363 ± 0.027 A |
| Sperm+ E. coli DH5α + PBS | 0.543 ± 0.047 B |
| Sperm + E. coli DH5α + lgG | 0.534 ± 0.119 B |
| Sperm + E. coli DH5α + anti-pBD129 antibody | 0.748 ± 0.060 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Wang, M.; Li, J.; Li, C.; Pan, X.; Meng, L.; Li, L.; Wei, H.; Zhang, S. Involvement of Porcine β-Defensin 129 in Sperm Capacitation and Rescue of Poor Sperm in Genital Tract Infection. Int. J. Mol. Sci. 2022, 23, 9441. https://doi.org/10.3390/ijms23169441
Zeng F, Wang M, Li J, Li C, Pan X, Meng L, Li L, Wei H, Zhang S. Involvement of Porcine β-Defensin 129 in Sperm Capacitation and Rescue of Poor Sperm in Genital Tract Infection. International Journal of Molecular Sciences. 2022; 23(16):9441. https://doi.org/10.3390/ijms23169441
Chicago/Turabian StyleZeng, Fanwen, Mingming Wang, Ju Li, Chengde Li, Xueqing Pan, Li Meng, Li Li, Hengxi Wei, and Shouquan Zhang. 2022. "Involvement of Porcine β-Defensin 129 in Sperm Capacitation and Rescue of Poor Sperm in Genital Tract Infection" International Journal of Molecular Sciences 23, no. 16: 9441. https://doi.org/10.3390/ijms23169441
APA StyleZeng, F., Wang, M., Li, J., Li, C., Pan, X., Meng, L., Li, L., Wei, H., & Zhang, S. (2022). Involvement of Porcine β-Defensin 129 in Sperm Capacitation and Rescue of Poor Sperm in Genital Tract Infection. International Journal of Molecular Sciences, 23(16), 9441. https://doi.org/10.3390/ijms23169441






