Histology of Cerebral Clots in Cryptogenic Stroke Varies According to the Presence of a Patent Foramen Ovale
Abstract
:1. Introduction
2. Results
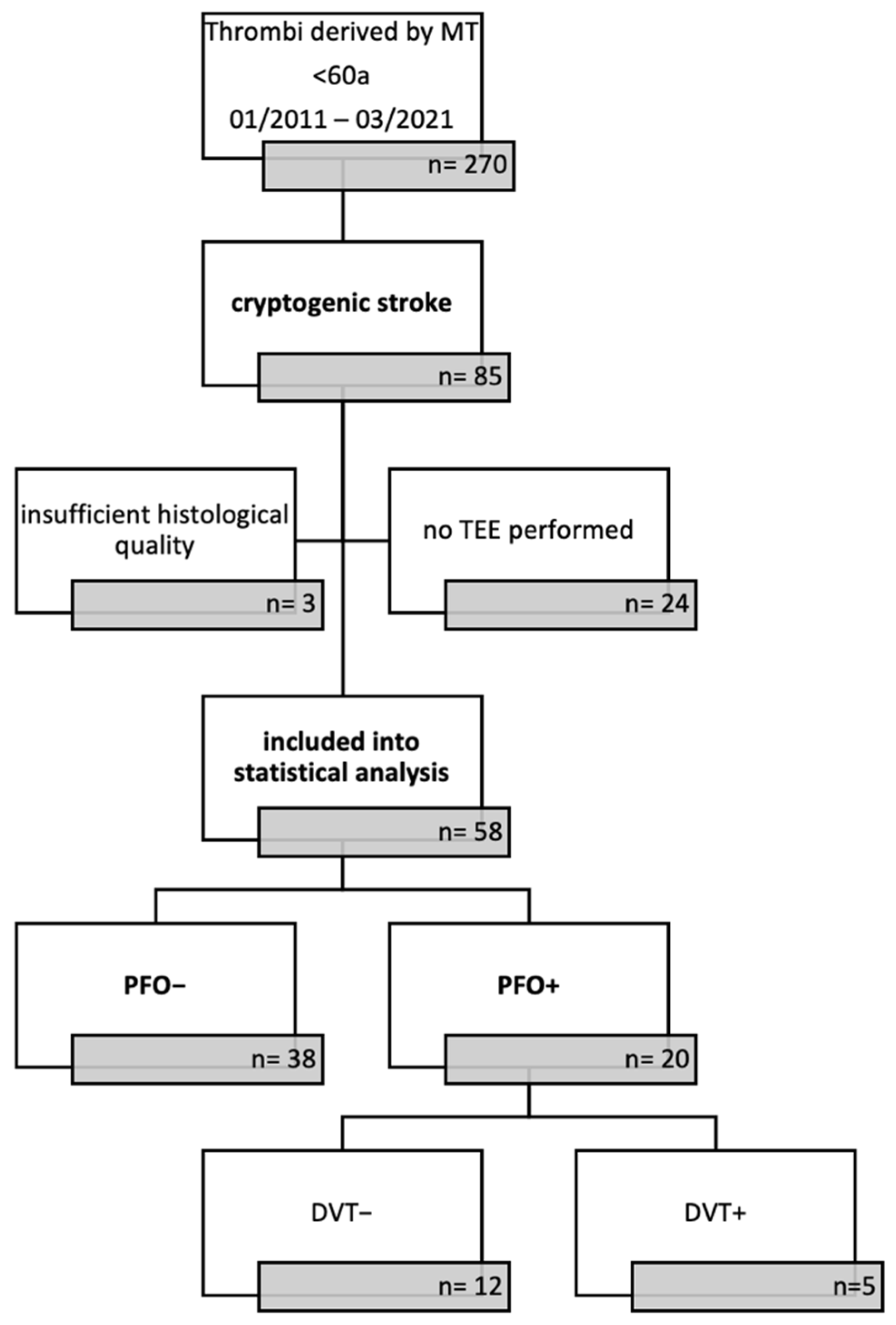
2.1. Patient Cohort
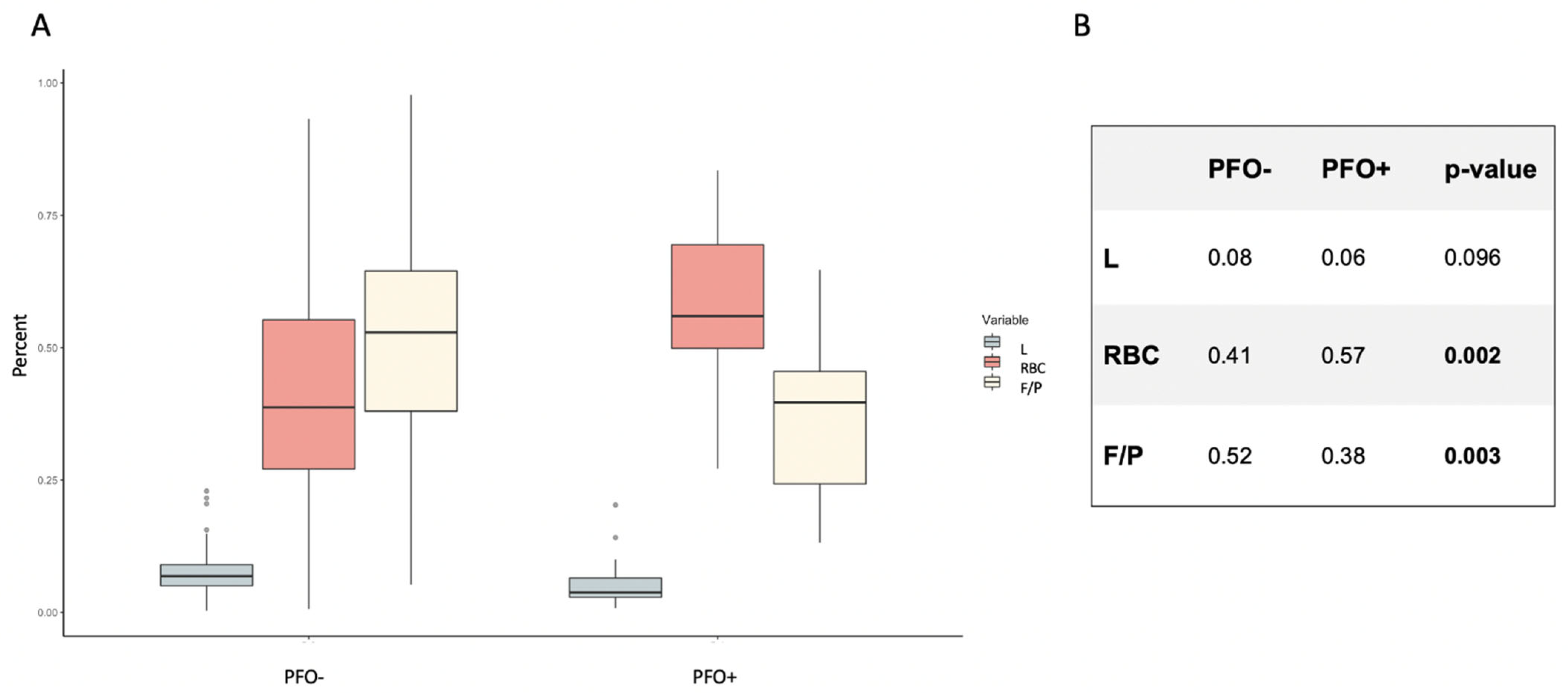
2.2. Histological Clot Composition According to PFO Status
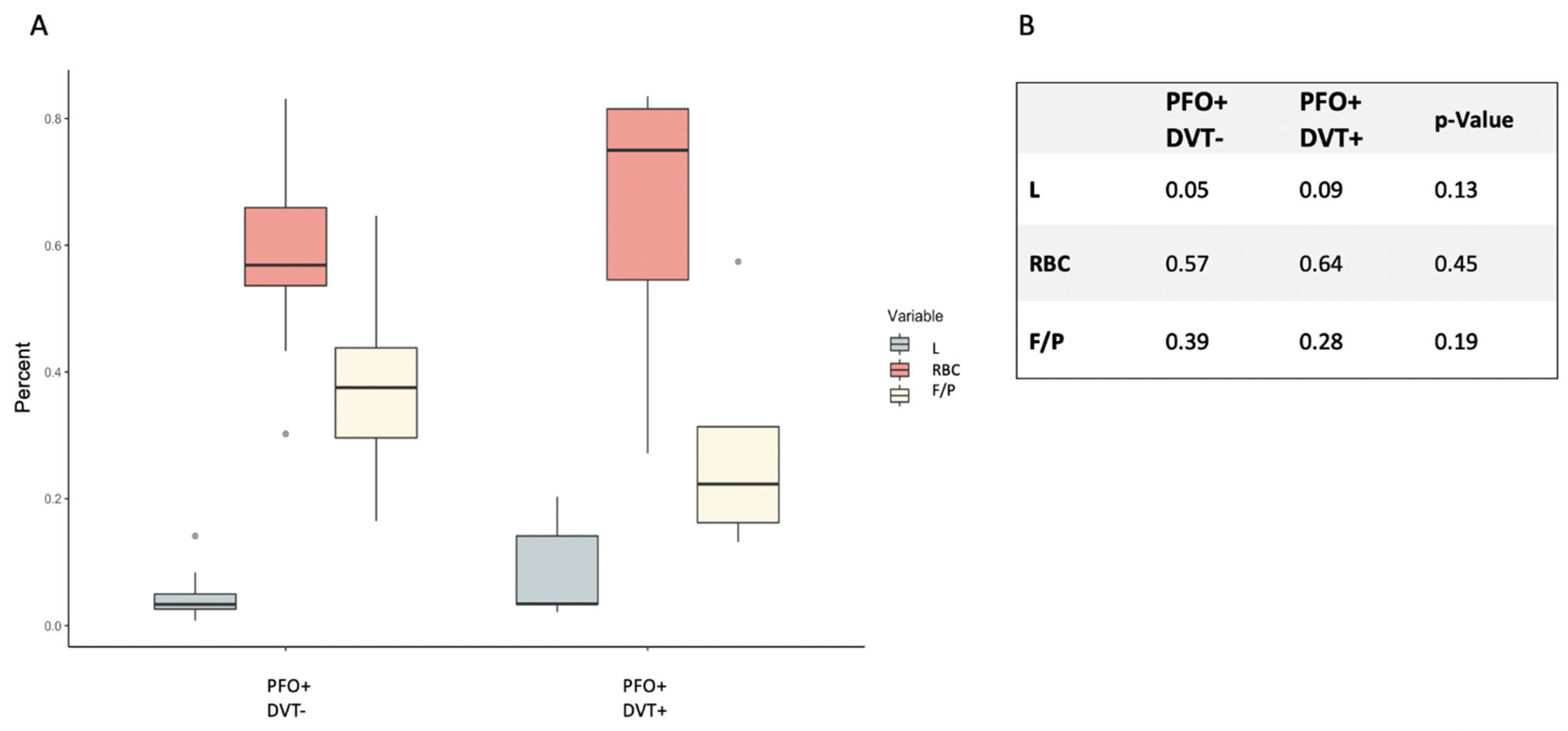
2.3. Histological Clot Composition in the PFO+ Patients with Respect to Proven DVT
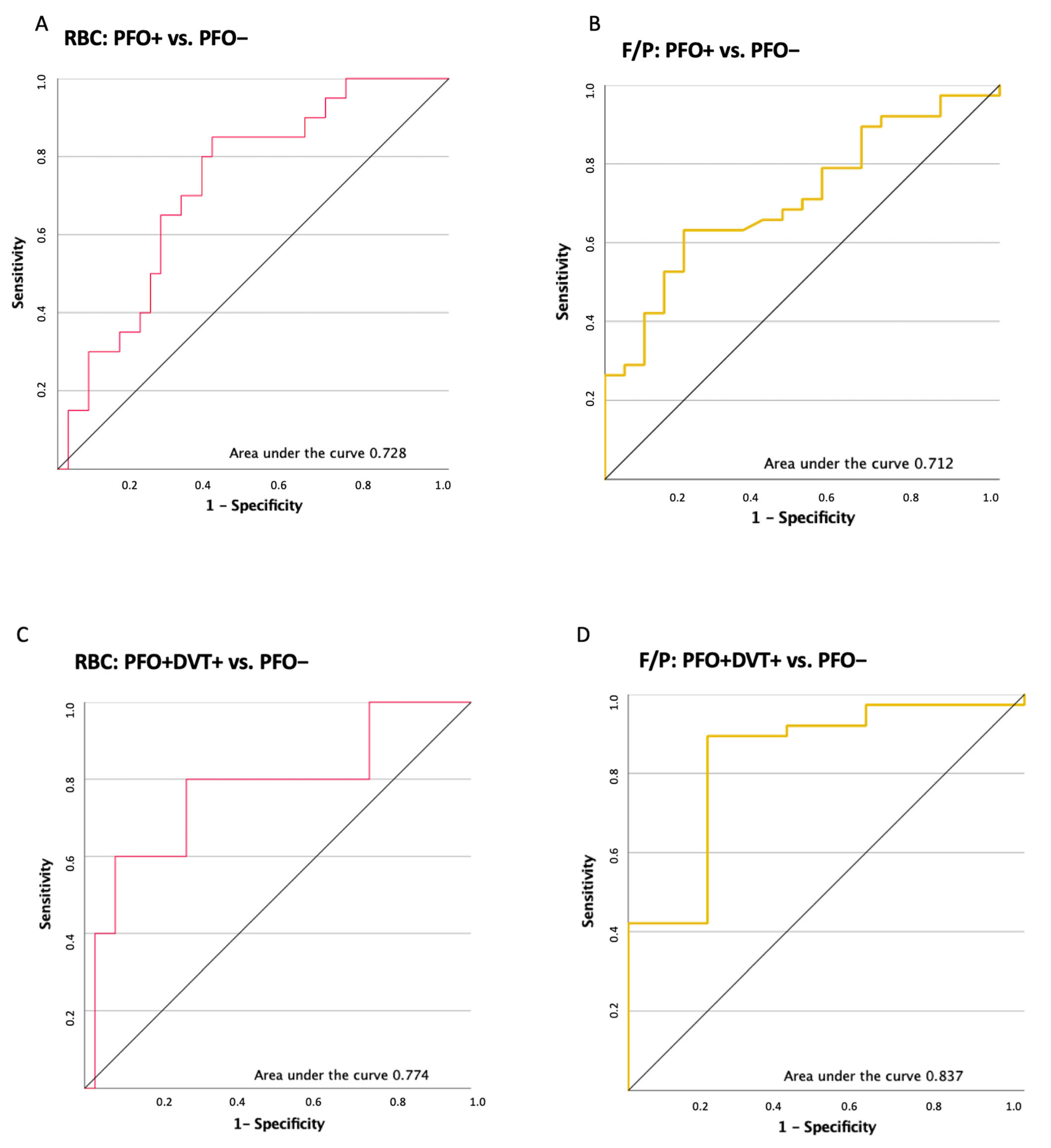
2.4. ROC Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Standard Treatment Protocol
4.3. Histologic Work-Up
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, C.H.; Chen, C.-H.; Lin, Y.-H.; Lee, C.-W.; Tsai, L.-K.; Tang, S.-C.; Shun, C.-T.; Jeng, J.-S. Fibrin and Platelet-Rich Composition in Retrieved Thrombi Hallmarks Stroke with Active Cancer. Stroke 2020, 51, 3723–3727. [Google Scholar] [CrossRef] [PubMed]
- Goebel, J.; Gaida, B.J.; Wanke, I.; Kleinschnitz, C.; Koehrmann, M.; Forsting, M.; Moenninghoff, C.; Radbruch, A.; Junker, A. Is Histologic Thrombus Composition in Acute Stroke Linked to Stroke Etiology or to Interventional Parameters? AJNR Am. J. Neuroradiol. 2020, 41, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Sporns, P.B.; Hanning, U.; Schwindt, W.; Velasco, A.; Minnerup, J.; Zoubi, T.; Heindel, W.; Jeibmann, A.; Niederstadt, T.U. Ischemic Stroke: What Does the Histological Composition Tell Us about the Origin of the Thrombus? Stroke 2017, 48, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.; Dai, D.; Wang, S.; Douglas, A.; Kadirvel, R.; Layton, K.F.; Thacker, I.C.; Gounis, M.J.; Chueh, J.Y.; Puri, A.S.; et al. Platelet-Rich Emboli in Cerebral Large Vessel Occlusion Are Associated with a Large Artery Atherosclerosis Source. Stroke 2019, 50, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Simons, N.; Mitchell, P.; Dowling, R.; Gonzales, M.; Yan, B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. J. Neuroradiol. 2015, 42, 86–92. [Google Scholar] [CrossRef]
- Brinjikji, W.; Nogueira, R.G.; Kvamme, P.; Layton, K.F.; Almandoz, J.E.D.; Hanel, R.A.; Pereira, V.M.; Almekhlafi, M.A.; Yoo, A.J.; Jahromi, B.S.; et al. Association between clot composition and stroke origin in mechanical thrombectomy patients: Analysis of the Stroke Thromboembolism Registry of Imaging and Pathology. J. NeuroInterv. Surg. 2021, 13, 594. [Google Scholar] [CrossRef]
- Boeckh-Behrens, T.; Kleine, J.F.; Zimmer, C.; Neff, F.; Scheipl, F.; Pelisek, J.; Schirmer, L.; Nguyen, K.; Karatas, D.; Poppert, H. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke 2016, 47, 1864–1871. [Google Scholar] [CrossRef] [Green Version]
- Boeckh-Behrens, T.; Schubert, M.; Förschler, A.; Prothmann, S.; Kreiser, K.; Zimmer, C.; Riegger, J.; Bauer, J.; Neff, F.; Kehl, V.; et al. The Impact of Histological Clot Composition in Embolic Stroke. Clin. Neuroradiol. 2016, 26, 189–197. [Google Scholar] [CrossRef]
- Lechat, P.; Mas, J.; Lascault, G.; Loron, P.; Theard, M.; Klimczac, M.; Drobinski, G.; Thomas, D.; Grosgogeat, Y. Prevalence of Patent Foramen Ovale in Patients with Stroke. N. Engl. J. Med. 1988, 318, 1148–1152. [Google Scholar] [CrossRef]
- Windecker, S.; Stortecky, S.; Meier, B. Paradoxical embolism. J. Am. Coll. Cardiol. 2014, 64, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Søndergaard, L.; Kasner, S.E.; Rhodes, J.F.; Andersen, G.; Iversen, H.K.; Nielsen-Kudsk, J.E.; Settergren, M.; Sjöstrand, C.; Roine, R.O.; Hildick-Smith, D.; et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N. Engl. J. Med. 2017, 377, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kasner, S.E.; Rhodes, J.F.; Andersen, G.; Iversen, H.K.; Nielsen-Kudsk, J.E.; Settergren, M.; Sjöstrand, C.; Roine, R.O.; Hildick-Smith, D.; Spence, J.D.; et al. Five-Year Outcomes of PFO Closure or Antiplatelet Therapy for Cryptogenic Stroke. N. Engl. J. Med. 2021, 384, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Carroll, J.D.; Thaler, D.E.; Smalling, R.W.; MacDonald, L.A.; Marks, D.S.; Tirschwell, D.L. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N. Engl. J. Med. 2017, 377, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Song, J.K.; Kim, J.S.; Heo, R.; Lee, S.; Kim, D.H.; Song, J.M.; Kang, D.H.; Kwon, S.U.; Kang, D.W.; et al. Cryptogenic Stroke and High-Risk Patent Foramen Ovale: The DEFENSE-PFO Trial. J. Am. Coll. Cardiol. 2018, 71, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Yuriditsky, E.; Narula, N.; Jacobowitz, G.R.; Moreira, A.L.; Maldonado, T.S.; Horowitz, J.M.; Sadek, M.; Barfield, M.E.; Rockman, C.B.; Garg, K.; et al. Histologic assessment of lower extremity deep vein thrombus from patients undergoing percutaneous mechanical thrombectomy. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 18–25. [Google Scholar] [CrossRef]
- Essig, F.; Kollikowski, A.M.; Pham, M.; Solymosi, L.; Stoll, G.; Haeusler, K.G.; Kraft, P.; Schuhmann, M.K. Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7387. [Google Scholar] [CrossRef]
- Saver, J.L.; Mattle, H.P.; Thaler, D. Patent Foramen Ovale Closure versus Medical Therapy for Cryptogenic Ischemic Stroke. Stroke 2018, 49, 1541–1548. [Google Scholar] [CrossRef]
- Sacco, R.L.; Ellenberg, J.H.; Mohr, J.P.; Tatemichi, T.K.; Hier, D.; Price, T.R.; Wolf, P.A. Infarcts of undetermined cause: The NINCDS stroke data bank. Ann. Neurol. 1989, 25, 382–390. [Google Scholar] [CrossRef]
- Diener, H.-C.; Grau, A.J.; Baldus, S.; Ghanem, A.; Gröschel, K.; Liebetrau, C.; Massberg, S.; Möllmann, H.; Nef, H.; Sander, D.; et al. Kryptogener schlaganfall und offenes foramen ovale, S2e-Leitlinie. In Leitlinien für Diagnostik und Therapie in der Neurologie; Deutsche Gesellschaft für Neurologie: Berlin, Germany, 2018; Available online: www.dgn.org/leitlinien (accessed on 18 July 2022).
- Sousa, C.S.; Aguiar de Sousa, D. Patent Foramen Ovale Closure after Cryptogenic Stroke—Assessing the Evidence; European Stroke Organisation: Basel, Switzerland, 2017; Available online: www.eso-stroke.org (accessed on 18 July 2022).
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Morais, L.A.; de Sousa, L.; Fiarresga, A.; Martins, J.D.; Timoteo, A.T.; Monteiro, A.V.; Soares, C.; Agapito, A.; Pinto, F.; Ferreira, R.C. RoPE Score as a Predictor of Recurrent Ischemic Events after Percutaneous Patent Foramen Ovale Closure. Int. Heart J. 2018, 59, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, R.; Takaya, Y.; Akagi, T.; Watanabe, N.; Ikeda, M.; Nakagawa, K.; Toh, N.; Ito, H. Identification of High-Risk Patent Foramen Ovale Associated with Cryptogenic Stroke: Development of a Scoring System. J. Am. Soc. Echocardiogr. 2019, 32, 811–816. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Mereuta, O.M.; Doyle, K.M.; Kallmes, D.F.; Brinjikji, W. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome. J. Neurosurg. Sci. 2019, 63, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Berndt, M.; Poppert, H.; Steiger, K.; Pelisek, J.; Oberdieck, P.; Maegerlein, C.; Zimmer, C.; Wunderlich, S.; Friedrich, B.; Boeckh-Behrens, T.; et al. Thrombus Histology of Basilar Artery Occlusions: Are There Differences to the Anterior Circulation? Clin. Neuroradiol. 2021, 31, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Sohn, H.; Sun, B.J.; Song, J.-K.; Kang, D.-W.; Kim, J.S.; Kwon, S.U. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013, 44, 3350–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendley, S.A.; Dimov, A.; Bhargava, A.; Snoddy, E.; Mansour, D.; Afifi, R.O.; Wool, G.D.; Zha, Y.; Sammet, S.; Lu, Z.F.; et al. Assessment of histological characteristics, imaging markers, and rt-PA susceptibility of ex vivo venous thrombi. Sci. Rep. 2021, 11, 22805. [Google Scholar] [CrossRef]
- Saver, J.L. Cryptogenic Stroke. N. Engl. J. Med. 2016, 374, 2065–2074. [Google Scholar] [CrossRef]
- Schneider, B.; Zienkiewicz, T.; Jansen, V.; Hofmann, T.; Noltenius, H.; Meinertz, T. Diagnosis of patent foramen ovale by transesophageal echocardiography and correlation with autopsy findings. Am. J. Cardiol. 1996, 77, 1202–1209. [Google Scholar] [CrossRef]
- Wells, P.S.; Hirsh, J.; Anderson, D.; Lensing, A.; Foster, G.; Kearon, C.; Weitz, J.; D’Ovidio, R.; Cogo, A.; Prandoni, P.; et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet 1995, 345, 1326–1330. [Google Scholar] [CrossRef]
- Stritt, M.; Stalder, A.K.; Vezzali, E. Orbit Image Analysis: An open-source whole slide image analysis tool. PLoS Comput. Biol. 2020, 16, e1007313. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Wang, S.; Dai, D.; Murphree, D.H., Jr.; Pandit, A.; Douglas, A.; Rizvi, A.; Kadirvel, R.; Gilvarry, M.; McCarthy, R.; et al. Orbit image analysis machine learning software can be used for the histological quantification of acute ischemic stroke blood clots. PLoS ONE 2019, 14, e0225841. [Google Scholar] [CrossRef]

| Total | PFO– | PFO+ | Comparison p-Value | |
|---|---|---|---|---|
| Number of patients | 58 | 38 | 20 | |
| Age Median Mean Minimum | 47.8 50.0 21 | 48.7 50.0 21 | 50.5 45.9 22 | 0.68 |
| Gender Female Male | 30/51.7% 28/48.3% | 20/52.6% 18/47.4% | 12/60.0% 8/40.0% | 0.78 |
| Arterial hypertension | 15/25.9% | 13/34.2% | 2/10.0% | 0.06 |
| Diabetes mellitus | 6/10.3% | 6/15.8% | 0/0% | 0.08 |
| Regular cigarette smoking | 15/25.9% | 13/34.2% | 2/10.0% | 0.06 |
| Risk for venous thrombosis Glucocorticoid therapy Immobilization * Hypercoagulopathy ** Active Malignancy | 13/22.4 % | 3/7.9% 1/2.6% 2/5.3% | 10/50.0% 2/10.0% 4/20.0% 3/15.0% 1/5.0% | <0.001 |
| Localization of occlusion Anterior circ. Posterior circ. | 53/91.4% 5/8.6% | 35/92.1% 3/7.9% | 18/90.0% 2/10.0% | 1.0 |
| Prior vasoactive treatment None apt oac | 47 10 1 | 32 5 1 | 15 5 - | 0.54 |
| Median NIHSS at admission | 13 | 14 | 12 | 0.79 |
| Bridging Thrombolysis | 36/62.0% | 27/71.1% | 9/45.0% | 0.09 |
| MT mTICI >2b | 47/81.0% | 30/78.9% | 17/85.0% | 0.73 |
| Mean time to MT (min) | 380 | 386 | 368 | 0.81 |
| mRS at discharge 0–2 3–6 | 34/58.6% 24/41.4% | 21/55.3% 17/44.7% | 13/65.0% 7/35.0% | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Härtl, J.; Berndt, M.; Poppert, H.; Liesche-Starnecker, F.; Steiger, K.; Wunderlich, S.; Boeckh-Behrens, T.; Ikenberg, B. Histology of Cerebral Clots in Cryptogenic Stroke Varies According to the Presence of a Patent Foramen Ovale. Int. J. Mol. Sci. 2022, 23, 9474. https://doi.org/10.3390/ijms23169474
Härtl J, Berndt M, Poppert H, Liesche-Starnecker F, Steiger K, Wunderlich S, Boeckh-Behrens T, Ikenberg B. Histology of Cerebral Clots in Cryptogenic Stroke Varies According to the Presence of a Patent Foramen Ovale. International Journal of Molecular Sciences. 2022; 23(16):9474. https://doi.org/10.3390/ijms23169474
Chicago/Turabian StyleHärtl, Johanna, Maria Berndt, Holger Poppert, Friederike Liesche-Starnecker, Katja Steiger, Silke Wunderlich, Tobias Boeckh-Behrens, and Benno Ikenberg. 2022. "Histology of Cerebral Clots in Cryptogenic Stroke Varies According to the Presence of a Patent Foramen Ovale" International Journal of Molecular Sciences 23, no. 16: 9474. https://doi.org/10.3390/ijms23169474
APA StyleHärtl, J., Berndt, M., Poppert, H., Liesche-Starnecker, F., Steiger, K., Wunderlich, S., Boeckh-Behrens, T., & Ikenberg, B. (2022). Histology of Cerebral Clots in Cryptogenic Stroke Varies According to the Presence of a Patent Foramen Ovale. International Journal of Molecular Sciences, 23(16), 9474. https://doi.org/10.3390/ijms23169474







