Performance Analysis Based on Sustainability Exergy Indicators of High-Temperature Proton Exchange Membrane Fuel Cell
Abstract
:1. Introduction
2. Results and Analysis
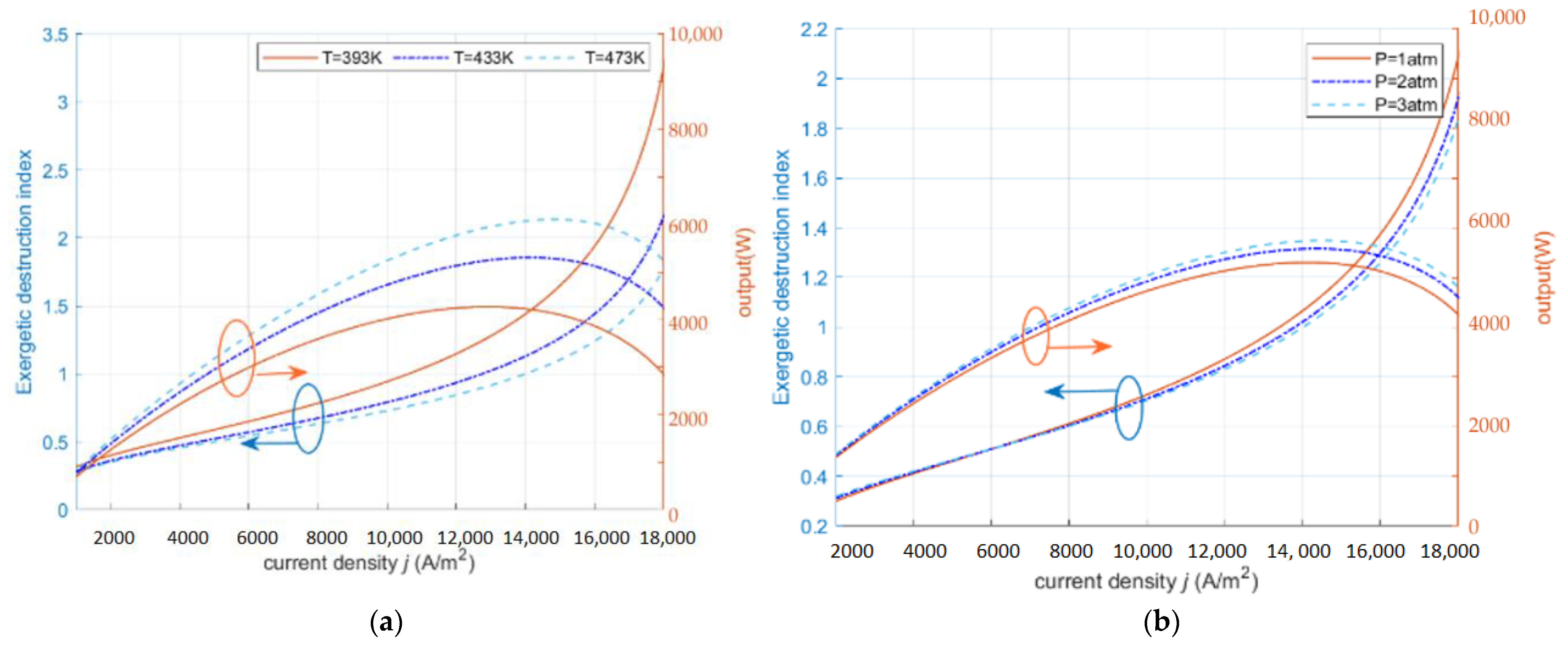
2.1. Exergy Destruction Index ()
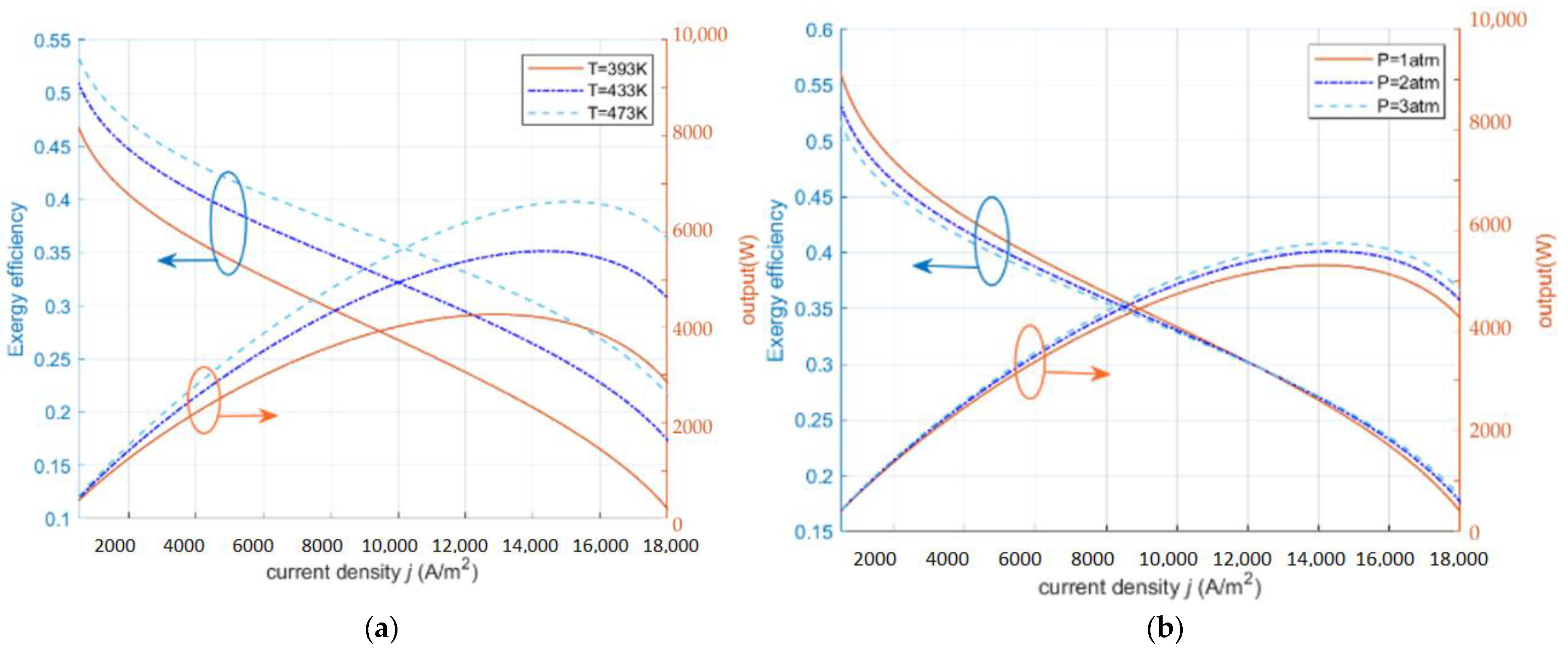
2.2. Exergy Sustainability Index ()
2.3. Exergy Sustainability Indicators
3. Thermodynamic Model
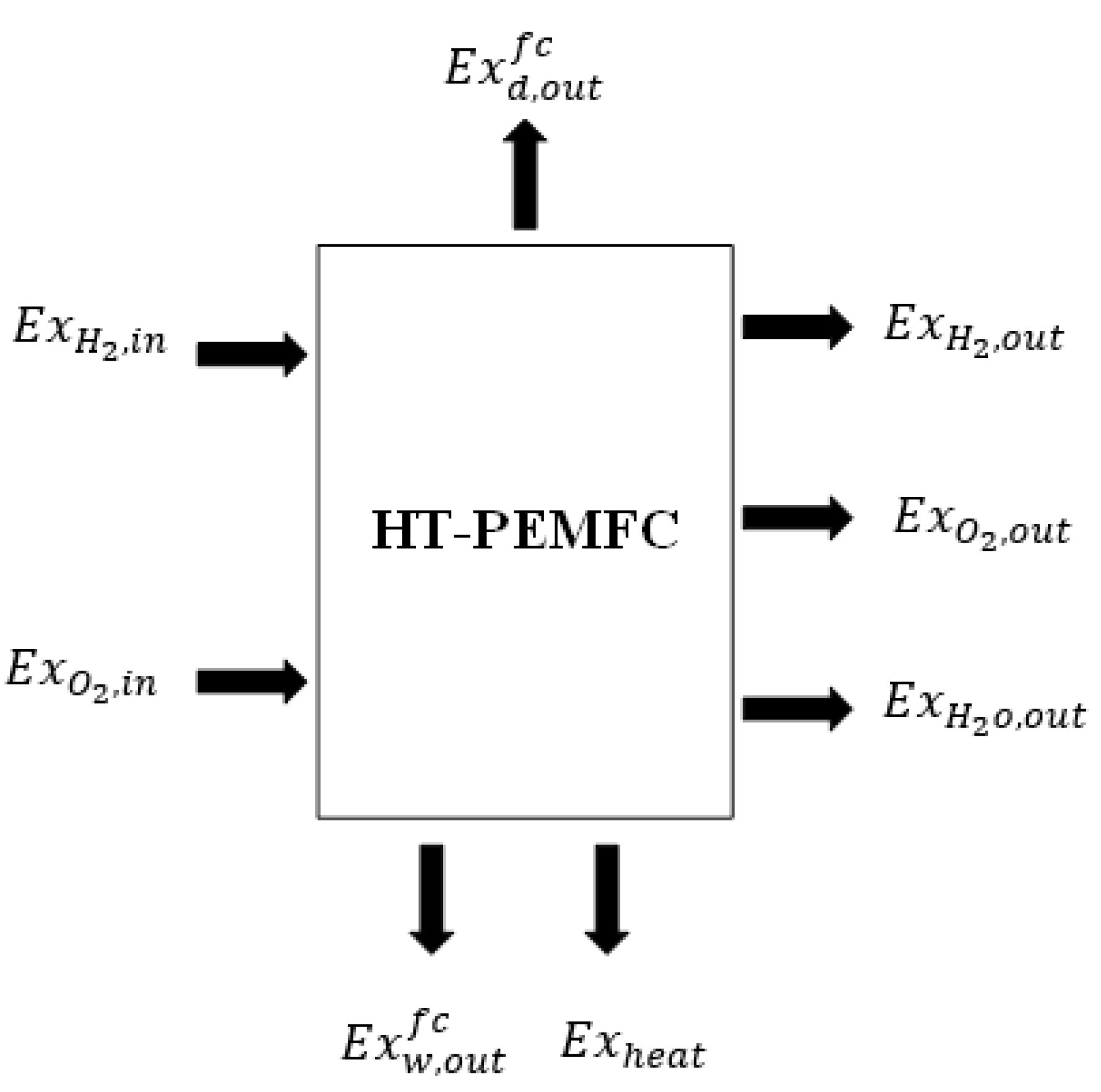
3.1. Working Principle of HT-PEMFC
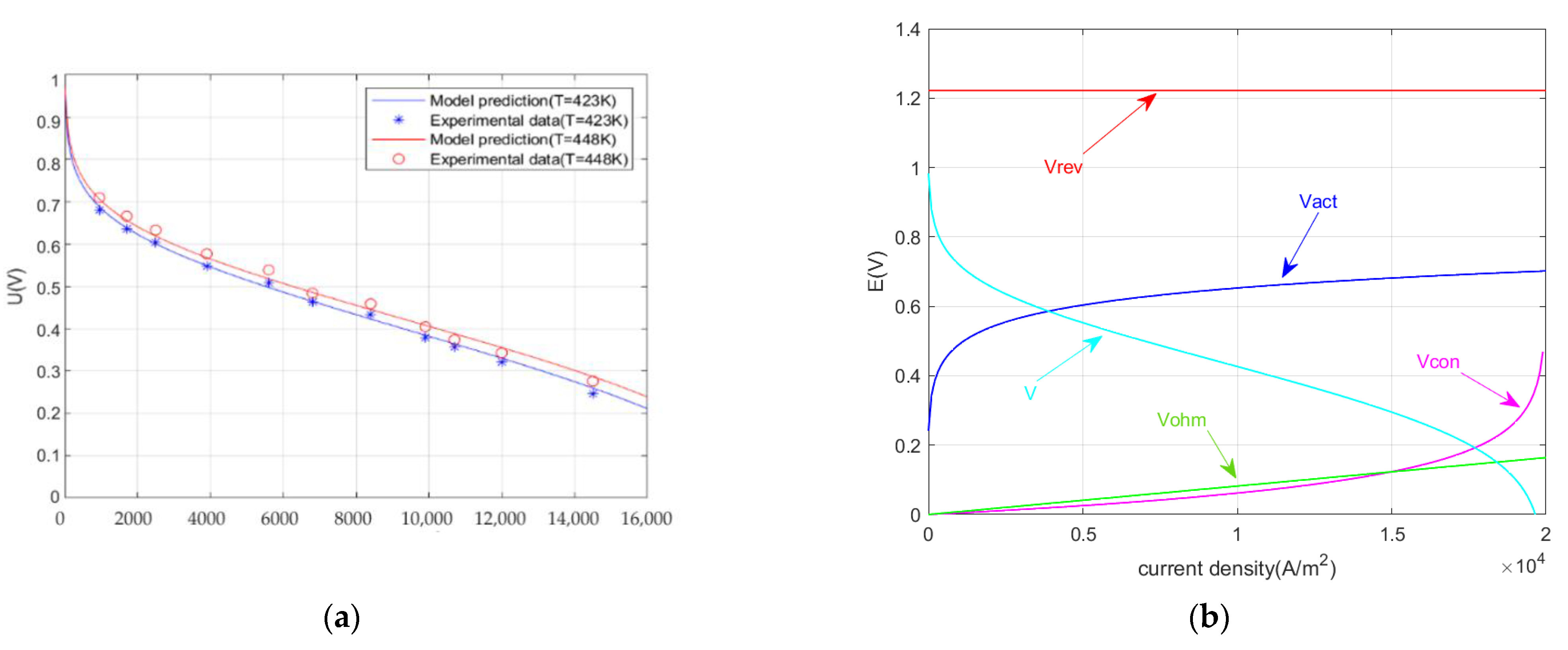
3.2. Reversible Potential of HT-PEMFC
- The fuel cell operates in a steady state.
- The reactants are ideally compressible gases and there are no reactants left after the full reaction.
- The operating pressure and relative humidity are constant during the reaction.
- The effect of the generated water on the relative humidity is not considered.
- Only physical and chemical exergy is considered, no potential exergy and kinetic exergy are considered.
- Leakage current loss and polarization loss are considered.
3.3. Overpotential of HT-PEMFC
3.4. Exergy Balance Model
3.5. HT-PEMFC Exergy Sustainability Index Derivation Process
4. Conclusions
- The reliability of the HT-PEMFC model is proved by comparing the model with the experimental data. Through the parameterization studies, the suitable increase of temperature is beneficial to the improvement of HT-PEMFC output performance. With the decrease of the plasmonic membrane thickness, the output performance is improved.
- At low exergy efficiency, the output power of HT-PEMFC takes the maximum value and starts to decrease when the exergy efficiency exceeds 0.27.
- The operating conditions of HT-PEMFC can be improved by increasing the inlet pressure, changing the diffusion rate of the gas, appropriately increasing the operating temperature, and using thin proton exchange membranes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.G. Researches on Green Features and Category Architecture of Green Strategies of Renewable-Resource-Based Enterprises: A Case Study of Forestry Enterprise. J. Nanjing For. Univ. Nat. Sci. Ed. 2020, 44, 1–8. [Google Scholar] [CrossRef]
- Liu, S.Q.; Jia, L.M. Review on Sustainable Development of Forest-based Biodiesel. J. Nanjing For. Univ. Nat. Sci. Ed. 2020, 44, 216–224. [Google Scholar] [CrossRef]
- Yan, Z.X.; Yang, H.Y.; Fan, S.F.; Wu, W.L.; Lyu, L.F.; Li, W.L. Analysis of the Expression of Sucrose Phosphate Synthase Genes Duringthe Development of Blackberry Fruit. J. Nanjing For. Univ. Nat. Sci. Ed. 2022, 46, 179–186. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, Y.; Pan, Z.; Lu, Q. Review on the Battery Model and SOC Estimation Method. Processes 2021, 9, 1685. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, Q.; Zheng, Y. Review on the Selection of Health Indicator for Lithium Ion Batteries. Machines 2022, 10, 512. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, H.; Lu, Z. A Novel 10-Parameter Motor Efficiency Model Based on I-SA and Its Comparative Application of Energy Utilization Efficiency in Different Driving Modes for Electric Tractor. Agriculture 2022, 12, 362. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High Temperature Proton Exchange Membrane Fuel Cells: Progress in Advanced Materials and Key Technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Xie, J.Y.; Xu, X.; Cai, B.; Zhang, H.G. Responses of Forest Soil Labile Nitrogen Pool and Nitrogen Cycle to the Changes of Carbon Input under Carbon Neutrality. J. Nanjing For. Univ. Nat. Sci. Ed. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Sonne, C.; Xia, C.; Lam, S.S. Is Engineered Wood China’s Way to Carbon Neutrality? J. Bioresour. Bioprod. 2022, 7, 83–84. [Google Scholar] [CrossRef]
- Zheng, C.; Xu, D.C.; Cao, J.; Li, L.; Wen, B. Design of Lightweight Electric Forestry Monorail Vehicle. J. For. Eng. 2021, 6, 140–146. [Google Scholar] [CrossRef]
- Alpaydin, G.U.; Devrim, Y.; Colpan, C.O. Performance of an HT-PEMFC Having a Catalyst with Graphene and Multiwalled Carbon Nanotube Support. Int. J. Energy Res. 2019, 43, 3578–3589. [Google Scholar] [CrossRef]
- Jörissen, L.; Garche, J. Polymer Electrolyte Membrane Fuel Cells. Hydrog. Fuel Cell Technol. Mark. Perspect. 2015, 88, 239–281. [Google Scholar]
- Harikishan Reddy, E.; Jayanti, S. Thermal Management Strategies for a 1 KWe Stack of a High Temperature Proton Exchange Membrane Fuel Cell. Appl. Therm. Eng. 2012, 48, 465–475. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like Chitosan Porous Carbon Spheres/MXene Composite with High Specific Capacitance and Rate Performance for Supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Zheng, Y. Regenerative Braking Control Strategy with Real-Time Wavelet Transform for Composite Energy Buses. Machines 2022, 10, 673. [Google Scholar] [CrossRef]
- Reddy, E.H.; Monder, D.S.; Jayanti, S. Parametric Study of an External Coolant System for a High Temperature Polymer Electrolyte Membrane Fuel Cell. Appl. Therm. Eng. 2013, 58, 155–164. [Google Scholar] [CrossRef]
- Ewulonu, C.M.; Liu, X.; Wu, M.; Yong, H. Lignin-Containing Cellulose Nanomaterials: A Promising New Nanomaterial for Numerous Applications. J. Bioresour. Bioprod. 2019, 4, 3–10. [Google Scholar] [CrossRef]
- Yang, H.Q.; Yu, Z.H. Research Trends and Future Key Issues of Global Harvested Wood Products Carbon Science. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 119–228. [Google Scholar] [CrossRef]
- Lee, D.; Lim, J.W.; Lee, D.G. Cathode/Anode Integrated Composite Bipolar Plate for High-Temperature PEMFC. Compos. Struct. 2017, 167, 144–151. [Google Scholar] [CrossRef]
- Ding, L.; Han, X.; Cao, L.; Chen, Y.; Ling, Z.; Han, J.; He, S.; Jiang, S. Characterization of Natural Fiber from Manau Rattan (Calamus Manan) as a Potential Reinforcement for Polymer-Based Composites. J. Bioresour. Bioprod. 2021, 10, 512. [Google Scholar] [CrossRef]
- Li, Q.F.; Rudbeck, H.C.; Chromik, A.; Jensen, J.O.; Pan, C.; Steenberg, T.; Calverley, M.; Bjerrum, N.J.; Kerres, J. Properties, Degradation and High Temperature Fuel Cell Test of Different Types of PBI and PBI Blend Membranes. J. Memb. Sci. 2010, 347, 260–270. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the Phosphoric Acid-Doping Level in a Polybenzimidazole Membrane on the Cell Performance of High-Temperature Proton Exchange Membrane Fuel Cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Pinar, F.J.; Cañizares, P.; Rodrigo, M.A.; Úbeda, D.; Lobato, J. Long-Term Testing of a High-Temperature Proton Exchange Membrane Fuel Cell Short Stack Operated with Improved Polybenzimidazole-Based Composite Membranes. J. Power Sources 2015, 274, 177–185. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K.; Kujawa, J. A Review on Ionic Liquids-Based Membranes for Middle and High Temperature Polymer Electrolyte Membrane Fuel Cells (PEM FCs). Int. J. Mol. Sci. 2021, 22, 5430. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next Generation of Proton-Exchange Membrane Fuel Cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, S.; Shanmugam, V.M.; Radhakrsihnan, S.; Manisankar, P. Novel Perovskite Structured Calcium Titanate-PBI Composite Membranes for High-Temperature PEM Fuel Cells: Synthesis and Characterizations. Int. J. Hydrogen Energy 2018, 43, 4763–4772. [Google Scholar] [CrossRef]
- Ma, C.H.; Luo, Y.H.; Li, J.H.; Huang, Y.X.; Jiang, N.; Guo, W.Q. Study on the Enrichment of Isoxaziridin from Acanthopanax Senticosus by Macroporous Resin Immobilized with Ionic Liquid. J. For. Eng. 2021, 6, 117–122. [Google Scholar] [CrossRef]
- Ma, C.H.; Sun, J.D.; Li, W.; Luo, S.; Liu, S.X. Application Progress of Ionic Liquids in the Field of Lignin Depolymerization. J. For. Eng. 2021, 6, 14–26. [Google Scholar] [CrossRef]
- Chen, M.; Gao, X. Theoretical, Experimental and Numerical Diagnose of Critical Power Point of Thermoelectric Generators. Energy 2014, 78, 364–372. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A Comprehensive Review of PBI-Based High Temperature PEM Fuel Cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Gao, X.; Andreasen, S.J.; Chen, M.; Kær, S.K. Numerical Model of a Thermoelectric Generator with Compact Plate-Fin Heat Exchanger for High Temperature PEM Fuel Cell Exhaust Heat Recovery. Int. J. Hydrogen Energy 2012, 37, 8490–8498. [Google Scholar] [CrossRef]
- Esfeh, H.K.; Hamid, M.K.A. Temperature Effect on Proton Exchange Membrane Fuel Cell Performance Part II: Parametric Study. Energy Procedia 2014, 61, 2617–2620. [Google Scholar] [CrossRef]
- Miansari, M.; Sedighi, K.; Amidpour, M.; Alizadeh, E.; Miansari, M. Experimental and Thermodynamic Approach on Proton Exchange Membrane Fuel Cell Performance. J. Power Sources 2009, 190, 356–361. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Xu, B.; Ma, Z. Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use. Processes 2019, 7, 419. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, X.; Zhang, H.; Hou, S. Energetic, Exergetic and Ecological Analyses of a High-Temperature Proton Exchange Membrane Fuel Cell Based on a Phosphoric-Acid-Doped Polybenzimidazole Membrane. Sustain. Energy Technol. Assess. 2020, 38, 100671. [Google Scholar] [CrossRef]
- Khan, S.S.; Shareef, H.; Ibrahim, A.A. Improved Semi-Empirical Model of Proton Exchange Membrane Fuel Cell Incorporating Fault Diagnostic Feature. J. Mod. Power Syst. Clean Energy 2021, 9, 1566–1573. [Google Scholar] [CrossRef]
- Nalbant, Y.; Colpan, C.O.; Devrim, Y. Energy and Exergy Performance Assessments of a High Temperature-Proton Exchange Membrane Fuel Cell Based Integrated Cogeneration System. Int. J. Hydrogen Energy 2020, 45, 3584–3594. [Google Scholar] [CrossRef]
- Toghyani, S.; Baniasadi, E.; Afshari, E. Numerical Simulation and Exergoeconomic Analysis of a High Temperature Polymer Exchange Membrane Electrolyzer. Int. J. Hydrogen Energy 2019, 44, 31731–31744. [Google Scholar] [CrossRef]
- Ye, L.; Jia, B.; Yin, Y. Modeling and Analysis of HT-PEMFC System Based on Reformed Hydrogen. J. Tianjin Univ. Sci. Technol. 2015, 48, 341–346. [Google Scholar] [CrossRef]
- Ay, M.; Midilli, A.; Dincer, I. Thermodynamic Modelling of a Proton Exchange Membrane Fuel Cell. Int. J. Exergy 2006, 3, 16–44. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Ma, Z.; Zheng, M.; Lu, Z. Exergetic Performance Coefficient Analysis and Optimization of a High-Temperature Proton Exchange Membrane Fuel Cell. Membranes 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Midilli, A.; Dincer, I. Development of Some Exergetic Parameters for PEM Fuel Cells for Measuring Environmental Impact and Sustainability. Int. J. Hydrogen Energy 2009, 34, 3858–3872. [Google Scholar] [CrossRef]
- Sousa, T.; Mamlouk, M.; Scott, K. An Isothermal Model of a Laboratory Intermediate Temperature Fuel Cell Using PBI Doped Phosphoric Acid Membranes. Chem. Eng. Sci. 2010, 65, 2513–2530. [Google Scholar] [CrossRef]
- Cheddie, D.; Munroe, N. Analytical Correlations for Intermediate Temperature PEM Fuel Cells. J. Power Sources 2006, 160, 299–304. [Google Scholar] [CrossRef]
- Olapade, P.O.; Meyers, J.P.; Borup, R.L.; Mukundan, R. Parametric Study of the Morphological Proprieties of HT-PEMFC Components for Effective Membrane Hydration. J. Electrochem. Soc. 2011, 158, B639. [Google Scholar] [CrossRef]
- Lee, W.Y.; Kim, M.; Sohn, Y.J.; Kim, S.G. Power Optimization of a Combined Power System Consisting of a High-Temperature Polymer Electrolyte Fuel Cell and an Organic Rankine Cycle System. Energy 2016, 113, 1062–1070. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Ma, Z.; Xu, B.; Lu, Z.; Li, Y.; Zheng, M. Ecological Performance Optimization of a High Temperature Proton Exchange Membrane Fuel Cell. Mathematics 2021, 9, 1332. [Google Scholar] [CrossRef]
- Xu, B.; Li, D.; Ma, Z.; Zheng, M.; Li, Y. Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics 2021, 9, 1792. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, H.; Zhao, J.; Wang, F.; Wang, J.; Miao, H.; Yuan, J. Performance Evaluation of an Integrated High-Temperature Proton Exchange Membrane Fuel Cell and Absorption Cycle System for Power and Heating/Cooling Cogeneration. Energy Convers. Manag. 2019, 181, 292–301. [Google Scholar] [CrossRef]
- Haghighat Mamaghani, A.; Najafi, B.; Casalegno, A.; Rinaldi, F. Optimization of an HT-PEM Fuel Cell Based Residential Micro Combined Heat and Power System: A Multi-Objective Approach. J. Clean. Prod. 2018, 180, 126–138. [Google Scholar] [CrossRef]
- Ay, M.; Midilli, A.; Dincer, I. Exergetic Performance Analysis of a PEM Fuel Cell. Int. J. Energy Res. 2006, 30, 307–321. [Google Scholar] [CrossRef]
- Chitsaz, A.; Haghghi, M.A.; Hosseinpour, J. Thermodynamic and Exergoeconomic Analyses of a Proton Exchange Membrane Fuel Cell (PEMFC) System and the Feasibility Evaluation of Integrating with a Proton Exchange Membrane Electrolyzer (PEME). Energy Convers. Manag. 2019, 186, 487–499. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Aris, A.M.; Shabani, B. PEM Fuel Cell Heat Recovery for Preheating Inlet Air in Standalone Solar-Hydrogen Systems for Telecommunication Applications: An Exergy Analysis. Int. J. Hydrogen Energy 2016, 41, 2987–3003. [Google Scholar] [CrossRef]
- Obara, S.; Tanno, I.; Kito, S.; Hoshi, A.; Sasaki, S. Exergy Analysis of the Woody Biomass Stirling Engine and PEM-FC Combined System with Exhaust Heat Reforming. Int. J. Hydrogen Energy 2008, 33, 2289–2299. [Google Scholar] [CrossRef]
- Taner, T. Energy and Exergy Analyze of PEM Fuel Cell: A Case Study of Modeling and Simulations. Energy 2018, 143, 284–294. [Google Scholar] [CrossRef]
- Cohce, M.K.; Dincer, I.; Rosen, M.A. Energy and Exergy Analyses of a Biomass-Based Hydrogen Production System. Bioresour. Technol. 2011, 102, 8466–8474. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Xu, B.; Ma, Z.; Li, Y.; Li, D. Performance Analysis Based on Sustainability Exergy Indicators of High-Temperature Proton Exchange Membrane Fuel Cell. Int. J. Mol. Sci. 2022, 23, 10111. https://doi.org/10.3390/ijms231710111
Guo X, Xu B, Ma Z, Li Y, Li D. Performance Analysis Based on Sustainability Exergy Indicators of High-Temperature Proton Exchange Membrane Fuel Cell. International Journal of Molecular Sciences. 2022; 23(17):10111. https://doi.org/10.3390/ijms231710111
Chicago/Turabian StyleGuo, Xinjia, Bing Xu, Zheshu Ma, Yanju Li, and Dongxu Li. 2022. "Performance Analysis Based on Sustainability Exergy Indicators of High-Temperature Proton Exchange Membrane Fuel Cell" International Journal of Molecular Sciences 23, no. 17: 10111. https://doi.org/10.3390/ijms231710111
APA StyleGuo, X., Xu, B., Ma, Z., Li, Y., & Li, D. (2022). Performance Analysis Based on Sustainability Exergy Indicators of High-Temperature Proton Exchange Membrane Fuel Cell. International Journal of Molecular Sciences, 23(17), 10111. https://doi.org/10.3390/ijms231710111






