CRISPR-Based Genome Editing and Its Applications in Woody Plants
Abstract
:1. Introduction
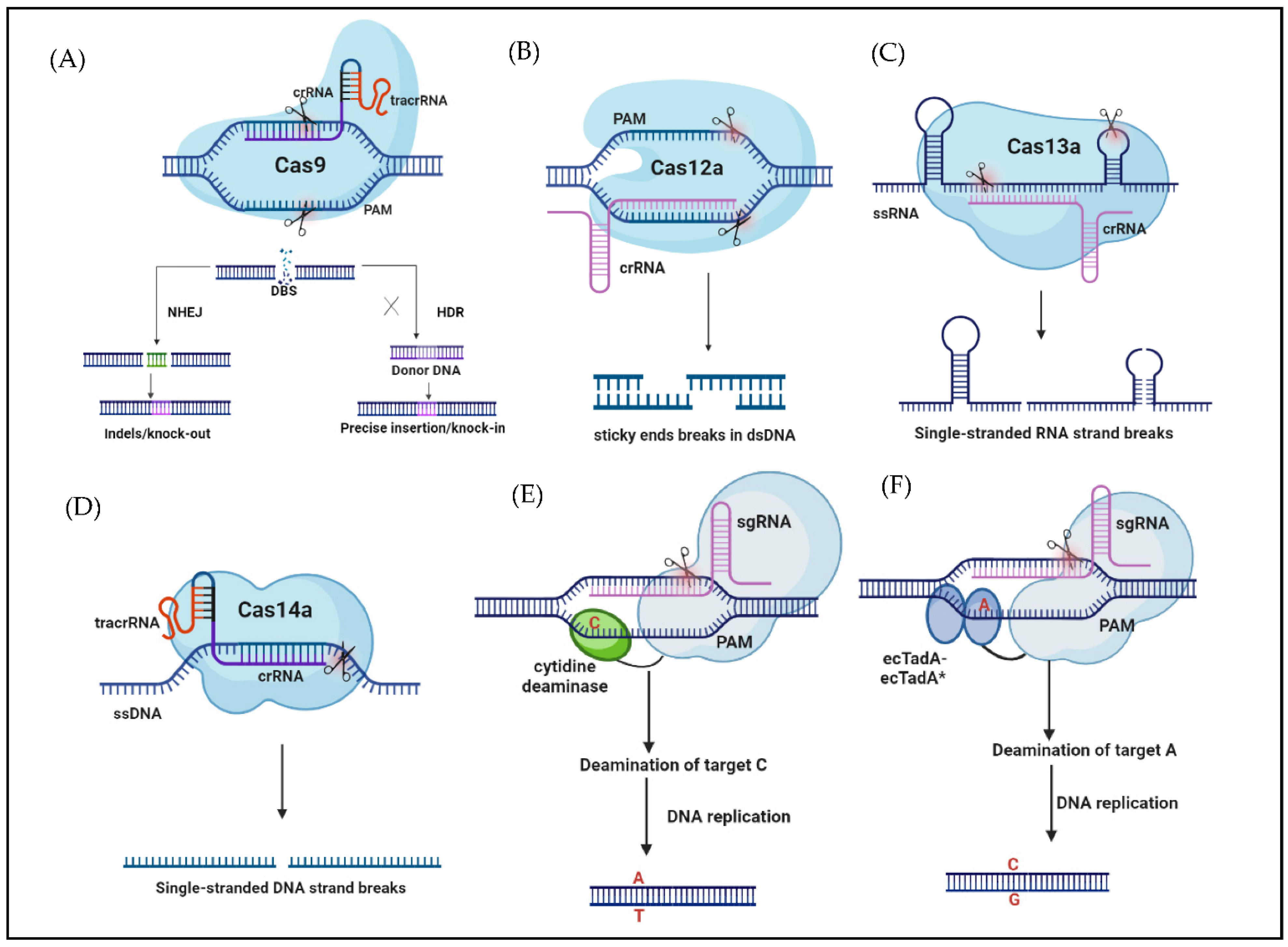
2. CRISPR/Cas-Based Systems and Editors
2.1. CRISPR/Cas System
2.2. New Editors Based on CRISPR/Cas
2.2.1. CRISPR/Cas12
2.2.2. CRISPR/Cas13
2.2.3. CRISPR/Cas14
2.2.4. Base Editing (BE)
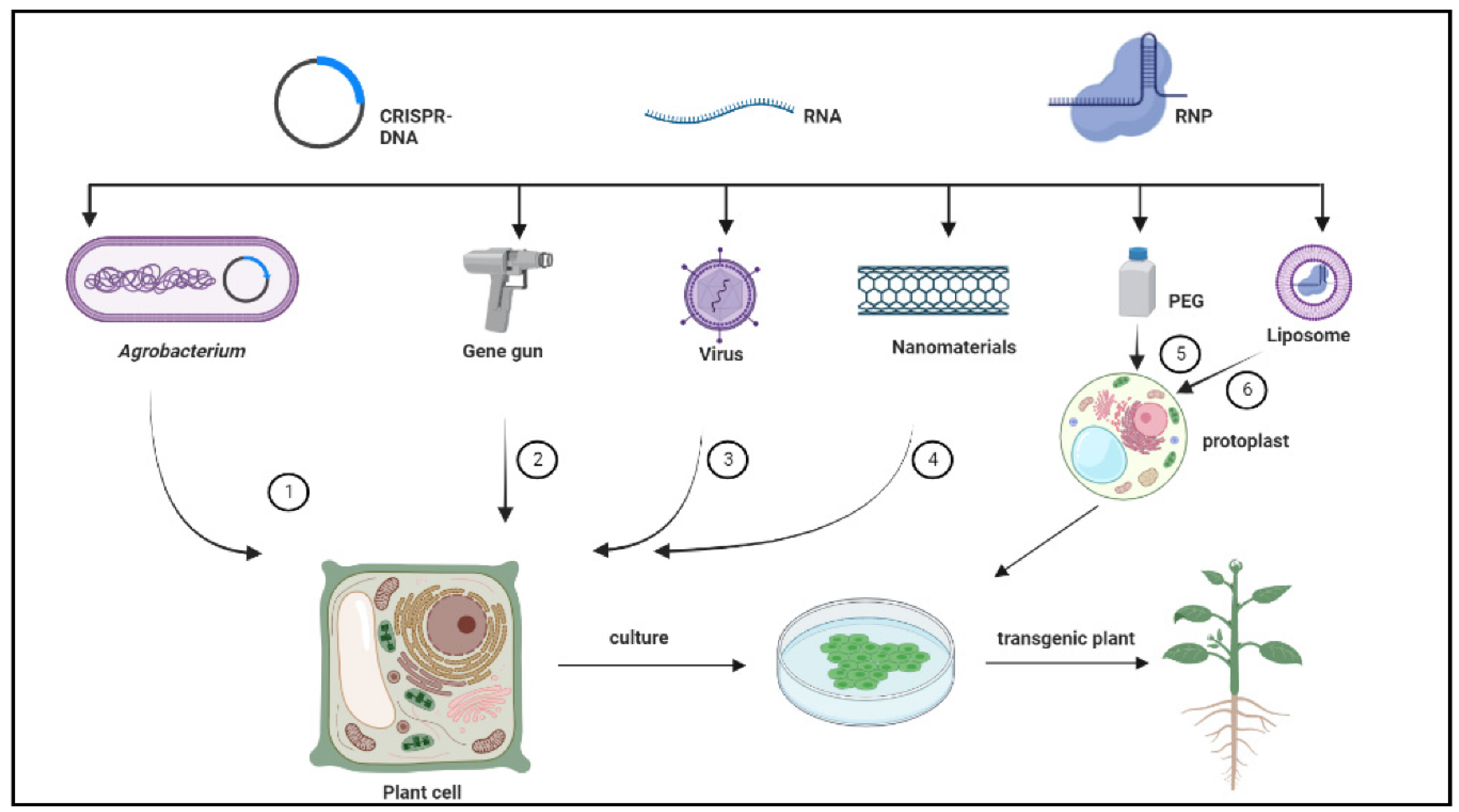
3. CRISPR-Based Delivery Systems in Woody Plants
| Species Name | Target Gene | Tool | Trait Performance | Transformation Method | Editing Efficiency | References | |
|---|---|---|---|---|---|---|---|
| Timber properties | Populus | 4CL1, 4CL2 | CRISPR/Cas9 | Decreased lignin content, discoloration of stems | Agrobacterium-mediated | 100 | [61] |
| Populus tremula × P. alba | CSE1, CSE2 | CRISPR/Cas9 | Reduced lignin and increased cellulose | Agrobacterium-mediated | _ | [62] | |
| Populus tomentosa | PtoMYB156 | CRISPR/Cas9 | Negative regulation of secondary wall formation | Agrobacterium-mediated | 48 | [63] | |
| Populus trichocarpa | PtrHSFB3-1 PtrMYB092 | CRISPR/Cas9 | Reduced lignin and increased cellulose | Agrobacterium-mediated | _ | [64] | |
| Populus tomentosa | PtoDWF4 | CRISPR/Cas9 | Reduced xylem development | Agrobacterium-mediated | _ | [65] | |
| Populus tomentosa | PtoDET2 | CRISPR/Cas9 | Xylem development and reduced wall thickness | Agrobacterium-mediated | _ | [66] | |
| Populus tremula L. × Populus tremuloides Michx. | VNS | CRISPR/Cas9 | Secondary cell wall thinning | Agrobacterium-mediated | _ | [67] | |
| Flowering | Hevea brasiliensis | HbFT, HbTFL1 | CRISPR/Cas9 | bloom early | PEG-mediated | 3.74–20.11 | [48] |
| Apple | MdTFL1.1 | CRISPR/Cas9 | bloom early | Agrobacterium-mediated | 93 | [68] | |
| Pear | PcTFL1.1 | CRISPR/Cas9 | bloom early | Agrobacterium-mediated | 9 | [68] | |
| kiwifruit A. chinensis | AcCEN4, AcCEN | CRISPR/Cas9 | bloom early | Agrobacterium-mediated | 30–75 | [69] | |
| Biological stress | Populus trichocarpa | PtrWRKY18, PtrWRKY35 | CRISPR/Cas9 | Melampsora resistance | Agrobacterium-mediated | _ | [70] |
| Cassava | ncbp-1, ncbp-2 | CRISPR/Cas9 | Cassava Brown Spot Virus resistance | Agrobacterium-mediated | 91 | [71] | |
| Theobroma cacao | TcNPR3 | CRISPR/Cas9 | Phytophthora resistance | Agrobacterium-mediated | 27 | [72] | |
| grape (Vitis vinifera) | VvWRKY52 | CRISPR/Cas9 | Botrytis cinerea resistance | Agrobacterium-mediated | 5.55–27.78 | [73] | |
| Plasmopara viticola | VvPR4b | CRISPR/Cas9 | Grapevine downy mildew resistance | Agrobacterium-mediated | 20.16 | [74] | |
| Duncan grape | CsLOB1 | CRISPR/Cas9 | citrus canker resistance | Agrobacterium-mediated | 14.29–81.25 | [75] | |
| Wanjincheng orange | CsLOB1 | CRISPR/Cas9 | citrus canker resistance | Agrobacterium-mediated | 11.5–64.7 | [76] | |
| Duncan grape | CsLOB1 | CRISPR/Cas9 | citrus canker resistance | Agrobacterium-mediated | 23.80–89.36 | [77] | |
| Wanjincheng orange | CsWRKY22 | CRISPR/Cas9 | citrus canker resistance | Agrobacterium-mediated | 68.2–85.7 | [78] | |
| Pear and apple | ALS | CRISPR/Cas9 base editing | herbicide-resistant | Agrobacterium-mediated | - | [79] | |
| Abiotic stress | Populus alba var. pyramidalis | PalWRKY77 | CRISPR/Cas9 | salt resistant | Agrobacterium-mediated | _ | [80] |
| Populus trichocarpa | PtrADA2b-3 | CRISPR/Cas9 | drought resistance | Agrobacterium-mediated | _ | [81] | |
| Populus | PdNF-YB21 | CRISPR/Cas9 | drought resistance | Agrobacterium-mediated | _ | [82] | |
| Secondary metabolism | Sweet orange | CsPDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 3.2–3.9 | [83] |
| Populus | PtoPDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 51.7 | [84] | |
| Apple | PDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 31.8 | [85] | |
| Vitis vinifera L., cv. Neo Muscat | VvPDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | _ | [86] | |
| Cassava | MePDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 90–100 | [87] | |
| citrus | PDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 45.5–75 | [88] | |
| Coffea canephora | CcPDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 30.4 | [89] | |
| Cotton | GhCLA1 | CRISPR/Cas9 | albinism | Agrobacterium-mediated | 66.7–100 | [90] | |
| Green bamboo | PDS | CRISPR/Cas9 | albinism | PEG-mediated | 12.5 | [91] | |
| Walnut | JrPDS | CRISPR/Cas9 | albinism | Agrobacterium-mediated | _ | [92] | |
| Populus | PDS | CRISPR/Cas12 | albinism | Agrobacterium-mediated | _ | [93] | |
| Populus | MYB115 | CRISPR/Cas9 | Reduced proanthocyanidin accumulation | Agrobacterium-mediated | 93.33–100 | [94] | |
| Populus tomentosa | PtrMYB57 | CRISPR/Cas9 | Increased anthocyanins and procyanidins | Agrobacterium-mediated | _ | [95] | |
| Populus | JMJ25 | CRISPR/Cas9 | Increased anthocyanin accumulation | Agrobacterium-mediated | _ | [96] | |
| Populus | UGT71L1 | CRISPR/Cas9 | Partial reduction in salicylin content | Agrobacterium-mediated | 40 | [97] | |
| Pomegranate | PgUGT84A23, PgUGT84A24 | CRISPR/Cas9 | Reduced punicalagin content | Agrobacterium-mediated | _ | [98] | |
| Grape | IdnDH | CRISPR/Cas9 | Reduced tartaric acid content | Agrobacterium-mediated | 100 | [99] | |
| tea [Camellia sinensis (L.) o. Kuntze] | CsHB1 | CRISPR/Cas9 | Decrease in caffeine | Agrobacterium-mediated | _ | [100] | |
| Growth and development | Jatropha curcas | JcCYP735A | CRISPR/Cas9 | Plant height reduction | Agrobacterium-mediated | _ | [101] |
| Ma bamboo (Dendrocalamus latiflorus Munro) | GRG1 | CRISPR/Cas9 | Plant height increase | Agrobacterium-mediated | 40 | [102] | |
| Parasponia andersonii | PanHK4, PanEIN2, PanNSP1, PanNSP2 | CRISPR/Cas9 | nodulation, bisexual flowers | Agrobacterium-mediated | 48–89 | [103] | |
| Vitis vinifera | VvCCD7, VvCCD8 | CRISPR/Cas9 | increased stem branching | Agrobacterium-mediated | 66.7 | [104] | |
| Populus | BRANCHED1 BRANCHED2 | CRISPR/Cas9 | Enhance the growth of shoots | Agrobacterium-mediated | _ | [105] | |
| Cotton | GhARG | CRISPR/Cas9 | Promote lateral root formation | Agrobacterium-mediated | 10–98 | [106] |
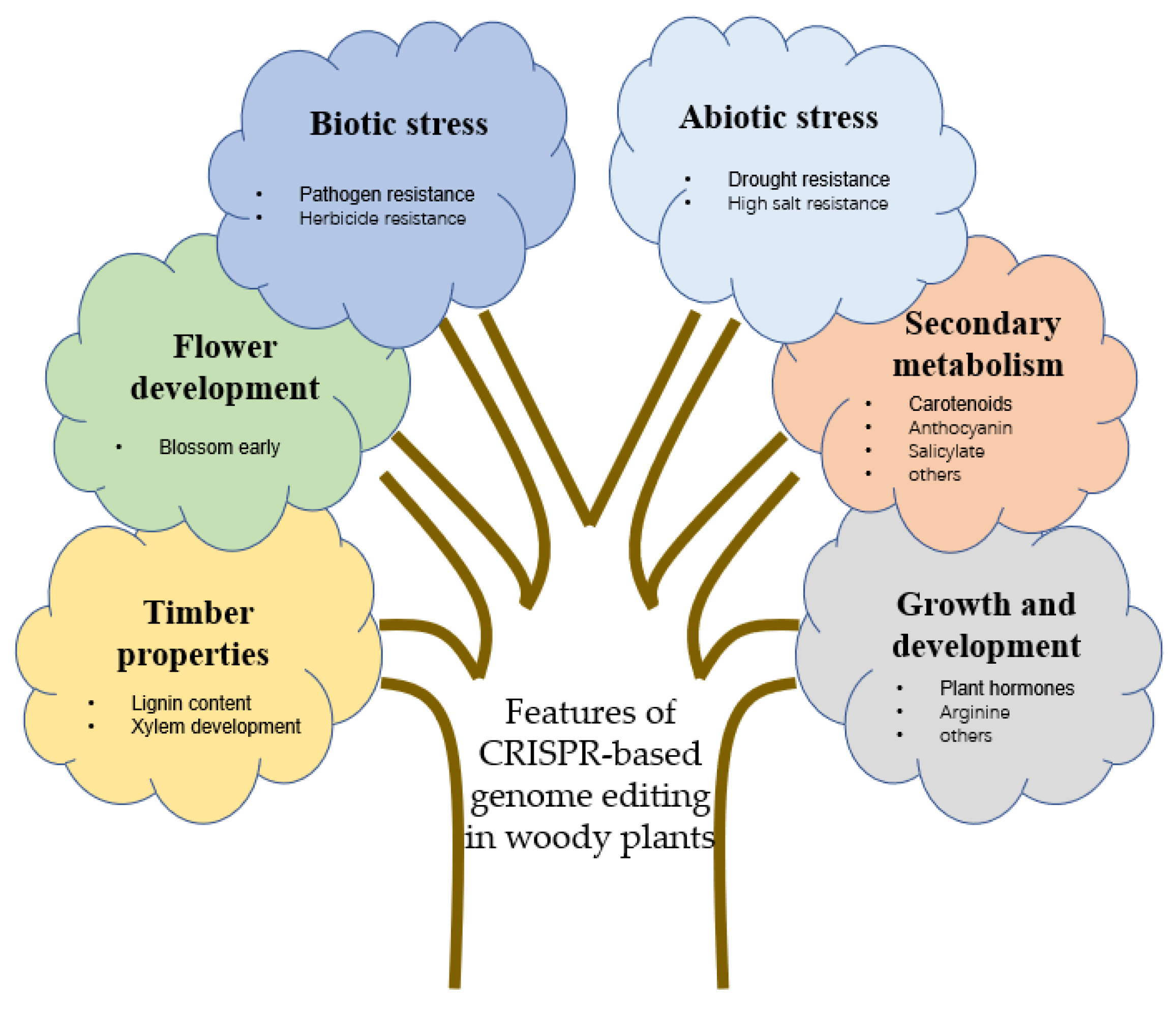
4. Applications of Gene Editing Technology in Woody Plants
4.1. Timber Properties of Woody Plants
4.2. Flower Development of Woody Plants
4.3. Improvement of Stress Resistance
4.3.1. Biotic Stress
4.3.2. Abiotic Stress
4.4. Secondary Metabolism
4.5. Growth and Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trumbore, S.; Brando, P.; Hartmann, H. Forest Health and Global Change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Sterck, L.; Rombauts, S.; Jansson, S.; Sterky, F.; Rouzé, P.; Van De Peer, Y. EST Data Suggest That Poplar Is an Ancient Polyploid. New Phytol. 2005, 167, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Hallingbäck, H.R.; Sánchez, L. Performance of Seven Tree Breeding Strategies under Conditions of Inbreeding Depression. G3 Genes Genomes Genet. 2016, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Kajita, S.; Osakabe, K. Genetic Engineering of Woody Plants: Current and Future Targets in a Stressful Environment. Physiol. Plant 2011, 142, 105–117. [Google Scholar] [CrossRef]
- Harfouche, A.; Meilan, R.; Altmane, A. Tree Genetic Engineering and Applications to Sustainable Forestry and Biomass Production. Trends Biotechnol. 2011, 29, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering Drought Resistance in Forest Trees. Front. Plant Sci. 2019, 9, 1875. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Janz, D.; Teichmann, T.; Lipka, V. Poplar Genetic Engineering: Promoting Desirable Wood Characteristics and Pest Resistance. Appl. Microbiol. Biotechnol. 2013, 97, 5669–5679. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, Y.; Zhang, Q.; Xiao, D.; Yang, M.; Wang, J. Expression of Multiple Exogenous Insect Resistance and Salt Tolerance Genes in Populus nigra L. Front. Plant Sci. 2020, 11, 1123. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, X.; Dong, Y.; Zhang, J.; Wang, J.; Yang, M. Exogenous Gene Expression and Insect Resistance in Dual Bt Toxin Populus × euramericana ‘Neva’ Transgenic Plants. Front. Plant Sci. 2021, 12, 660226. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, C.; Xiao, D.; Liang, Y.; Wang, Y. Advances and Perspectives of Transgenic Technology and Biotechnological Application in Forest Trees. Front. Plant Sci. 2021, 12, 786328. [Google Scholar] [CrossRef]
- Movahedi, A.; Wei, H.; Zhou, X.; Fountain, J.C.; Chen, Z.-H.; Mu, Z.; Sun, W.; Zhang, J.; Li, D.; Guo, B.; et al. Precise Exogenous Insertion and Sequence Replacements in Poplar by Simultaneous HDR Overexpression and NHEJ Suppression Using CRISPR-Cas9. Hortic. Res. 2022, 7, uhac154. [Google Scholar] [CrossRef]
- Ahmar, S.; Ballesta, P.; Ali, M.; Mora-Poblete, F. Achievements and Challenges of Genomics-Assisted Breeding in Forest Trees: From Marker-Assisted Selection to Genome Editing. Int. J. Mol. Sci. 2021, 22, 10583. [Google Scholar] [CrossRef]
- Ahmar, S.; Saeed, S.; Khan, M.H.U.; Khan, S.U.; Mora-Poblete, F.; Kamran, M.; Faheem, A.; Maqsood, A.; Rauf, M.; Saleem, S.; et al. A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement. Int. J. Mol. Sci. 2020, 21, 5665. [Google Scholar] [CrossRef]
- Carroll, D. Genome Engineering with Zinc-Finger Nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef]
- Lloyd, A.; Plaisier, C.L.; Carroll, D.; Drews, G.N. Targeted Mutagenesis in Arabidopsis Using Zinc-Finger Nucleases. Proc. Natl. Acad. Sci. USA 2005, 102, 2232–2237. [Google Scholar] [CrossRef]
- Peer, R.; Rivlin, G.; Golobovitch, S.; Lapidot, M.; Gal-On, A.; Vainstein, A.; Tzfira, T.; Flaishman, M.A. Targeted Mutagenesis Using Zinc-Finger Nucleases in Perennial Fruit Trees. Planta 2015, 241, 941–951. [Google Scholar] [CrossRef]
- Lu, H.; Klocko, A.L.; Dow, M.; Ma, C.; Amarasinghe, V.; Strauss, S.H. Low Frequency of Zinc-Finger Nuclease-Induced Mutagenesis in Populus. Mol. Breed. 2016, 36, 121. [Google Scholar] [CrossRef]
- Mussolino, C.; Cathomen, T. TALE Nucleases: Tailored Genome Engineering Made Easy. Curr. Opin. Biotechnol. 2012, 23, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA Double-Strand Breaks with TAL Effector Nucleases. Genetics 2010, 186, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA Maturation by Trans-Encoded Small RNA and Host Factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–822. [Google Scholar] [CrossRef]
- Wang, H.; La Russa, M.; Qi, L.S. CRISPR/Cas9 in Genome Editing and Beyond. Annu. Rev. Biochem. 2016, 85, 227–264. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 System: A New-Fangled Dawn in Gene Editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Manghwar, H.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas System: Recent Advances and Future Prospects for Genome Editing. Trends Plant Sci. 2019, 24, 1102–1125. [Google Scholar] [CrossRef]
- Bewg, W.P.; Ci, D.; Tsai, C.J. Genome Editing in Trees: From Multiple Repair Pathways to Long-Term Stability. Front. Plant Sci. 2018, 871, 1732. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 2017, 37, 67–68. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.G.; Kamoun, S. Targeted Mutagenesis in the Model Plant Nicotiana Benthamiana Using Cas9 RNA-Guided Endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted Genome Modification of Crop Plants Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Li, J.-F.; Aach, J.; Norville, J.E.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and Homologous Recombination-Mediated Plant Genome Editing via Guide RNA/Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Qin, R.; Li, H.; Li, D.; Li, L.; Wei, P.; Yang, J. Generation of Targeted Mutant Rice Using a CRISPR-Cpf1 System. Plant Biotechnol. J. 2017, 15, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Jonathan, S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Cui, T.; Feng, G.; Guo, L.; Xu, K.; Gao, Q.; Li, T.; Li, J.; Zhou, Q.; Li, W. Repurposing CRISPR-Cas12b for Mammalian Genome Engineering. Cell Discov. 2018, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Qiao, X.; Zhao, Y.; Zhang, Z.; Gao, Y.; Shi, L.; Du, H.; Wang, L.; Zhang, Y.J.; Zhang, Y.; et al. Targeted Mutagenesis in Arabidopsis thaliana Using CRISPR-Cas12b/C2c1. J. Integr. Plant Biol. 2020, 62, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Ren, Q.; Pan, C.; He, Y.; Zhang, Y.; Liu, S.; Zhong, Z.; Wang, J.; Malzahn, A.A.; Wu, J.; et al. CRISPR–Cas12b Enables Efficient Plant Genome Engineering. Nat. Plants 2020, 6, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Zou, X.; Duan, S.; Li, Z.; Deng, Z.; Luo, J.; Lee, S.Y.; Chen, S. Advances in CRISPR-Cas Systems for RNA Targeting, Tracking and Editing. Biotechnol. Adv. 2019, 37, 708–729. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA Targeting with CRISPR-Cas13a Omar. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef]
- Aman, R.; Ali, Z.; Butt, H.; Mahas, A.; Aljedaani, F.; Khan, M.Z.; Ding, S.; Mahfouz, M. RNA Virus Interference via CRISPR/Cas13a System in Plants. Genome Biol. 2018, 19, 1. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, F.; Zhong, Z.; Chen, R.; Wang, Y.; Chang, L.; Bock, R.; Nie, B.; Zhang, J. Generation of Virus-Resistant Potato Plants by RNA Genome Targeting. Plant Biotechnol. J. 2019, 17, 1814–1822. [Google Scholar] [CrossRef] [Green Version]
- Abudayyeh, O.O.; Gootenberg, J.S.; Kellner, M.J.; Zhang, F. Nucleic Acid Detection of Plant Genes Using CRISPR-Cas13. Cris. J. 2019, 2, 165–171. [Google Scholar] [CrossRef]
- Khan, M.Z.; Haider, S.; Mansoor, S.; Amin, I. Targeting Plant SsDNA Viruses with Engineered Miniature CRISPR-Cas14a. Trends Biotechnol. 2019, 37, 800–804. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.L.; Wang, D.; Gao, C. Precise Base Editing in Rice, Wheat and Maize with a Cas9-Cytidine Deaminase Fusion. Nat. Biotechnol. 2017, 35, 438–440. [Google Scholar] [CrossRef]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.L.; Gao, C. Efficient C-to-T Base Editing in Plants Using a Fusion of Ncas9 and Human APOBEC3A. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef]
- Yang, B.; Yang, L.; Chen, J. Development and Application of Base Editors. Cris. J. 2019, 2, 91–104. [Google Scholar] [CrossRef]
- Mao, Y.; Botella, J.R.; Zhu, J.K. Heritability of Targeted Gene Modifications Induced by Plant-Optimized CRISPR Systems. Cell Mol. Life Sci. 2017, 74, 1075–1093. [Google Scholar] [CrossRef]
- Fan, Y.; Xin, S.; Dai, X.; Yang, X.; Huang, H.; Hua, Y. Efficient Genome Editing of Rubber Tree (Hevea brasiliensis) Protoplasts Using CRISPR/Cas9 Ribonucleoproteins. Ind. Crop. Prod. 2020, 146, 112146. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.H.; Koo, O.J.; Kim, S.; Kim, J.S.; Velasco, R.; Kanchiswamy, C.N. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M.; et al. CRISPR–Cas9-Mediated Genome Editing in Apple and Grapevine. Nat. Protoc. 2018, 13, 2844–2863. [Google Scholar] [CrossRef]
- Liu, W.; Rudis, M.R.; Cheplick, M.H.; Millwood, R.J.; Yang, J.P.; Ondzighi-Assoume, C.A.; Montgomery, G.A.; Burris, K.P.; Mazarei, M.; Chesnut, J.D.; et al. Lipofection-Mediated Genome Editing Using DNA-Free Delivery of the Cas9/GRNA Ribonucleoprotein into Plant Cells. Plant Cell Rep. 2020, 39, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant Gene Editing through de Novo Induction of Meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Sharma, A.; Paredes-López, O. Factors Affecting Genetic Transformation by Particle Bombardment of the Prickly Pear Cactus (O. ficus-indica). 3 Biotech 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.J.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing Crop Transformation in the Era of Genome Editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Mark Cigan, A. Genome Editing in Maize Directed by CRISPR-Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2016, 7, 13274. [Google Scholar] [CrossRef] [PubMed]
- Banakar, R.; Schubert, M.; Collingwood, M.; Vakulskas, C.; Eggenberger, A.L.; Wang, K. Comparison of CRISPR-Cas9/Cas12a Ribonucleoprotein Complexes for Genome Editing Efficiency in the Rice Phytoene Desaturase (OsPDS) Gene. Rice 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, S.; Li, Z.; Li, H.; Song, W.; Zhao, H.; Lai, J.; Xia, L.; Li, D.; Zhang, Y. A Barley Stripe Mosaic Virus-Based Guide RNA Delivery System for Targeted Mutagenesis in Wheat and Maize. Mol. Plant Pathol. 2019, 20, 1463–1474. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Liu, H.; Li, Z. Highly Efficient DNA-Free Plant Genome Editing Using Virally Delivered CRISPR–Cas9. Nat. Plants 2020, 6, 773–779. [Google Scholar] [CrossRef]
- Doyle, C.; Higginbottom, K.; Swift, T.; Winfield, M.; Bellas, C.; Benito-Alifonso, D.; Fletcher, T.; Galan, M.C.; Edwards, K.; Whitney, H. A Simple Method for Spray-on Gene Editing in Planta. bioRxiv 2019. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.J.; et al. High Aspect Ratio Nanomaterials Enable Delivery of Functional Genetic Material without DNA Integration in Mature Plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef]
- Zhou, X.; Jacobs, T.B.; Xue, L.J.; Harding, S.A.; Tsai, C.J. Exploiting SNPs for Biallelic CRISPR Mutations in the Outcrossing Woody Perennial Populus Reveals 4-Coumarate: CoA Ligase Specificity and Redundancy. New Phytol. 2015, 208, 298–301. [Google Scholar] [CrossRef]
- de Vries, L.; Brouckaert, M.; Chanoca, A.; Kim, H.; Regner, M.R.; Timokhin, V.I.; Sun, Y.; De Meester, B.; Van Doorsselaere, J.; Goeminne, G.; et al. CRISPR-Cas9 Editing of CAFFEOYL SHIKIMATE ESTERASE 1 and 2 Shows Their Importance and Partial Redundancy in Lignification in Populus Tremula × P. alba. Plant Biotechnol. J. 2021, 19, 2221–2234. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Ran, L.; Li, C.; Fan, D.; Luo, K. PtoMYB156 Is Involved in Negative Regulation of Phenylpropanoid Metabolism and Secondary Cell Wall Biosynthesis during Wood Formation in Poplar. Sci. Rep. 2017, 7, 41209. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Yu, J.; Wang, Z.; Sun, Y.; Li, S.; Lin, Y.C.J.; Chiang, V.L.; Li, W.; Wang, J.P. Transcriptional Reprogramming of Xylem Cell Wall Biosynthesis in Tension Wood. Plant Physiol. 2021, 186, 250–269. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; Xu, D.; Yang, C.; Li, C.; Luo, K. Molecular Cloning and Characterization of a Brassinosteriod Biosynthesis-Related Gene PtoDWF4 from Populus Tomentosa. Tree Physiol. 2018, 38, 1424–1436. [Google Scholar] [CrossRef]
- Fan, C.; Yu, H.; Qin, S.; Li, Y.; Alam, A.; Xu, C.; Fan, D.; Zhang, Q.; Wang, Y.; Zhu, W.; et al. Brassinosteroid Overproduction Improves Lignocellulose Quantity and Quality to Maximize Bioethanol Yield under Green-like Biomass Process in Transgenic Poplar. Biotechnol. Biofuels 2020, 13, 9. [Google Scholar] [CrossRef]
- Takata, N.; Awano, T.; Nakata, M.T.; Sano, Y.; Sakamoto, S.; Mitsuda, N.; Taniguchi, T. Populus NST/SND Orthologs Are Key Regulators of Secondary Cell Wall Formation in Wood Fibers, Phloem Fibers and Xylem Ray Parenchyma Cells. Tree Physiol. 2019, 39, 514–525. [Google Scholar] [CrossRef]
- Charrier, A.; Vergne, E.; Dousset, N.; Richer, A.; Petiteau, A.; Chevreau, E. Efficient Targeted Mutagenesis in Apple and First Time Edition of Pear Using the CRISPR-Cas9 System. Front. Plant Sci. 2019, 10, 40. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wang, T.; Voogd, C.; Jeon, S.; Drummond, R.S.M.; Gleave, A.P.; Allan, A.C. Mutagenesis of Kiwifruit CENTRORADIALIS-like Genes Transforms a Climbing Woody Perennial with Long Juvenility and Axillary Flowering into a Compact Plant with Rapid Terminal Flowering. Plant Biotechnol. J. 2019, 17, 869–880. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, L.; Ma, X.; Zhao, X.; Jiao, B.; Li, C.; Luo, K. The WRKY Transcription Factors PtrWRKY18 and PtrWRKY35 Promote Melampsora Resistance in Populus. Tree Physiol. 2017, 37, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous CRISPR/Cas9-Mediated Editing of Cassava EIF4E Isoforms NCBP-1 and NCBP-2 Reduces Cassava Brown Streak Disease Symptom Severity and Incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma Cacao. Front. Plant Sci. 2018, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Grape in the First Generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Jiao, Y.T.; Wang, Y.T.; Zhang, N.; Wang, B.B.; Liu, R.Q.; Yin, X.; Xu, Y.; Liu, G.T. CRISPR/Cas9-Mediated VvPR4b Editing Decreases Downy Mildew Resistance in Grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Orbovic, V.; Jones, J.B.; Wang, N. Modification of the PthA4 Effector Binding Elements in Type I CsLOB1 Promoter Using Cas9/SgRNA to Produce Transgenic Duncan Grapefruit Alleviating XccΔpthA4: DCsLOB1.3 Infection. Plant Biotechnol. J. 2016, 14, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering Canker-Resistant Plants through CRISPR/Cas9-Targeted Editing of the Susceptibility Gene CsLOB1 Promoter in Citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Orbović, V.; Wang, N. CRISPR-LbCas12a-Mediated Modification of Citrus. Plant Biotechnol. J. 2019, 17, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, S.; Peng, A.; Xie, Z.; He, Y.; Zou, X. CRISPR/Cas9-Mediated Editing of CsWRKY22 Reduces Susceptibility to Xanthomonas Citri Subsp. Citri in Wanjincheng Orange (Citrus sinensis (L.) Osbeck). Plant Biotechnol. Rep. 2019, 13, 501–510. [Google Scholar] [CrossRef]
- Malabarba, J.; Chevreau, E.; Dousset, N.; Veillet, F.; Moizan, J.; Vergne, E. New Strategies to Overcome Present CRISPR/Cas9 Limitations in Apple and Pear: Efficient Dechimerization and Base Editing. Int. J. Mol. Sci. 2021, 22, 319. [Google Scholar] [CrossRef]
- Jiang, Y.; Tong, S.; Chen, N.; Liu, B.; Bai, Q.; Chen, Y.; Bi, H.; Zhang, Z.; Lou, S.; Tang, H.; et al. The PalWRKY77 Transcription Factor Negatively Regulates Salt Tolerance and Abscisic Acid Signaling in Populus. Plant J. 2021, 105, 1258–1273. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.C.J.; Wang, P.; Zhang, B.; Li, M.; Chen, S.; Shi, R.; Tunlaya-Anukit, S.; Liu, X.; Wang, Z.; et al. The AREB1 Transcription Factor Influences Histone Acetylation to Regulate Drought Responses and Tolerance in Populus trichocarpa. Plant Cell 2019, 31, 663–686. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, X.; Han, X.; An, Y.; Lin, S.; Shen, C.; Wen, J.L.; Liu, C.; Yin, W.; et al. Root-Specific NF-Y Family Transcription Factor, PdNF-YB21, Positively Regulates Root Growth and Drought Resistance by Abscisic Acid-Mediated Indoylacetic Acid Transport in Populus. New Phytol. 2020, 227, 407–426. [Google Scholar] [CrossRef]
- Jia, H.; Nian, W. Targeted Genome Editing of Sweet Orange Using Cas9/SgRNA. PLoS ONE 2014, 9, e93806. [Google Scholar] [CrossRef]
- Fan, D.; Liu, T.; Li, C.; Jiao, B.; Li, S.; Hou, Y.; Luo, K. Efficient CRISPR/Cas9-Mediated Targeted Mutagenesis in Populus in the First Generation. Sci. Rep. 2015, 5, 12217. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, C.; Hirai, N.; Komori, S.; Wada, M.; Okada, K.; Osakabe, K.; Yamamoto, T.; Osakabe, Y. Efficient Genome Editing in Apple Using a CRISPR/Cas9 System. Sci. Rep. 2016, 6, 31481. [Google Scholar] [CrossRef]
- Nakajima, I.; Ban, Y.; Azuma, A.; Onoue, N.; Moriguchi, T.; Yamamoto, T.; Toki, S.; Endo, M. CRISPR/Cas9-Mediated Targeted Mutagenesis in Grape. PLoS ONE 2017, 12, e0177966. [Google Scholar] [CrossRef] [PubMed]
- Odipio, J.; Alicai, T.; Ingelbrecht, I.; Nusinow, D.A.; Bart, R.; Taylor, N.J. Efficient CRISPR/Cas9 Genome Editing of Phytoene Desaturase in Cassava. Front. Plant Sci. 2017, 8, 1780. [Google Scholar] [CrossRef]
- Zhang, F.; LeBlanc, C.; Irish, V.F.; Jacob, Y. Rapid and Efficient CRISPR/Cas9 Gene Editing in Citrus Using the YAO Promoter. Plant Cell Rep. 2017, 36, 1883–1887. [Google Scholar] [CrossRef]
- Breitler, J.C.; Dechamp, E.; Campa, C.; Zebral Rodrigues, L.A.; Guyot, R.; Marraccini, P.; Etienne, H. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis Has the Potential to Accelerate the Domestication of Coffea Canephora. Plant Cell. Tissue Organ. Cult. 2018, 134, 383–394. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Sun, L.; Ma, Y.; Xu, J.; Liang, S.; Deng, J.; Tan, J.; Zhang, Q.; Tu, L.; et al. High Efficient Multisites Genome Editing in Allotetraploid Cotton (Gossypium hirsutum) Using CRISPR/Cas9 System. Plant Biotechnol. J. 2018, 16, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.W.; Zhang, Y.; Zhang, R.; et al. Application of Protoplast Technology to CRISPR/Cas9 Mutagenesis: From Single-Cell Mutation Detection to Mutant Plant Regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Walawage, S.L.; Zaini, P.A.; Mubarik, M.S.; Martinelli, F.; Balan, B.; Caruso, T.; Leslie, C.A.; Dandekar, A.M. Deploying Genome Editing Tools for Dissecting the Biology of Nut Trees. Front. Sustain. Food Syst. 2019, 3, 100. [Google Scholar] [CrossRef]
- An, Y.; Geng, Y.; Yao, J.; Fu, C.; Lu, M.; Wang, C.; Du, J. Efficient Genome Editing in Populus Using CRISPR/Cas12a. Front. Plant Sci. 2020, 11, 593938. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The Transcription Factor MYB115 Contributes to the Regulation of Proanthocyanidin Biosynthesis and Enhances Fungal Resistance in Poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef]
- Wan, S.; Li, C.; Ma, X.; Luo, K.; Repressor, P.Á.; Cas, Á.C. PtrMYB57 Contributes to the Negative Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Poplar. Plant Cell Rep. 2017, 36, 1263–1276. [Google Scholar] [CrossRef]
- Fan, D.; Wang, X.; Tang, X.; Ye, X.; Ren, S.; Wang, D.; Luo, K. Histone H3K9 Demethylase JMJ25 Epigenetically Modulates Anthocyanin Biosynthesis in Poplar. Plant J. 2018, 96, 1121–1136. [Google Scholar] [CrossRef]
- Fellenberg, C.; Corea, O.; Yan, L.; Archinuk, F.; Piirtola, E.; Gordon, H. Discovery of Salicyl Benzoate UDP-Glycosyltransferase, a Central Enzyme in Poplar Salicinoid Phenolic Glycoside Biosynthesis. Plant J. 2020, 102, 99–115. [Google Scholar] [CrossRef]
- Chang, L.; Wu, S.; Tian, L. Effective Genome Editing and Identification of a Regiospecific Gallic Acid 4-O-Glycosyltransferase in Pomegranate (Punica Granatum L.). Hortic. Res. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Chardonnay (Vitis Vinifera L.). Sci. Rep. 2016, 6, 32289. [Google Scholar] [CrossRef]
- Ma, W.; Kang, X.; Liu, P.; Zhang, Y.; Lin, X.; Li, B.; Chen, Z. The Analysis of Transcription Factor CsHB1 Effects on Caffeine Accumulation in Tea Callus through CRISPR/Cas9 Mediated Gene Editing. Process. Biochem. 2021, 101, 304–311. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, L.; Fu, Q.; Xu, Z.F. Identification and Expression Analysis of Cytokinin Metabolic Genes IPTs, CYP735A and CKXs in the Biofuel Plant Jatropha Curcas. PeerJ. 2018, 6, e4812. [Google Scholar] [CrossRef]
- Ye, S.; Chen, G.; Kohnen, M.V.; Wang, W.; Cai, C.; Ding, W.S.; Wu, C.; Gu, L.; Zheng, Y.; Ma, X.; et al. Robust CRISPR/Cas9 Mediated Genome Editing and Its Application in Manipulating Plant Height in the First Generation of Hexaploid Ma Bamboo (Dendrocalamus latiflorus Munro). Plant Biotechnol. J. 2020, 18, 1501–1503. [Google Scholar] [CrossRef]
- Van Zeijl, A.; Wardhani, T.A.K.; Seifi Kalhor, M.; Rutten, L.; Bu, F.; Hartog, M.; Linders, S.; Fedorova, E.E.; Bisseling, T.; Kohlen, W.; et al. CRISPR/Cas9-Mediated Mutagenesis of Four Putative Symbiosis Genes of the Tropical Tree Parasponia Andersonii Reveals Novel Phenotypes. Front. Plant Sci. 2018, 9, 284. [Google Scholar] [CrossRef]
- Ren, C.; Guo, Y.; Kong, J.; Lecourieux, F.; Dai, Z.; Li, S.; Liang, Z. Knockout of VvCCD8 Gene in Grapevine Affects Shoot Branching. BMC Plant Biol. 2020, 20, 47. [Google Scholar] [CrossRef]
- Muhr, M.; Paulat, M.; Awwanah, M.; Brinkkötter, M.; Teichmann, T. CRISPR/Cas9-Mediated Knockout of Populus BRANCHED1 and BRANCHED2 Orthologs Reveals a Major Function in Bud Outgrowth Control. Tree Physiol. 2018, 38, 1588–1597. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Z.; Liang, C.; Meng, Z.; Wang, Y.; Sun, G.; Zhu, T.; Cai, Y.; Guo, S.; Zhang, R.; et al. Increased Lateral Root Formation by CRISPR/Cas9-Mediated Editing of Arginase Genes in Cotton. Sci. China Life Sci. 2017, 60, 524–527. [Google Scholar] [CrossRef]
- Dai, Y.; Hu, G.; Dupas, A.; Medina, L.; Blandels, N.; Clemente, H.S.; Ladouce, N.; Badawi, M.; Hernandez-Raquet, G.; Mounet, F.; et al. Implementing the CRISPR/CAS9 Technology in Eucalyptus Hairy Roots Using Wood-Related Genes. Int. J. Mol. Sci. 2020, 21, 3408. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Holt, N.E.; Kaligotla, S.; Fuciman, M.; Cazzaniga, S.; Carbonera, D.; Frank, H.A.; Alric, J.; Bassi, R. Zeaxanthin Protects Plant Photosynthesis by Modulating Chlorophyll Triplet Yield in Specific Light-Harvesting Antenna Subunits. J. Biol. Chem. 2012, 287, 41820–41834. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, T.; Hwarari, D.; Li, D.; Movahedi, A.; Yang, L. CRISPR-Based Genome Editing and Its Applications in Woody Plants. Int. J. Mol. Sci. 2022, 23, 10175. https://doi.org/10.3390/ijms231710175
Min T, Hwarari D, Li D, Movahedi A, Yang L. CRISPR-Based Genome Editing and Its Applications in Woody Plants. International Journal of Molecular Sciences. 2022; 23(17):10175. https://doi.org/10.3390/ijms231710175
Chicago/Turabian StyleMin, Tian, Delight Hwarari, Dong’ao Li, Ali Movahedi, and Liming Yang. 2022. "CRISPR-Based Genome Editing and Its Applications in Woody Plants" International Journal of Molecular Sciences 23, no. 17: 10175. https://doi.org/10.3390/ijms231710175
APA StyleMin, T., Hwarari, D., Li, D., Movahedi, A., & Yang, L. (2022). CRISPR-Based Genome Editing and Its Applications in Woody Plants. International Journal of Molecular Sciences, 23(17), 10175. https://doi.org/10.3390/ijms231710175









