Connexins Signatures of the Neurovascular Unit and Their Physio-Pathological Functions
Abstract
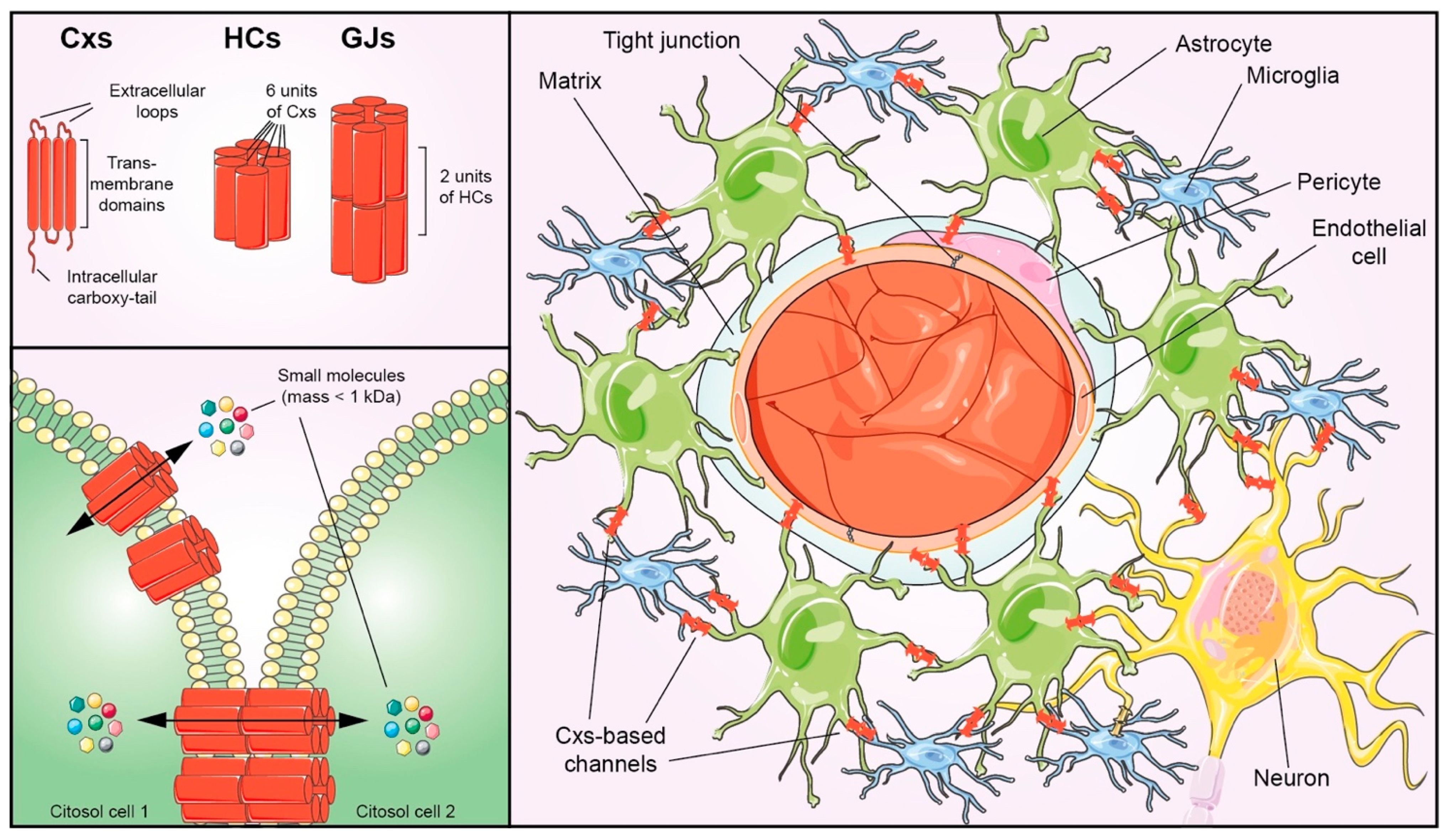
:1. Introduction
2. Connexins Signatures of NVU Components
2.1. Neurons
2.2. Nerve Glial Cells
2.2.1. Astrocytes
2.2.2. Microglia
2.2.3. Oligodendrocytes
2.3. Brain Microvascular Endothelial Cells (BMECs)
2.4. Pericytes
2.5. Smooth Muscle Cells (SMCs)
2.6. Brain-Specific Extracellular Matrix (BSECM)
3. Overview and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ApoE | Apolipoprotein E |
| APP | β-amyloid precursor protein |
| BBB | Blood brain barrier |
| BDNF | Brain-derived neurotropic factor |
| BMEC | Brain microvascular endothelial cell |
| BSECM | Brain-specific extracellular matrix |
| CNS | Central nervous system |
| Cx | Connexin |
| ECM | Extracellular matrix |
| EPO | Erythropoietin |
| GJ | Gap Junction |
| HC | Hemichannel |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IP3 | Inositol 1,4,5-trisphosphate |
| NVU | Neurovascular unit |
| PDGF-B | Platelet-derived growth factor-B |
| PS1 | Presenilin1 |
| SMC | Smooth muscle cell |
| TBI | Traumatic brain injury |
| TGF-β | Transforming growth factor-β |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
References
- Vicario, N.; Bernstock, J.D.; Spitale, F.M.; Giallongo, C.; Giunta, M.A.S.; Li Volti, G.; Gulisano, M.; Leanza, G.; Tibullo, D.; Parenti, R.; et al. Clobetasol Modulates Adult Neural Stem Cell Growth via Canonical Hedgehog Pathway Activation. Int. J. Mol. Sci. 2019, 20, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicario, N.; Spitale, F.M.; Tibullo, D.; Giallongo, C.; Amorini, A.M.; Scandura, G.; Spoto, G.; Saab, M.W.; D’Aprile, S.; Alberghina, C.; et al. Clobetasol promotes neuromuscular plasticity in mice after motoneuronal loss via sonic hedgehog signaling, immunomodulation and metabolic rebalancing. Cell Death Dis. 2021, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Vicario, N.; Calabrese, G.; Zappala, A.; Parenti, C.; Forte, S.; Graziano, A.C.E.; Vanella, L.; Pellitteri, R.; Cardile, V.; Parenti, R. Inhibition of Cx43 mediates protective effects on hypoxic/reoxygenated human neuroblastoma cells. J. Cell. Mol. Med. 2017, 21, 2563–2572. [Google Scholar] [CrossRef]
- Camiolo, G.; Barbato, A.; Giallongo, C.; Vicario, N.; Romano, A.; Parrinello, N.L.; Parenti, R.; Sandoval, J.C.; Garcia-Moreno, D.; Lazzarino, G.; et al. Iron regulates myeloma cell/macrophage interaction and drives resistance to bortezomib. Redox Biol. 2020, 36, 101611. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Longo, A.; Vicario, N.; Romano, A.; Barbato, A.; Di Rosa, M.; Barbagallo, I.; Anfuso, C.D.; Lupo, G.; Gulino, R.; et al. Ixazomib Improves Bone Remodeling and Counteracts sonic Hedgehog signaling Inhibition Mediated by Myeloma Cells. Cancers 2020, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, C.; Castrogiovanni, P.; Imbesi, R.; Tibullo, D.; Li Volti, G.; Barbagallo, I.; Vicario, N.; Musumeci, G.; Di Rosa, M. Middle-aged healthy women and Alzheimer’s disease patients present an overlapping of brain cell transcriptional profile. Neuroscience 2019, 406, 333–344. [Google Scholar] [CrossRef]
- Tibullo, D.; Giallongo, C.; Romano, A.; Vicario, N.; Barbato, A.; Puglisi, F.; Parenti, R.; Amorini, A.M.; Wissam Saab, M.; Tavazzi, B.; et al. Mitochondrial Functions, Energy Metabolism and Protein Glycosylation are Interconnected Processes Mediating Resistance to Bortezomib in Multiple Myeloma Cells. Biomolecules 2020, 10, 696. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Vicario, N.; Li, R.; Nan, L.; Totsch, S.K.; Schlappi, C.; Gessler, F.; Han, X.; Parenti, R.; Beierle, E.A.; et al. Safety and efficacy of oncolytic HSV-1 G207 inoculated into the cerebellum of mice. Cancer Gene Ther. 2020, 27, 246–255. [Google Scholar] [CrossRef]
- Torrisi, F.; Vicario, N.; Spitale, F.M.; Cammarata, F.P.; Minafra, L.; Salvatorelli, L.; Russo, G.; Cuttone, G.; Valable, S.; Gulino, R.; et al. The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance. Cancers 2020, 12, 2860. [Google Scholar] [CrossRef]
- Parenti, R.; Puzzo, L.; Vecchio, G.M.; Gravina, L.; Salvatorelli, L.; Musumeci, G.; Vasquez, E.; Magro, G. Immunolocalization of Wilms’ Tumor protein (WT1) in developing human peripheral sympathetic and gastroenteric nervous system. Acta Histochem. 2014, 116, 48–54. [Google Scholar] [CrossRef]
- Calabrese, V.; Dattilo, S.; Petralia, A.; Parenti, R.; Pennisi, M.; Koverech, G.; Calabrese, V.; Graziano, A.; Monte, I.; Maiolino, L.; et al. Analytical approaches to the diagnosis and treatment of aging and aging-related disease: Redox status and proteomics. Free Radic. Res. 2015, 49, 511–524. [Google Scholar] [CrossRef]
- Parenti, R.; Cicirata, F.; Panto, M.R.; Serapide, M.F. The projections of the lateral reticular nucleus to the deep cerebellar nuclei. An experimental analysis in the rat. Eur. J. Neurosci. 1996, 8, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Panto, M.R.; Cicirata, F.; Angaut, P.; Parenti, R.; Serapide, F. The projection from the primary motor and somatic sensory cortex to the basilar pontine nuclei. A detailed electrophysiological and anatomical study in the rat. J. Hirnforsch. 1995, 36, 7–19. [Google Scholar]
- Cicirata, F.; Parenti, R.; Spinella, F.; Giglio, S.; Tuorto, F.; Zuffardi, O.; Gulisano, M. Genomic organization and chromosomal localization of the mouse Connexin36 (mCx36) gene. Gene 2000, 251, 123–130. [Google Scholar] [CrossRef]
- Vicario, N.; Turnaturi, R.; Spitale, F.M.; Torrisi, F.; Zappala, A.; Gulino, R.; Pasquinucci, L.; Chiechio, S.; Parenti, C.; Parenti, R. Intercellular communication and ion channels in neuropathic pain chronicization. Inflamm. Res. 2020, 69, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Vicario, N.; Zappala, A.; Calabrese, G.; Gulino, R.; Parenti, C.; Gulisano, M.; Parenti, R. Connexins in the Central Nervous System: Physiological Traits and Neuroprotective Targets. Front. Physiol. 2017, 8, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Guldenagel, M.; Deutsch, U.; Sohl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Bruzzone, R.; White, T.W.; Goodenough, D.A. The cellular Internet: On-line with connexins. Bioessays 1996, 18, 709–718. [Google Scholar] [CrossRef]
- Retamal, M.A.; Froger, N.; Palacios-Prado, N.; Ezan, P.; Saez, P.J.; Saez, J.C.; Giaume, C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 2007, 27, 13781–13792. [Google Scholar] [CrossRef]
- Nijjar, S.; Maddison, D.; Meigh, L.; de Wolf, E.; Rodgers, T.; Cann, M.J.; Dale, N. Opposing modulation of Cx26 gap junctions and hemichannels by CO2. J. Physiol. 2021, 599, 103–118. [Google Scholar] [CrossRef]
- Khan, A.K.; Jagielnicki, M.; McIntire, W.E.; Purdy, M.D.; Dharmarajan, V.; Griffin, P.R.; Yeager, M. A Steric ″Ball-and-Chain″ Mechanism for pH-Mediated Regulation of Gap Junction Channels. Cell Rep. 2020, 31, 107482. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, E.; Decrock, E.; De Bock, M.; Yamasaki, H.; Naus, C.C.; Evans, W.H.; Leybaert, L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol. Biol. Cell 2007, 18, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xin, Y.; He, Z.; Hu, W. Function of Connexins in the Interaction between Glial and Vascular Cells in the Central Nervous System and Related Neurological Diseases. Neural Plast. 2018, 2018, 6323901. [Google Scholar] [CrossRef]
- Decrock, E.; De Bock, M.; Wang, N.; Bultynck, G.; Giaume, C.; Naus, C.C.; Green, C.R.; Leybaert, L. Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cell. Mol. Life Sci. 2015, 72, 2823–2851. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Leybaert, L.; Giaume, C. Connexin Channels at the Glio-Vascular Interface: Gatekeepers of the Brain. Neurochem. Res. 2017, 42, 2519–2536. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnar, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Arcuino, G.; Takano, T.; Liu, Q.S.; Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 2003, 23, 9254–9262. [Google Scholar] [CrossRef]
- Pannasch, U.; Vargova, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Sykova, E.; Rouach, N. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef] [Green Version]
- Nakase, T.; Maeda, T.; Yoshida, Y.; Nagata, K. Ischemia alters the expression of connexins in the aged human brain. J. Biomed. Biotechnol. 2009, 2009, 147946. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stackhouse, T.L.; Mishra, A. Neurovascular Coupling in Development and Disease: Focus on Astrocytes. Front. Cell Dev. Biol. 2021, 9, 702832. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Hirsch, I.B.; Cryer, P.E. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am. J. Physiol. 1996, 270, H554–H559. [Google Scholar] [CrossRef]
- Lindauer, U.; Leithner, C.; Kaasch, H.; Rohrer, B.; Foddis, M.; Fuchtemeier, M.; Offenhauser, N.; Steinbrink, J.; Royl, G.; Kohl-Bareis, M.; et al. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J. Cereb. Blood Flow Metab. 2010, 30, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Buxton, R.B. The thermodynamics of thinking: Connections between neural activity, energy metabolism and blood flow. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190624. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Giaume, C. Neurons set the tone of gap junctional communication in astrocytic networks. Neurochem. Int. 2004, 45, 265–272. [Google Scholar] [CrossRef]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef]
- Charveriat, M.; Naus, C.C.; Leybaert, L.; Saez, J.C.; Giaume, C. Connexin-Dependent Neuroglial Networking as a New Therapeutic Target. Front. Cell. Neurosci. 2017, 11, 174. [Google Scholar] [CrossRef]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Wong, W.S.; Clinton, B.K.; Kriegstein, A.R. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J. Neurosci. 2002, 22, 3161–3173. [Google Scholar] [CrossRef] [Green Version]
- Kilb, W.; Kirischuk, S.; Luhmann, H.J. Electrical activity patterns and the functional maturation of the neocortex. Eur. J. Neurosci. 2011, 34, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Schock, S.C.; Leblanc, D.; Hakim, A.M.; Thompson, C.S. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem. Biophys. Res. Commun. 2008, 368, 138–144. [Google Scholar] [CrossRef]
- Moore, A.R.; Zhou, W.L.; Sirois, C.L.; Belinsky, G.S.; Zecevic, N.; Antic, S.D. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc. Natl. Acad. Sci. USA 2014, 111, E3919–E3928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenti, R.; Gulisano, M.; Zappala, A.; Cicirata, F. Expression of connexin36 mRNA in adult rodent brain. Neuroreport 2000, 11, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, M.; Parenti, R.; Spinella, F.; Cicirata, F. Cx36 is dynamically expressed during early development of mouse brain and nervous system. Neuroreport 2000, 11, 3823–3828. [Google Scholar] [CrossRef]
- Condorelli, D.F.; Parenti, R.; Spinella, F.; Trovato Salinaro, A.; Belluardo, N.; Cardile, V.; Cicirata, F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur. J. Neurosci. 1998, 10, 1202–1208. [Google Scholar] [CrossRef]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.; Schousboe, A.; Haydon, P.G.; Stout, R.F., Jr.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial cells in (patho)physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef] [Green Version]
- Barker, A.J.; Ullian, E.M. New roles for astrocytes in developing synaptic circuits. Commun. Integr. Biol. 2008, 1, 207–211. [Google Scholar] [CrossRef]
- Bramanti, V.; Grasso, S.; Tibullo, D.; Giallongo, C.; Pappa, R.; Brundo, M.V.; Tomassoni, D.; Viola, M.; Amenta, F.; Avola, R. Neuroactive molecules and growth factors modulate cytoskeletal protein expression during astroglial cell proliferation and differentiation in culture. J. Neurosci. Res. 2016, 94, 90–98. [Google Scholar] [CrossRef]
- Iadecola, C.; Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007, 10, 1369–1376. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef] [Green Version]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Orellana, J.A.; Stehberg, J. Hemichannels: New roles in astroglial function. Front. Physiol. 2014, 5, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eroglu, C.; Barres, B.A. Regulation of synaptic connectivity by glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef]
- Cotman, C.W.; Nieto-Sampedro, M. Cell biology of synaptic plasticity. Science 1984, 225, 1287–1294. [Google Scholar] [CrossRef]
- Mazaud, D.; Capano, A.; Rouach, N. The many ways astroglial connexins regulate neurotransmission and behavior. Glia 2021, 69, 2527–2545. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, A.; Stout, R.; Timmermann, A.; de Menezes, L.; Guo, P.; Maass, K.; Seifert, G.; Steinhauser, C.; Spray, D.C.; Scemes, E. Cx43 carboxyl terminal domain determines AQP4 and Cx30 endfoot organization and blood brain barrier permeability. Sci. Rep. 2021, 11, 24334. [Google Scholar] [CrossRef]
- Drewes, L.R. Making connexons in the neurovascular unit. J. Cereb. Blood Flow Metab. 2012, 32, 1455–1456. [Google Scholar] [CrossRef] [Green Version]
- Tanigami, H.; Okamoto, T.; Yasue, Y.; Shimaoka, M. Astroglial integrins in the development and regulation of neurovascular units. Pain Res. Treat. 2012, 2012, 964652. [Google Scholar] [CrossRef]
- Munoz, M.F.; Puebla, M.; Figueroa, X.F. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front. Cell. Neurosci. 2015, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Theis, M.; Jauch, R.; Zhuo, L.; Speidel, D.; Wallraff, A.; Doring, B.; Frisch, C.; Sohl, G.; Teubner, B.; Euwens, C.; et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 2003, 23, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.E.; Zhao, Y.; Gulinello, M.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 2009, 29, 7743–7752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezan, P.; Andre, P.; Cisternino, S.; Saubamea, B.; Boulay, A.C.; Doutremer, S.; Thomas, M.A.; Quenech’du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of astroglial connexins weakens the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Dere, E.; De Souza-Silva, M.A.; Frisch, C.; Teubner, B.; Sohl, G.; Willecke, K.; Huston, J.P. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur. J. Neurosci. 2003, 18, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Pannasch, U.; Derangeon, M.; Chever, O.; Rouach, N. Astroglial gap junctions shape neuronal network activity. Commun. Integr. Biol. 2012, 5, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Hillen, A.E.J.; Burbach, J.P.H.; Hol, E.M. Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog. Neurobiol. 2018, 165–167, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Pannasch, U.; Ezan, P.; Rouach, N. Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos. Trans. R Soc. B Biol. Sci. 2014, 369, 20130596. [Google Scholar] [CrossRef] [Green Version]
- Ebong, E.E.; Depaola, N. Specificity in the participation of connexin proteins in flow-induced endothelial gap junction communication. Pflugers Arch. 2013, 465, 1293–1302. [Google Scholar] [CrossRef]
- Alvarez-Maubecin, V.; Garcia-Hernandez, F.; Williams, J.T.; Van Bockstaele, E.J. Functional coupling between neurons and glia. J. Neurosci. 2000, 20, 4091–4098. [Google Scholar] [CrossRef] [Green Version]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef] [Green Version]
- Striedinger, K.; Meda, P.; Scemes, E. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia 2007, 55, 652–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulay, A.C.; Saubamea, B.; Cisternino, S.; Mignon, V.; Mazeraud, A.; Jourdren, L.; Blugeon, C.; Cohen-Salmon, M. The Sarcoglycan complex is expressed in the cerebrovascular system and is specifically regulated by astroglial Cx30 channels. Front. Cell. Neurosci. 2015, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaume, C.; Leybaert, L.; Naus, C.C.; Saez, J.C. Connexin and pannexin hemichannels in brain glial cells: Properties, pharmacology, and roles. Front. Pharmacol. 2013, 4, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustmann, P.M.; Haase, C.G.; Romberg, S.; Hinkerohe, D.; Szlachta, D.; Smikalla, D.; Krause, D.; Dermietzel, R. Microglia activation influences dye coupling and Cx43 expression of the astrocytic network. Glia 2003, 42, 101–108. [Google Scholar] [CrossRef]
- Koulakoff, A.; Ezan, P.; Giaume, C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia 2008, 56, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Miyamoto, A.; Nabekura, J. Microglia: Actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013, 36, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Csaszar, E.; Lenart, N.; Cserep, C.; Kornyei, Z.; Fekete, R.; Posfai, B.; Balazsfi, D.; Hangya, B.; Schwarcz, A.D.; Szabadits, E.; et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 2022, 219, e20211071. [Google Scholar] [CrossRef]
- Gajardo-Gomez, R.; Labra, V.C.; Orellana, J.A. Connexins and Pannexins: New Insights into Microglial Functions and Dysfunctions. Front. Mol. Neurosci. 2016, 9, 86. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The ″quad-partite″ synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat.Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenti, R.; Campisi, A.; Vanella, A.; Cicirata, F. Immunocytochemical and RT-PCR analysis of connexin36 in cultures of mammalian glial cells. Arch. Ital. Biol. 2002, 140, 101–108. [Google Scholar] [PubMed]
- Moon, Y.; Choi, S.Y.; Kim, K.; Kim, H.; Sun, W. Expression of connexin29 and 32 in the penumbra region after traumatic brain injury of mice. Neuroreport 2010, 21, 1135–1139. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Eckardt, D.; Theis, M.; Willecke, K.; Bennett, M.V.; Saez, J.C. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc. Natl. Acad. Sci. USA 2001, 98, 4190–4195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez, P.J.; Shoji, K.F.; Retamal, M.A.; Harcha, P.A.; Ramirez, G.; Jiang, J.X.; von Bernhardi, R.; Saez, J.C. ATP is required and advances cytokine-induced gap junction formation in microglia in vitro. Mediat. Inflamm. 2013, 2013, 216402. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cepeda, C.; Chang, J.W.; Owens, G.C.; Huynh, M.N.; Chen, J.Y.; Tran, C.; Vinters, H.V.; Levine, M.S.; Mathern, G.W. In Rasmussen encephalitis, hemichannels associated with microglial activation are linked to cortical pyramidal neuron coupling: A possible mechanism for cellular hyperexcitability. CNS Neurosci. Ther. 2015, 21, 152–163. [Google Scholar] [CrossRef]
- Liu, Y.D.; Tang, G.; Qian, F.; Liu, L.; Huang, J.R.; Tang, F.R. Astroglial Connexins in Neurological and Neuropsychological Disorders and Radiation Exposure. Curr. Med. Chem. 2021, 28, 1970–1986. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, W.; Wang, L.; Jiang, J.; Nie, H.; Wang, B.; Wei, X.; Ying, W. Basal CD38/cyclic ADP-ribose-dependent signaling mediates ATP release and survival of microglia by modulating connexin 43 hemichannels. Glia 2014, 62, 943–955. [Google Scholar] [CrossRef]
- Orellana, J.A.; Figueroa, X.F.; Sanchez, H.A.; Contreras-Duarte, S.; Velarde, V.; Saez, J.C. Hemichannels in the neurovascular unit and white matter under normal and inflamed conditions. CNS Neurol. Disord. Drug Targets 2011, 10, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Parenti, R.; Cicirata, F.; Zappala, A.; Catania, A.; La Delia, F.; Cicirata, V.; Tress, O.; Willecke, K. Dynamic expression of Cx47 in mouse brain development and in the cuprizone model of myelin plasticity. Glia 2010, 58, 1594–1609. [Google Scholar] [CrossRef] [PubMed]
- Dermietzel, R.; Farooq, M.; Kessler, J.A.; Althaus, H.; Hertzberg, E.L.; Spray, D.C. Oligodendrocytes express gap junction proteins connexin32 and connexin45. Glia 1997, 20, 101–114. [Google Scholar] [CrossRef]
- Eiberger, J.; Kibschull, M.; Strenzke, N.; Schober, A.; Bussow, H.; Wessig, C.; Djahed, S.; Reucher, H.; Koch, D.A.; Lautermann, J.; et al. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia 2006, 53, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, P.; Blumcke, I.; Traub, O.; Dermietzel, R.; Willecke, K. Coexpression of connexin45 and -32 in oligodendrocytes of rat brain. J. Neurocytol. 1997, 26, 17–22. [Google Scholar] [CrossRef]
- Menichella, D.M.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Connexins are critical for normal myelination in the CNS. J. Neurosci. 2003, 23, 5963–5973. [Google Scholar] [CrossRef]
- Nagy, J.I.; Dudek, F.E.; Rash, J.E. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res. Rev. 2004, 47, 191–215. [Google Scholar] [CrossRef]
- Nagy, J.I.; Ionescu, A.V.; Lynn, B.D.; Rash, J.E. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 2003, 44, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Maglione, M.; Tress, O.; Haas, B.; Karram, K.; Trotter, J.; Willecke, K.; Kettenmann, H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 2010, 58, 1104–1117. [Google Scholar] [CrossRef]
- Orthmann-Murphy, J.L.; Freidin, M.; Fischer, E.; Scherer, S.S.; Abrams, C.K. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J. Neurosci. 2007, 27, 13949–13957. [Google Scholar] [CrossRef] [Green Version]
- Kamasawa, N.; Sik, A.; Morita, M.; Yasumura, T.; Davidson, K.G.; Nagy, J.I.; Rash, J.E. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: Implications for ionic homeostasis and potassium siphoning. Neuroscience 2005, 136, 65–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menichella, D.M.; Majdan, M.; Awatramani, R.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J. Neurosci. 2006, 26, 10984–10991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Li, T.; Yi, C.; Huang, N.; Koulakoff, A.; Weng, C.; Li, C.; Zhao, C.J.; Giaume, C.; Xiao, L. Connexin-based channels contribute to metabolic pathways in the oligodendroglial lineage. J. Cell Sci. 2016, 129, 1902–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, K.; Lo, E.H. An oligovascular niche: Cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 2009, 29, 4351–4355. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, G.; Ohtomo, R.; Takase, H.; Lok, J.; Arai, K. White-matter repair: Interaction between oligodendrocytes and the neurovascular unit. Brain Circ. 2018, 4, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, Z.; Tian, Y.; Zhang, H.; Fang, Y.; Yu, Z.; Wang, W.; Xie, M.; Ding, F. Inhibition of Astrocyte Connexin 43 Channels Facilitates the Differentiation of Oligodendrocyte Precursor Cells Under Hypoxic Conditions In Vitro. J. Mol. Neurosci. 2018, 64, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Keep, R.F.; Andjelkovic, A.V.; Xiang, J.; Stamatovic, S.M.; Antonetti, D.A.; Hua, Y.; Xi, G. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cereb. Blood Flow Metab. 2018, 38, 1255–1275. [Google Scholar] [CrossRef]
- Hautefort, A.; Pfenniger, A.; Kwak, B.R. Endothelial connexins in vascular function. Vasc. Biol. 2019, 1, H117–H124. [Google Scholar] [CrossRef] [Green Version]
- Hogan-Cann, A.D.; Lu, P.; Anderson, C.M. Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 10229–10231. [Google Scholar] [CrossRef] [Green Version]
- Stobart, J.L.; Lu, L.; Anderson, H.D.; Mori, H.; Anderson, C.M. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2013, 110, 3149–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccolo, E.; Lim, D.; Kheder, D.A.; Perna, A.; Catarsi, P.; Botta, L.; Rosti, V.; Riboni, L.; Sancini, G.; Tanzi, F.; et al. Acetylcholine induces intracellular Ca(2+) oscillations and nitric oxide release in mouse brain endothelial cells. Cell Calcium 2017, 66, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Akiyama, M.; Takeda, M.; Gabazza, E.C.; Hayashi, T.; Suzuki, K. Connexin32 is expressed in vascular endothelial cells and participates in gap-junction intercellular communication. Biochem. Biophys. Res. Commun. 2009, 382, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Akiyama, M.; Takeda, M.; Akita, N.; Yoshida, K.; Hayashi, T.; Suzuki, K. Connexin32 protects against vascular inflammation by modulating inflammatory cytokine expression by endothelial cells. Exp. Cell Res. 2011, 317, 348–355. [Google Scholar] [CrossRef]
- Sokoya, E.M.; Burns, A.R.; Setiawan, C.T.; Coleman, H.A.; Parkington, H.C.; Tare, M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H385–H393. [Google Scholar] [CrossRef]
- Seul, K.H.; Kang, K.Y.; Lee, K.S.; Kim, S.H.; Beyer, E.C. Adenoviral delivery of human connexin37 induces endothelial cell death through apoptosis. Biochem. Biophys. Res. Commun. 2004, 319, 1144–1151. [Google Scholar] [CrossRef]
- Pogoda, K.; Fuller, M.; Pohl, U.; Kameritsch, P. NO, via its target Cx37, modulates calcium signal propagation selectively at myoendothelial gap junctions. Cell Commun. Signal. 2014, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Arthur, F.E.; Shivers, R.R.; Bowman, P.D. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: An efficient in vitro model. Brain Res. 1987, 433, 155–159. [Google Scholar] [CrossRef]
- Rubin, L.L.; Hall, D.E.; Porter, S.; Barbu, K.; Cannon, C.; Horner, H.C.; Janatpour, M.; Liaw, C.W.; Manning, K.; Morales, J.; et al. A cell culture model of the blood-brain barrier. J. Cell Biol. 1991, 115, 1725–1735. [Google Scholar] [CrossRef] [Green Version]
- Cibelli, A.; Veronica Lopez-Quintero, S.; McCutcheon, S.; Scemes, E.; Spray, D.C.; Stout, R.F., Jr.; Suadicani, S.O.; Thi, M.M.; Urban-Maldonado, M. Generation and Characterization of Immortalized Mouse Cortical Astrocytes From Wildtype and Connexin43 Knockout Mice. Front. Cell. Neurosci. 2021, 15, 647109. [Google Scholar] [CrossRef]
- Wuestefeld, R.; Chen, J.; Meller, K.; Brand-Saberi, B.; Theiss, C. Impact of vegf on astrocytes: Analysis of gap junctional intercellular communication, proliferation, and motility. Glia 2012, 60, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, H.; Sun, W.; Nan, D.; Deng, J.; Jia, J.; Yu, Z.; Huang, Y. Connexin43 promotes angiogenesis through activating the HIF-1alpha/VEGF signaling pathway under chronic cerebral hypoperfusion. J. Cereb. Blood Flow Metab. 2021, 41, 2656–2675. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, Z.; Wu, M.; Yang, Y.; Zhang, J.; Ou, J.; Zuo, Z.; Wang, J.; Chen, Y. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1alpha via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res. Ther. 2016, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Asahara, T.; Takahashi, T.; Masuda, H.; Kalka, C.; Chen, D.; Iwaguro, H.; Inai, Y.; Silver, M.; Isner, J.M. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef] [Green Version]
- Gartner, C.; Ziegelhoffer, B.; Kostelka, M.; Stepan, H.; Mohr, F.W.; Dhein, S. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol. Res. 2012, 65, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Cho, J.H.; Reid, B.; Tseng, C.C.; He, L.; Tan, P.; Yeh, C.Y.; Wu, P.; Li, Y.; Widelitz, R.B.; et al. Calcium oscillations coordinate feather mesenchymal cell movement by SHH dependent modulation of gap junction networks. Nat. Commun. 2018, 9, 5377. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [Green Version]
- Dore-Duffy, P. Pericytes: Pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 2008, 14, 1581–1593. [Google Scholar] [CrossRef]
- Uemura, M.T.; Maki, T.; Ihara, M.; Lee, V.M.Y.; Trojanowski, J.Q. Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front. Aging Neurosci. 2020, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, L.B.; Tewari, B.P.; Dunkenberger, L.; Bond, S.; Savelli, A.; Darden, J.; Zhao, H.; Willi, C.; Kanodia, R.; Gude, R.; et al. Pericyte Progenitor Coupling to the Emerging Endothelium During Vasculogenesis via Connexin 43. Arter. Thromb. Vasc. Biol. 2022, 42, e96–e114. [Google Scholar] [CrossRef] [PubMed]
- Perrot, C.Y.; Herrera, J.L.; Fournier-Goss, A.E.; Komatsu, M. Prostaglandin E2 breaks down pericyte-endothelial cell interaction via EP1 and EP4-dependent downregulation of pericyte N-cadherin, connexin-43, and R-Ras. Sci. Rep. 2020, 10, 11186. [Google Scholar] [CrossRef] [PubMed]
- Mazare, N.; Gilbert, A.; Boulay, A.C.; Rouach, N.; Cohen-Salmon, M. Connexin 30 is expressed in a subtype of mouse brain pericytes. Brain Struct. Funct. 2018, 223, 1017–1024. [Google Scholar] [CrossRef]
- Silva, M.E.; Lange, S.; Hinrichsen, B.; Philp, A.R.; Reyes, C.R.; Halabi, D.; Mansilla, J.B.; Rotheneichner, P.; Guzman de la Fuente, A.; Couillard-Despres, S.; et al. Pericytes Favor Oligodendrocyte Fate Choice in Adult Neural Stem Cells. Front. Cell Neurosci. 2019, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, A.G.; Lange, S.; Silva, M.E.; Gonzalez, G.A.; Tempfer, H.; van Wijngaarden, P.; Zhao, C.; Di Canio, L.; Trost, A.; Bieler, L.; et al. Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination. Cell Rep. 2017, 20, 1755–1764. [Google Scholar] [CrossRef] [Green Version]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit-concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Quelhas, P.; Baltazar, G.; Cairrao, E. The Neurovascular Unit: Focus on the Regulation of Arterial Smooth Muscle Cells. Curr. Neurovasc. Res. 2019, 16, 502–515. [Google Scholar] [CrossRef]
- Pan, Q.; He, C.; Liu, H.; Liao, X.; Dai, B.; Chen, Y.; Yang, Y.; Zhao, B.; Bihl, J.; Ma, X. Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol. Brain 2016, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Filosa, J.A.; Morrison, H.W.; Iddings, J.A.; Du, W.; Kim, K.J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 2016, 323, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Mariana, M.; Roque, C.; Baltazar, G.; Cairrao, E. In Vitro Model for Ischemic Stroke: Functional Analysis of Vascular Smooth Muscle Cells. Cell. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Filiberto, A.C.; Spinosa, M.D.; Elder, C.T.; Su, G.; Leroy, V.; Ladd, Z.; Lu, G.; Mehaffey, J.H.; Salmon, M.D.; Hawkins, R.B.; et al. Endothelial pannexin-1 channels modulate macrophage and smooth muscle cell activation in abdominal aortic aneurysm formation. Nat. Commun. 2022, 13, 1521. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; He, W.; Yang, R.; Liu, L.; Zhang, Y.; Li, L.; Si, J.; Li, X.; Ma, K. Inhibition of Connexin 43 reverses ox-LDL-mediated inhibition of autophagy in VSMC by inhibiting the PI3K/Akt/mTOR signaling pathway. PeerJ 2022, 10, e12969. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Zhao, D. Modulation of Vascular Smooth Muscle Cell Multiplication, Apoptosis, and Inflammatory Damage by miR-21 in Coronary Heart Disease. Comput. Math. Methods Med. 2021, 2021, 6942699. [Google Scholar] [CrossRef] [PubMed]
- Faissner, A.; Pyka, M.; Geissler, M.; Sobik, T.; Frischknecht, R.; Gundelfinger, E.D.; Seidenbecher, C. Contributions of astrocytes to synapse formation and maturation-Potential functions of the perisynaptic extracellular matrix. Brain Res. Rev. 2010, 63, 26–38. [Google Scholar] [CrossRef]
- Michalski, D.; Spielvogel, E.; Puchta, J.; Reimann, W.; Barthel, H.; Nitzsche, B.; Mages, B.; Jager, C.; Martens, H.; Horn, A.K.E.; et al. Increased Immunosignals of Collagen IV and Fibronectin Indicate Ischemic Consequences for the Neurovascular Matrix Adhesion Zone in Various Animal Models and Human Stroke Tissue. Front. Physiol. 2020, 11, 575598. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Del Zoppo, G.J.; Milner, R.; Mabuchi, T.; Hung, S.; Wang, X.; Koziol, J.A. Vascular matrix adhesion and the blood-brain barrier. Biochem. Soc. Trans. 2006, 34, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Gatseva, A.; Sin, Y.Y.; Brezzo, G.; Van Agtmael, T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019, 63, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, M.J.; Damodarasamy, M.; Banks, W.A. The extracellular matrix of the blood-brain barrier: Structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers 2019, 7, 1651157. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Rozental, A.F.; Rozental, R.; Hopperstad, M.G.; Wu, J.K.; Vrionis, F.D.; Spray, D.C. Gap junctions: The ″kiss of death″ and the ″kiss of life″. Brain Res. Rev. 2000, 32, 308–315. [Google Scholar] [CrossRef]
- Spray, D.C.; Hanstein, R.; Lopez-Quintero, S.V.; Stout, R.F., Jr.; Suadicani, S.O.; Thi, M.M. Gap junctions and Bystander Effects: Good Samaritans and executioners. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Decrock, E.; Vinken, M.; De Vuyst, E.; Krysko, D.V.; D’Herde, K.; Vanhaecke, T.; Vandenabeele, P.; Rogiers, V.; Leybaert, L. Connexin-related signaling in cell death: To live or let die? Cell Death Differ. 2009, 16, 524–536. [Google Scholar] [CrossRef]
- Dermietzel, R.; Gao, Y.; Scemes, E.; Vieira, D.; Urban, M.; Kremer, M.; Bennett, M.V.; Spray, D.C. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res. Rev. 2000, 32, 45–56. [Google Scholar] [CrossRef]
- Rouach, N.; Avignone, E.; Meme, W.; Koulakoff, A.; Venance, L.; Blomstrand, F.; Giaume, C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol. Cell 2002, 94, 457–475. [Google Scholar] [CrossRef]
- Giaume, C.; Naus, C.C.; Saez, J.C.; Leybaert, L. Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol. Rev. 2021, 101, 93–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, S.; Liu, Y.; Siddique, R.; Zhang, R.; Yong, V.W.; Xue, M. Gap Junctions and Hemichannels Composed of Connexins and Pannexins Mediate the Secondary Brain Injury Following Intracerebral Hemorrhage. Biology 2021, 11, 27. [Google Scholar] [CrossRef]
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032. [Google Scholar] [CrossRef]
- Yu, H.; Cao, X.; Li, W.; Liu, P.; Zhao, Y.; Song, L.; Chen, J.; Chen, B.; Yu, W.; Xu, Y. Targeting connexin 43 provides anti-inflammatory effects after intracerebral hemorrhage injury by regulating YAP signaling. J. Neuroinflammation 2020, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Davidson, J.O.; Gunn, K.C.; Phillips, A.R.; Green, C.R.; Gunn, A.J. Role of Hemichannels in CNS Inflammation and the Inflammasome Pathway. Adv. Protein Chem. Struct. Biol. 2016, 104, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Brand-Schieber, E.; Werner, P.; Iacobas, D.A.; Iacobas, S.; Beelitz, M.; Lowery, S.L.; Spray, D.C.; Scemes, E. Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. J. Neurosci. Res. 2005, 80, 798–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markoullis, K.; Sargiannidou, I.; Gardner, C.; Hadjisavvas, A.; Reynolds, R.; Kleopa, K.A. Disruption of oligodendrocyte gap junctions in experimental autoimmune encephalomyelitis. Glia 2012, 60, 1053–1066. [Google Scholar] [CrossRef]
- Mei, X.; Ezan, P.; Giaume, C.; Koulakoff, A. Astroglial connexin immunoreactivity is specifically altered at beta-amyloid plaques in beta-amyloid precursor protein/presenilin1 mice. Neuroscience 2010, 171, 92–105. [Google Scholar] [CrossRef]
- Spitale, F.M.; Vicario, N.; Rosa, M.D.; Tibullo, D.; Vecchio, M.; Gulino, R.; Parenti, R. Increased expression of connexin 43 in a mouse model of spinal motoneuronal loss. Aging 2020, 12, 12598–12608. [Google Scholar] [CrossRef]
- Almad, A.A.; Doreswamy, A.; Gross, S.K.; Richard, J.P.; Huo, Y.; Haughey, N.; Maragakis, N.J. Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis. Glia 2016, 64, 1154–1169. [Google Scholar] [CrossRef] [Green Version]
- Almad, A.A.; Taga, A.; Joseph, J.; Gross, S.K.; Welsh, C.; Patankar, A.; Richard, J.P.; Rust, K.; Pokharel, A.; Plott, C.; et al. Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS. Proc. Natl. Acad. Sci. USA 2022, 119, e2107391119. [Google Scholar] [CrossRef]
- Harcha, P.A.; Garces, P.; Arredondo, C.; Fernandez, G.; Saez, J.C.; van Zundert, B. Mast Cell and Astrocyte Hemichannels and Their Role in Alzheimer’s Disease, ALS, and Harmful Stress Conditions. Int. J. Mol. Sci. 2021, 22, 1924. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Zhang, L.; Chen, B.; Yang, L.; Li, X.; Li, Y.; Yu, H. Inhibition of Connexin 43 Hemichannels Alleviates Cerebral Ischemia/Reperfusion Injury via the TLR4 Signaling Pathway. Front. Cell. Neurosci. 2018, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Yang, L.; Chen, J.; Chen, Y.; Zhang, L.; Wang, L.; Li, X.; Li, Y.; Yu, H. Inhibition of Connexin43 hemichannels with Gap19 protects cerebral ischemia/reperfusion injury via the JAK2/STAT3 pathway in mice. Brain Res. Bull. 2019, 146, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wei, X.; Xiang, J.; Gao, J.; Wang, L.; You, J.; Cai, Y.; Cai, D. Protection of erythropoietin against ischemic neurovascular unit injuries through the effects of connexin43. Biochem. Biophys. Res. Commun. 2015, 458, 656–662. [Google Scholar] [CrossRef]
- Freitas-Andrade, M.; Bechberger, J.; Wang, J.; Yeung, K.K.C.; Whitehead, S.N.; Hansen, R.S.; Naus, C.C. Danegaptide Enhances Astrocyte Gap Junctional Coupling and Reduces Ischemic Reperfusion Brain Injury in Mice. Biomolecules 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Wallerstein, A.; Abdul-Muneer, P.M. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019, 317, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, C.G.; Ivanova, E.; Sagdullaev, B.T. Of neurons and pericytes: The neuro-vascular approach to diabetic retinopathy. Vis. Neurosci. 2020, 37, E005. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Sohl, G.; Theis, M.; Willecke, K.; Naus, C.C. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am. J. Pathol. 2004, 164, 2067–2075. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Yi, C.; Luo, X.; Xu, S.; Yu, Z.; Tang, Y.; Zhu, W.; Du, Y.; Jia, L.; Zhang, Q.; et al. Glial gap junctional communication involvement in hippocampal damage after middle cerebral artery occlusion. Ann. Neurol. 2011, 70, 121–132. [Google Scholar] [CrossRef]
- Freitas, A.S.; Xavier, A.L.; Furtado, C.M.; Hedin-Pereira, C.; Froes, M.M.; Menezes, J.R. Dye coupling and connexin expression by cortical radial glia in the early postnatal subventricular zone. Dev. Neurobiol. 2012, 72, 1482–1497. [Google Scholar] [CrossRef]
- Liu, X.; Bolteus, A.J.; Balkin, D.M.; Henschel, O.; Bordey, A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia 2006, 54, 394–410. [Google Scholar] [CrossRef]
- Li, T.; Niu, J.; Yu, G.; Ezan, P.; Yi, C.; Wang, X.; Koulakoff, A.; Gao, X.; Chen, X.; Saez, J.C.; et al. Connexin 43 deletion in astrocytes promotes CNS remyelination by modulating local inflammation. Glia 2020, 68, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Glen, C.M.; McDevitt, T.C.; Kemp, M.L. Dynamic intercellular transport modulates the spatial patterning of differentiation during early neural commitment. Nat. Commun. 2018, 9, 4111. [Google Scholar] [CrossRef] [PubMed]
- Morioka, N.; Nakamura, Y.; Zhang, F.F.; Hisaoka-Nakashima, K.; Nakata, Y. Role of Connexins in Chronic Pain and Their Potential as Therapeutic Targets for Next-Generation Analgesics. Biol. Pharm. Bull. 2019, 42, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Vicario, N.; Pasquinucci, L.; Spitale, F.M.; Chiechio, S.; Turnaturi, R.; Caraci, F.; Tibullo, D.; Avola, R.; Gulino, R.; Parenti, R.; et al. Simultaneous Activation of Mu and Delta Opioid Receptors Reduces Allodynia and Astrocytic Connexin 43 in an Animal Model of Neuropathic Pain. Mol. Neurobiol. 2019, 56, 7338–7354. [Google Scholar] [CrossRef] [PubMed]
- Vicario, N.; Denaro, S.; Turnaturi, R.; Longhitano, L.; Spitale, F.M.; Spoto, S.; Marrazzo, A.; Zappala, A.; Tibullo, D.; Li Volti, G.; et al. Mu and Delta Opioid Receptor Targeting Reduces Connexin 43-Based Heterocellular Coupling during Neuropathic Pain. Int. J. Mol. Sci. 2022, 23, 5864. [Google Scholar] [CrossRef]
- Torrisi, F.; Alberghina, C.; D’Aprile, S.; Pavone, A.M.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Di Rosa, M.; Zappala, A.; Cammarata, F.P.; et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines 2022, 10, 806. [Google Scholar] [CrossRef]
- Torrisi, F.; Alberghina, C.; Lo Furno, D.; Zappala, A.; Valable, S.; Li Volti, G.; Tibullo, D.; Vicario, N.; Parenti, R. Connexin 43 and Sonic Hedgehog Pathway Interplay in Glioblastoma Cell Proliferation and Migration. Biology 2021, 10, 767. [Google Scholar] [CrossRef]
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- Murphy, S.F.; Varghese, R.T.; Lamouille, S.; Guo, S.; Pridham, K.J.; Kanabur, P.; Osimani, A.M.; Sharma, S.; Jourdan, J.; Rodgers, C.M.; et al. Connexin 43 Inhibition Sensitizes Chemoresistant Glioblastoma Cells to Temozolomide. Cancer Res. 2016, 76, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicario, N.; Parenti, R. Connexins Signatures of the Neurovascular Unit and Their Physio-Pathological Functions. Int. J. Mol. Sci. 2022, 23, 9510. https://doi.org/10.3390/ijms23179510
Vicario N, Parenti R. Connexins Signatures of the Neurovascular Unit and Their Physio-Pathological Functions. International Journal of Molecular Sciences. 2022; 23(17):9510. https://doi.org/10.3390/ijms23179510
Chicago/Turabian StyleVicario, Nunzio, and Rosalba Parenti. 2022. "Connexins Signatures of the Neurovascular Unit and Their Physio-Pathological Functions" International Journal of Molecular Sciences 23, no. 17: 9510. https://doi.org/10.3390/ijms23179510






