Targeting Melanin in Melanoma with Radionuclide Therapy
Abstract
:1. Introduction
2. Methodology
3. Targeting Melanin Antigen in Melanoma with Radiolabeled Antibodies
4. Targeting Melanin with Small Molecules
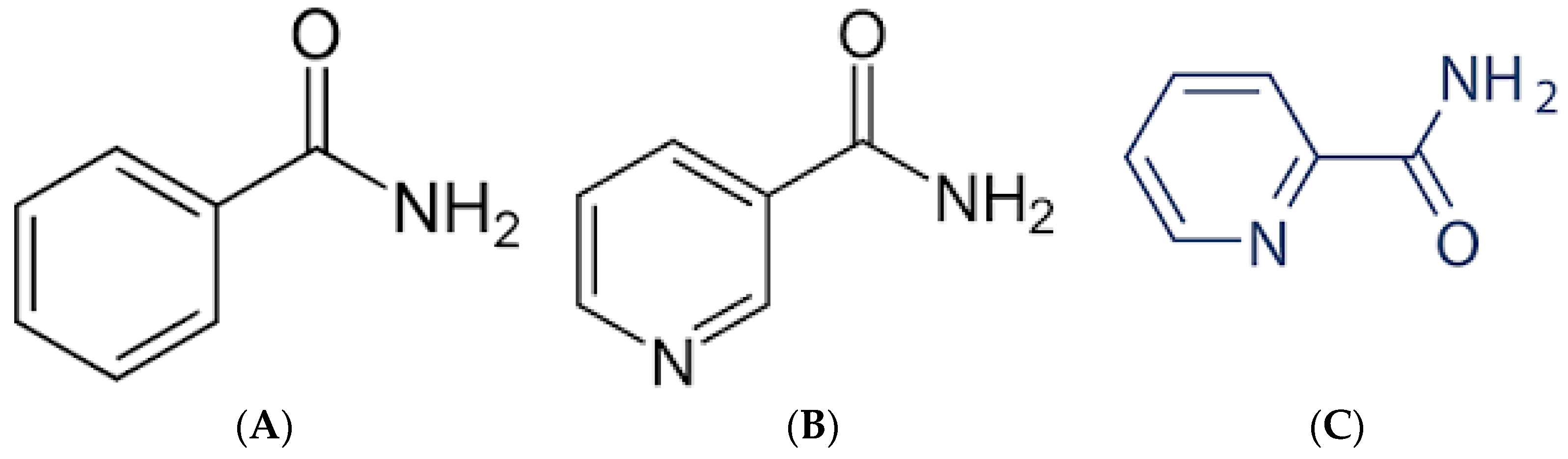
4.1. Benzamides
“the study will include a maximum of 36 patients. This study will begin with a preselection part that consists of an injection of [131I]ICF01012 at a diagnostic dose (185 MBq) in order to preselect patients who will receive the therapeutic dose according to the dosimetry results: binding of [131I]ICF01012 on at least a tumoral lesion and an acceptable radiation absorbed dose to major organs. The second phase will consist of a therapeutic part with a single administration of [131I]ICF01012 at a therapeutic dose. This part is a dose escalation model (4 levels of therapeutic dose to be tested)”.
4.2. Nicotinamides and Picolinamides
5. Conclusions
6. Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Statistics Center: Melanoma of the Skin. Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Melanoma%20of%20the%20skin (accessed on 4 March 2022).
- Understanding Cancer: Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics#:~:text=The%20most%20common%20cancers%20(listed,endometrial%20cancer%2C%20leukemia%2C%20pancreatic%20cancer (accessed on 4 March 2022).
- Ragusa, F.; Ferrari, S.M.; Elia, G.; Paparo, S.R.; Balestri, E.; Botrini, C.; Patrizio, A.; Mazzi, V.; Guglielmi, G.; Foddis, R.; et al. Combination Strategies Involving Immune Checkpoint Inhibitors and Tyrosine Kinase or BRAF Inhibitors in Aggressive Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 5731. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Moser, J.C.; Wei, G.; Colonna, S.V.; Grossmann, K.F.; Patel, S.; Hyngstrom, J.R. Comparative-effectiveness of pembrolizumab vs. nivolumab for patients with metastatic melanoma. Acta Oncol. 2020, 59, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Philips, G.K.; Atkins, M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int. Immunol. 2015, 27, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelliott, E.J.; McArthur, G.A.; Oliaro, J.; Sheppard, K.E. Immunomodulatory Effects of BRAF, MEK, and CDK4/6 Inhibitors: Implications for Combining Targeted Therapy and Immune Checkpoint Blockade for the Treatment of Melanoma. Front. Immunol. 2021, 12, 661737. [Google Scholar] [CrossRef]
- Ribas, A.; Kim, K.B.; Schuchter, L.M.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Flaherty, K.T.; Moschos, S.J.; et al. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. J. Clin. Oncol. 2011, 29, 8509. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Larkin, J.M.G.; Haanen, J.B.A.G.; Ribas, A.; Hogg, D.; O’Day, S.; Ascierto, P.A.; Testori, A.; et al. Phase III randomized, open-label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600EBRAF-mutated melanoma. J. Clin. Oncol. 2011, 29, LBA4. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, Randomised Phase 3 Trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Gualandri, L.; Betti, R.; Crosti, C. Clinical features of 36 cases of amelanotic melanomas and considerations about the relationship between histologic subtypes and diagnostic delay. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 283–287. [Google Scholar] [CrossRef]
- Thomas, N.E.; Kricker, A.; Waxweiler, W.T.; Dillon, P.M.; Busman, K.J.; From, L.; Groben, P.A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: A population-based study. JAMA Dermatol. 2014, 150, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Kondoh, H.; Hiratsuka, J.; Mishima, Y. Enhanced Melanogenesis Induced by Tyrosinase Gene-Transfer Increases Boron-Uptake and Killing Effect of Boron Neutron Capture Therapy for Amelanotic Melanoma. Pigment Cell Res. 1998, 11, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989, 2, 388–389. [Google Scholar] [CrossRef]
- Mishima, Y.; Ichihashi, M.; Hatta, S.; Honda, C.; Yamamura, K.; Nakagawa, T. New thermal neutron capture therapy for malignant melanoma: Melanogenesis-seeking 10B molecule-melanoma cell interaction from in vitro to first clinical trial. Pigment Cell Res. 1989, 2, 226–234. [Google Scholar] [CrossRef]
- Fukuda, H. Response of Normal Tissues to Boron Neutron Capture Therapy (BNCT) with (10)B-Borocaptate Sodium (BSH) and (10)B-Paraboronophenylalanine (BPA). Cells 2021, 10, 2883. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H. Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results. Cells 2021, 10, 2881. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Brożyna, A.A.; Janjetovic, Z.; Brooks, D.L.P.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brożyna, A.A.; Jozwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum. Pathol. 2011, 42, 618–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malo, M.E.; Frank, C.; Khokhoev, E.; Gorbunov, A.; Dontsov, A.; Garg, R.; Dadachova, E. Mitigating effects of sublethal and lethal whole-body gamma irradiation in a mouse model with soluble melanin. J. Radiol. Prot. 2022, 42, 011508. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.E.; Frank, C.; Dadachova, E. Assessing Melanin Capabilities in Radiation Shielding and Radioadaptation. J. Med. Imaging Radiat. Sci. 2019, 50, S2. [Google Scholar] [CrossRef]
- Malo, M.E.; Bryan, R.A.; Shuryak, I.; Dadachova, E. Morphological changes in melanized and non-melanized Cryptococcus neoformans cells post exposure to sparsely and densely ionizing radiation demonstrate protective effect of melanin. Fungal Biol. 2018, 122, 449–456. [Google Scholar] [CrossRef]
- Selbmann, L.; Pacelli, C.; Zucconi, L.; Dadachova, E.; Moeller, R.; de Vera, J.P.; Onofri, S. Resistance of an Antarctic cryptoendolithic black fungus to radiation gives new insights of astrobiological relevance. Fungal Biol. 2018, 122, 546–554. [Google Scholar] [CrossRef]
- Schweitzer, A.D.; Revskaya, E.; Chu, P.; Pazo, V.; Friedman, M.; Nosanchuk, J.D.; Cahill, S.; Frases, S.; Casadevall, A.; Dadachova, E. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1494–1502. [Google Scholar] [CrossRef] [Green Version]
- Revskaya, E.; Chu, P.; Howell, R.C.; Schweitzer, A.D.; Bryan, R.A.; Harris, M.; Gerfen, G.; Jiang, Z.; Jandl, T.; Kim, K.; et al. Compton Scattering by Internal Shields Based on Melanin-Containing Mushrooms Provides Protection of Gastrointestinal Tract from Ionizing Radiation. Cancer Biother. Radiopharm. 2012, 27, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Pawelek, J. Animals under the sun: Effects of ultraviolet radiation on mammalian skin. Clin. Dermatol. 1998, 16, 503–515. [Google Scholar] [CrossRef]
- Brożyna, A.A.; VanMiddlesworth, L.; Slominski, A.T. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer 2008, 123, 1448–1456. [Google Scholar] [CrossRef]
- Dadachova, E.; Nosanchuk, J.D.; Shi, L.; Schweitzer, A.D.; Frenkel, A.; Nosanchuk, J.S.; Casadevall, A. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin. Proc. Natl. Acad. Sci. USA 2004, 101, 14865–14870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarna, T.; Pilas, B.; Land, E.J.; Truscott, T.G. Interaction of radicals from water radiolysis with melanin. Biochim. Biophys. Acta 1986, 883, 162–167. [Google Scholar] [CrossRef]
- Rózanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Pilas, B.; Sarna, T.; Kalyanaraman, B.; Swartz, H.M. The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radic. Biol. Med. 1988, 4, 285–293. [Google Scholar] [CrossRef]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Rosas, Á.L.; Nosanchuk, J.D.; Feldmesser, M.; Cox, G.M.; McDade, H.C.; Casadevall, A. Synthesis of Polymerized Melanin by Cryptococcus neoformans in Infected Rodents. Infect. Immun. 2000, 68, 2845–2853. [Google Scholar] [CrossRef] [Green Version]
- Dadachova, E.; Revskaya, E.; Sesay, M.A.; Damania, H.; Boucher, R.; Sellers, R.S.; Howell, R.C.; Burns, L.; Thornton, G.B.; Natarajan, A.; et al. Pre-clinical evaluation and efficacy studies of a melanin-binding IgM antibody labeled with 188Re against experimental human metastatic melanoma in nude mice. Cancer Biol. Ther. 2008, 7, 1116–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, A.D.; Rakesh, V.; Revskaya, E.; Datta, A.; Casadevall, A.; Dadachova, E. Computational model predicts effective delivery of 188-Re-labeled melanin-binding antibody to metastatic melanoma tumors with wide range of melanin concentrations. Melanoma Res. 2007, 17, 291–303. [Google Scholar] [CrossRef]
- Klein, M.; Shibli, N.; Friedmann, N.; Thornton, G.; Chisin, R.; Lotem, M. Imaging of metastatic melanoma (MM) with a 188Rhenium (188Re)-labeled melanin binding antibody. J. Nucl. Med. 2008, 49, 52P. [Google Scholar]
- Klein, M.; Lotem, M.; Peretz, T.; Zwas, S.T.; Mizrachi, S.; Liberman, Y.; Chisin, R.; Schachter, J.; Ron, I.G.; Iosilevsky, G.; et al. Safety and efficacy of 188-rhenium-labeled antibody to melanin in patients with metastatic melanoma. J. Ski. Cancer 2013, 2013, 828329. [Google Scholar] [CrossRef] [Green Version]
- Jandl, T.; Revskaya, E.; Jiang, Z.; Bryan, R.A.; Casadevall, A.; Dadachova, E. Complement-dependent cytotoxicity of an antibody to melanin in radioimmunotherapy of metastatic melanoma. Immunotherapy 2013, 5, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Zabierowski, S.E.; Herlyn, M. Melanoma stem cells: The dark seed of melanoma. J. Clin. Oncol. 2008, 26, 2890–2894. [Google Scholar] [CrossRef] [PubMed]
- Jandl, T.; Revskaya, E.; Jiang, Z.; Harris, M.; Dorokhova, O.; Tsukrov, D.; Casadevall, A.; Dadachova, E. Melanoma stem cells in experimental melanoma are killed by radioimmunotherapy. Nucl. Med. Biol. 2013, 40, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Jeyakumar, A.; Ray, A.; Revskaya, E.; Jiang, Z.; Bryan, R.A.; Allen, K.J.H.; Jiao, R.; Malo, M.E.; Gomez, B.L.; et al. Structure-function analysis and therapeutic efficacy of antibodies to fungal melanin for melanoma radioimmunotherapy. Sci. Rep. 2018, 8, 5466. [Google Scholar] [CrossRef]
- Allen, K.J.H.; Jiao, R.; Malo, M.E.; Frank, C.; Dadachova, E. Biodistribution of a Radiolabeled Antibody in Mice as an Approach to Evaluating Antibody Pharmacokinetics. Pharmaceutics 2018, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.J.H.; Jiao, R.; Malo, M.E.; Frank, C.; Fisher, D.R.; Rickles, D.; Dadachova, E. Comparative Radioimmunotherapy of Experimental Melanoma with Novel Humanized Antibody to Melanin Labeled with 213Bismuth and 177Lutetium. Pharmaceutics 2019, 11, 348. [Google Scholar] [CrossRef] [Green Version]
- Jiao, R.; Allen, K.J.H.; Malo, M.E.; Rickles, D.; Dadachova, E. Evaluating the Combination of Radioimmunotherapy and Immunotherapy in a Melanoma Mouse Model. Int. J. Mol. Sci. 2020, 21, 773. [Google Scholar] [CrossRef] [Green Version]
- Malo, M.E.; Allen, K.J.H.; Jiao, R.; Frank, C.; Rickles, D.; Dadachova, E. Mechanistic Insights into Synergy between Melanin-Targeting Radioimmunotherapy and Immunotherapy in Experimental Melanoma. Int. J. Mol. Sci. 2020, 21, 8721. [Google Scholar] [CrossRef]
- Labarre, P.; Papon, J.; Moreau, M.-F.; Moins, N.; Bayle, M.; Veyre, A.; Madelmont, J.-C. Melanin affinity of N-(2-diethylaminoethyl)-4-iodobenzamide, an effective melanoma imaging agent. Melanoma Res. 2002, 12, 15–21. [Google Scholar] [CrossRef]
- Michelot, J.M.; Moreau, M.F.; Veyre, A.J.; Bonafous, J.F.; Bacin, F.J.; Madelmont, J.C.; Bussiere, F.; Souteyrand, P.A.; Mauclaire, L.P.; Chossat, F.M.; et al. Phase II scintigraphic clinical trial of malignant melanoma and metastases with iodine-123-N-(2-diethylaminoethyl 4-iodobenzamide). J. Nucl. Med. 1993, 34, 1260–1266. [Google Scholar] [PubMed]
- Brandau, W.; Niehoff, T.; Pulawski, P.; Jonas, M.; Dutschka, K.; Sciuk, J.; Coenen, H.H.; Schober, O. Structure distribution relationship of iodine-123-iodobenzamides as tracers for the detection of melanotic melanoma. J. Nucl. Med. 1996, 37, 1865–1871. [Google Scholar] [PubMed]
- Moins, N.; D’Incan, M.; Bonafous, J.; Bacin, F.; Labarre, P.; Moreau, M.F.; Mestas, D.; Noirault, E.; Chossat, F.; Berthommier, E.; et al. 123I-N-(2-diethylaminoethyl)-2-iodobenzamide: A potential imaging agent for cutaneous melanoma staging. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Mier, W.; Kratochwil, C.; Hassel, J.C.; Giesel, F.L.; Beijer, B.; Babich, J.W.; Friebe, M.; Eisenhut, M.; Enk, A.; Haberkorn, U. Radiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. J. Nucl. Med. 2014, 55, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyal, J.L.; Barrett, J.A.; Marquis, J.C.; Chen, J.; Hillier, S.M.; Maresca, K.P.; Boyd, M.; Gage, K.; Nimmagadda, S.; Kronauge, J.F.; et al. Preclinical evaluation of an 131I-labeled benzamide for targeted radiotherapy of metastatic melanoma. Cancer Res. 2010, 70, 4045–4053. [Google Scholar] [CrossRef] [Green Version]
- Billaud, E.M.; Rbah-Vidal, L.; Vidal, A.; Besse, S.; Tarrit, S.; Askienazy, S.; Maisonial, A.; Moins, N.; Madelmont, J.C.; Miot-Noirault, E.; et al. Synthesis, radiofluorination, and in vivo evaluation of novel fluorinated and iodinated radiotracers for PET imaging and targeted radionuclide therapy of melanoma. J. Med. Chem. 2013, 56, 8455–8467. [Google Scholar] [CrossRef]
- Gardette, M.; Viallard, C.; Paillas, S.; Guerquin-Kern, J.L.; Papon, J.; Moins, N.; Labarre, P.; Desbois, N.; Wong-Wah-Chung, P.; Palle, S.; et al. Evaluation of two (125)I-radiolabeled acridine derivatives for Auger-electron radionuclide therapy of melanoma. Investig. New Drugs 2014, 32, 587–597. [Google Scholar] [CrossRef]
- Degoul, F.; Borel, M.; Jacquemot, N.; Besse, S.; Communal, Y.; Mishellany, F.; Papon, J.; Penault-Llorca, F.; Donnarieix, D.; Doly, M.; et al. In vivo efficacy of melanoma internal radionuclide therapy with a 131I-labelled melanin-targeting heteroarylcarboxamide molecule. Int. J. Cancer 2013, 133, 1042–1053. [Google Scholar] [CrossRef]
- Billaud, E.M.; Maisonial-Besset, A.; Rbah-Vidal, L.; Vidal, A.; Besse, S.; Béquignat, J.B.; Decombat, C.; Degoul, F.; Audin, L.; Deloye, J.B.; et al. Synthesis, radiolabeling and preliminary in vivo evaluation of multimodal radiotracers for PET imaging and targeted radionuclide therapy of pigmented melanoma. Eur. J. Med. Chem. 2015, 92, 818–838. [Google Scholar] [CrossRef]
- Rouanet, J.; Quintana, M.; Auzeloux, P.; Cachin, F.; Degoul, F. Benzamide derivative radiotracers targeting melanin for melanoma imaging and therapy: Preclinical/clinical development and combination with other treatments. Pharmacol. Ther. 2021, 224, 107829. [Google Scholar] [CrossRef]
- Viallard, C.; Perrot, Y.; Boudhraa, Z.; Jouberton, E.; Miot-Noirault, E.; Bonnet, M.; Besse, S.; Mishellany, F.; Cayre, A.; Maigne, L.; et al. [123I]ICF01012 melanoma imaging and [131I]ICF01012 dosimetry allow adapted internal targeted radiotherapy in preclinical melanoma models. Eur. J. Dermatol. 2015, 25, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jouberton, E.; Perrot, Y.; Dirat, B.; Billoux, T.; Auzeloux, P.; Cachin, F.; Chezal, J.M.; Filaire, M.; Labarre, P.; Miot-Noirault, E.; et al. Radiation dosimetry of [(131) I]ICF01012 in rabbits: Application to targeted radionuclide therapy for human melanoma treatment. Med. Phys. 2018, 45, 5251–5262. [Google Scholar] [CrossRef] [PubMed]
- Thivat, E.; Rouanet, J.; Auzeloux, P.; Sas, N.; Jouberton, E.; Levesque, S.; Billoux, T.; Mansard, S.; Molnar, I.; Chanchou, M.; et al. Phase I study of [131I] ICF01012, a targeted radionuclide therapy, in metastatic melanoma: MELRIV-1 protocol. BMC Cancer 2022, 22, 417. [Google Scholar] [CrossRef]
- Viallard, C.; Chezal, J.M.; Mishellany, F.; Ranchon-Cole, I.; Pereira, B.; Herbette, A.; Besse, S.; Boudhraa, Z.; Jacquemot, N.; Cayre, A.; et al. Targeting DNA repair by coDbait enhances melanoma targeted radionuclide therapy. Oncotarget 2016, 7, 12927–12936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akil, H.; Rouanet, J.; Viallard, C.; Besse, S.; Auzeloux, P.; Chezal, J.M.; Miot-Noirault, E.; Quintana, M.; Degoul, F. Targeted Radionuclide Therapy Decreases Melanoma Lung Invasion by Modifying Epithelial-Mesenchymal Transition-Like Mechanisms. Transl. Oncol. 2019, 12, 1442–1452. [Google Scholar] [CrossRef]
- Rouanet, J.; Benboubker, V.; Akil, H.; Hennino, A.; Auzeloux, P.; Besse, S.; Pereira, B.; Delorme, S.; Mansard, S.; D’Incan, M.; et al. Immune checkpoint inhibitors reverse tolerogenic mechanisms induced by melanoma targeted radionuclide therapy. Cancer Immunol. Immunother. 2020, 69, 2075–2088. [Google Scholar] [CrossRef]
- Akil, H.; Quintana, M.; Raymond, J.H.; Billoux, T.; Benboubker, V.; Besse, S.; Auzeloux, P.; Delmas, V.; Petit, V.; Larue, L.; et al. Efficacy of Targeted Radionuclide Therapy Using [(131)I]ICF01012 in 3D Pigmented BRAF- and NRAS-Mutant Melanoma Models and In Vivo NRAS-Mutant Melanoma. Cancers 2021, 13, 1426. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, C.H.; Shen, C.C.; Chen, C.L.; Liu, R.S.; Lin, M.H.; Wang, H.E. Synthesis and evaluation of ¹²³/¹³¹I-Iochlonicotinamide as a novel SPECT probe for malignant melanoma. Bioorg. Med. Chem. 2015, 23, 2261–2269. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, L.; Gai, Y.; Liu, Q.; Yin, L.; Jiang, Y.; Wang, Y.; Zhang, Y.; Lan, X. Targeted radiotherapy of pigmented melanoma with (131)I-5-IPN. J. Exp. Clin. Cancer Res. 2018, 37, 306. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, Y.Y.; Lo, Y.H.; Lin, M.H.; Chang, C.H.; Chen, C.L.; Wang, H.E.; Wu, C.Y. Evaluation of Radioiodinated Fluoronicotinamide/Fluoropicolinamide-Benzamide Derivatives as Theranostic Agents for Melanoma. Int. J. Mol. Sci. 2020, 21, 6597. [Google Scholar] [CrossRef]
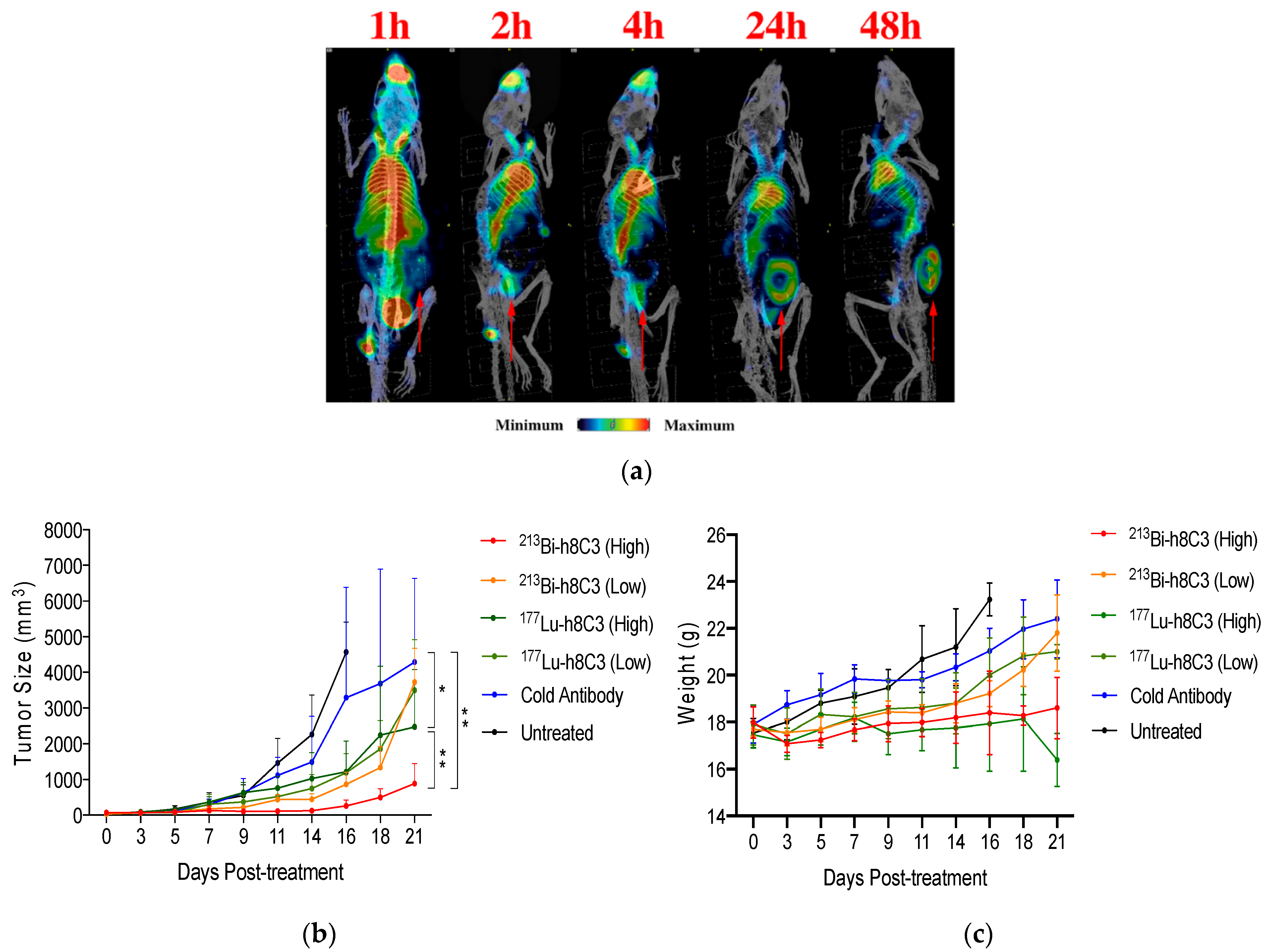
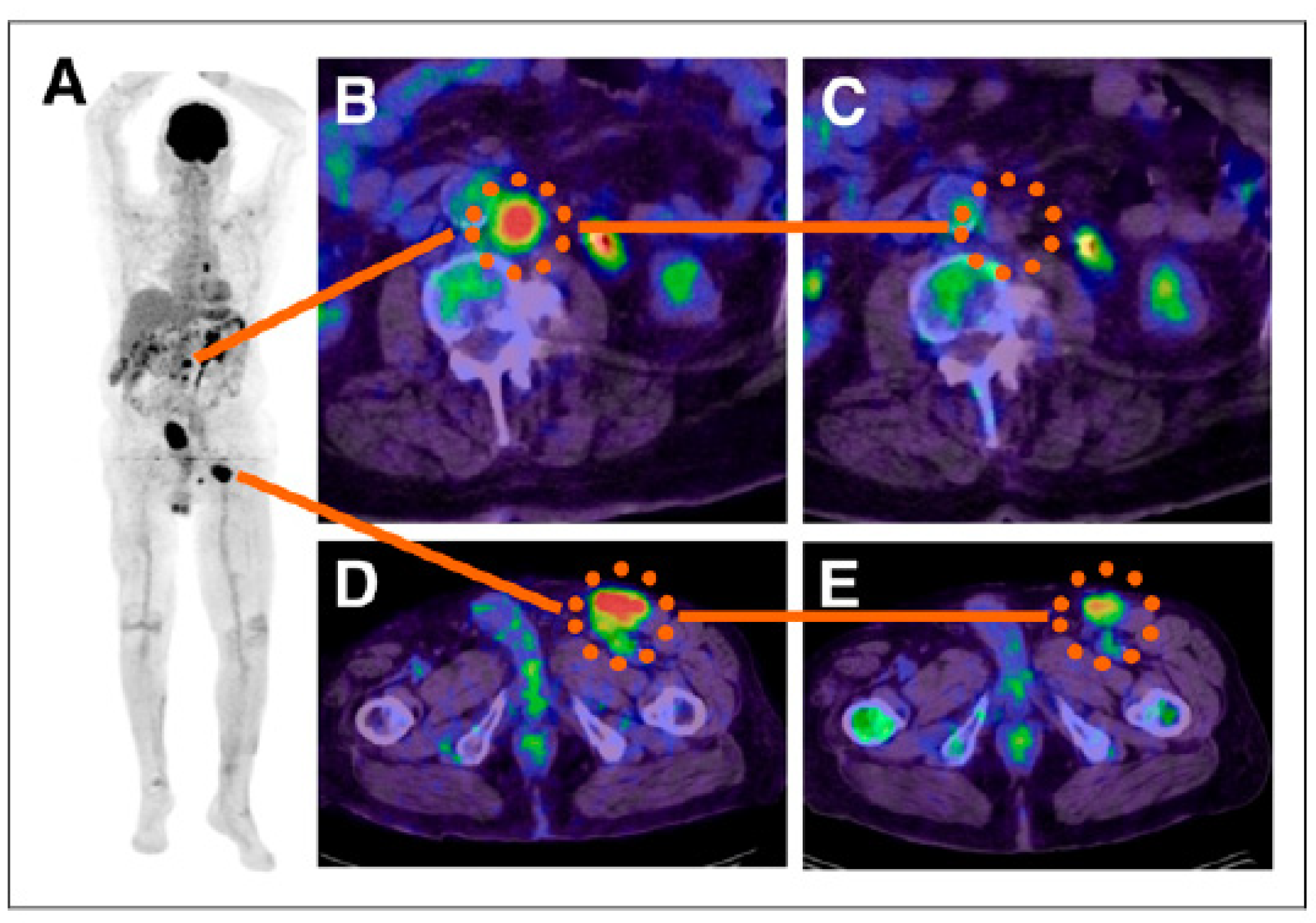

| Studies and Results | Reference |
|---|---|
| First in man study of 131I-BA52. The dose of 12.2 Gy/GBq was delivered to the tumor and the highest off-target dose was 3.1 Gy/GBq, the drug was well-tolerated and deemed safe with no acute or mid-term toxicities. | [56] |
| [131I]ICF01012 administration in the range of 14.8–22.2 MBq resulted in decreased tumor growth and only transient hematological toxicity in B16-BL6 melanoma mice. No damage to the retina of an eye was recorded in 30% of the treated mice while in the remaining 70% of the animals, the damage was only seen in the optic nerve area. | [60] |
| Radiation dosimetry study of [123I]ICF01012 in murine melanoma showed that fractionated treatment with 3 × 25 MBq of [131I]ICF01012 delivered 53.2 Gy to the tumor. | [63] |
| Further radiation dosimetry evaluation of [131I]ICF01012 was conducted by performing the SPECT/CT and ex vivo measurements in healthy rabbits. The doses delivered to the eyes and liver were dose-limiting with 45.8 ± 7.9 Gy/GBq and 6.38 ± 0.50 Gy/GBq, respectively. However, the conversion of the former into the dose to the human retina resulted in a significantly lower value of 3.07 ± 0.70 Gy/GBq. | [64] |
| On-going clinical trial NCT03784625, which was initiated in 2019 [65]. According to clinicaltrials.gov: “the study will include a maximum of 36 patients. This study will begin with a preselection part that consists of an injection of [131I]ICF01012 at a diagnostic dose (185 MBq) in order to preselect patients who will receive the therapeutic dose according to the dosimetry results: binding of [131I]ICF01012 on at least a tumoral lesion and an acceptable radiation absorbed dose to major organs. The second phase will consist of a therapeutic part with a single administration of [131I]ICF01012 at a therapeutic dose. This part is a dose escalation model (4 levels of therapeutic dose to be tested)”. | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, K.J.H.; Malo, M.E.; Jiao, R.; Dadachova, E. Targeting Melanin in Melanoma with Radionuclide Therapy. Int. J. Mol. Sci. 2022, 23, 9520. https://doi.org/10.3390/ijms23179520
Allen KJH, Malo ME, Jiao R, Dadachova E. Targeting Melanin in Melanoma with Radionuclide Therapy. International Journal of Molecular Sciences. 2022; 23(17):9520. https://doi.org/10.3390/ijms23179520
Chicago/Turabian StyleAllen, Kevin J. H., Mackenzie E. Malo, Rubin Jiao, and Ekaterina Dadachova. 2022. "Targeting Melanin in Melanoma with Radionuclide Therapy" International Journal of Molecular Sciences 23, no. 17: 9520. https://doi.org/10.3390/ijms23179520
APA StyleAllen, K. J. H., Malo, M. E., Jiao, R., & Dadachova, E. (2022). Targeting Melanin in Melanoma with Radionuclide Therapy. International Journal of Molecular Sciences, 23(17), 9520. https://doi.org/10.3390/ijms23179520







