Low-Dose Near-Infrared Light-Activated Mitochondria-Targeting Photosensitizers for PDT Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. Photocytotoxicity
2.2. DNA Damage
2.3. Cellular Uptake of LC31, MLC31, and DMLC31Pt
2.4. Distribution Coefficient (logP)
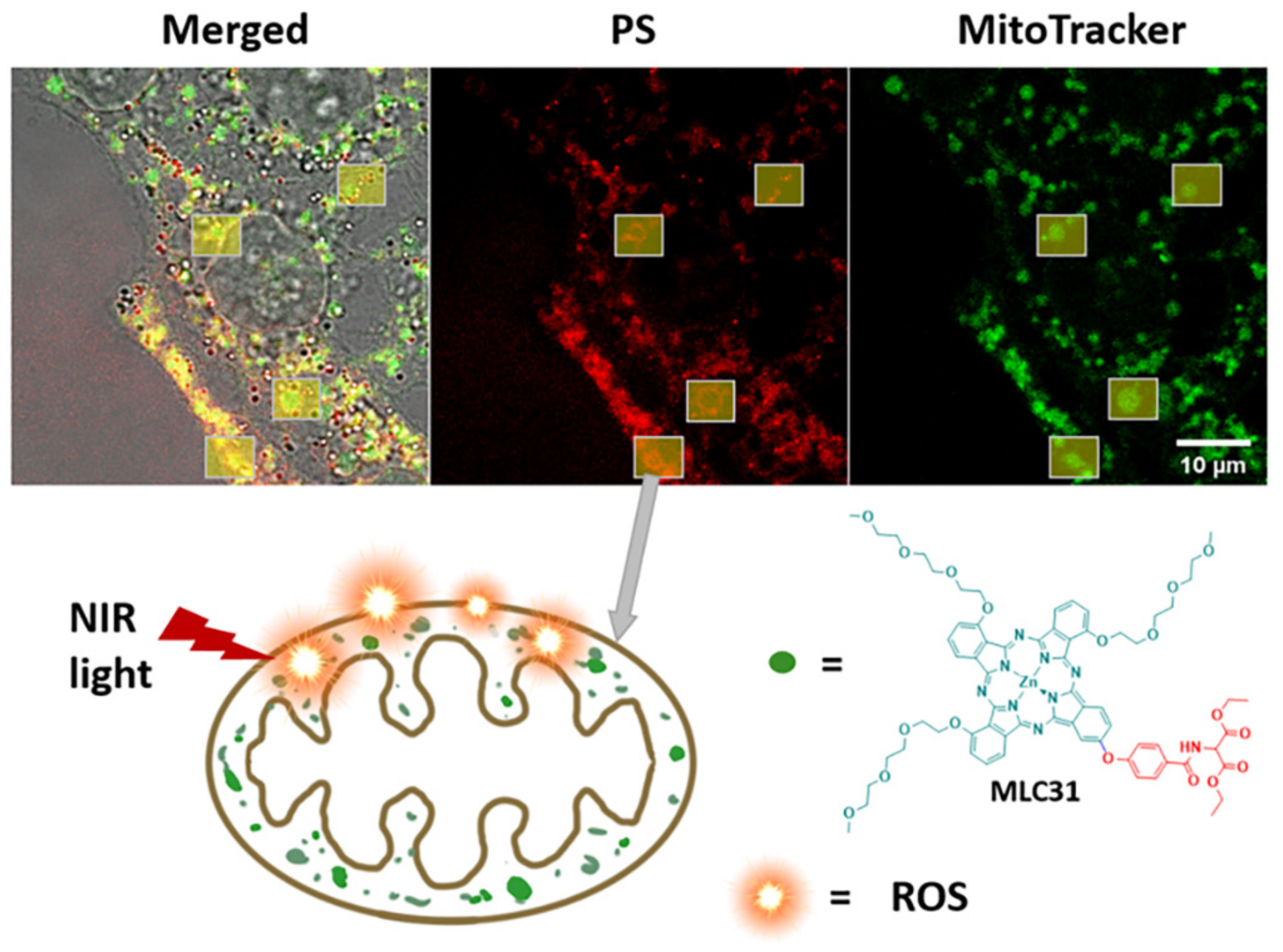
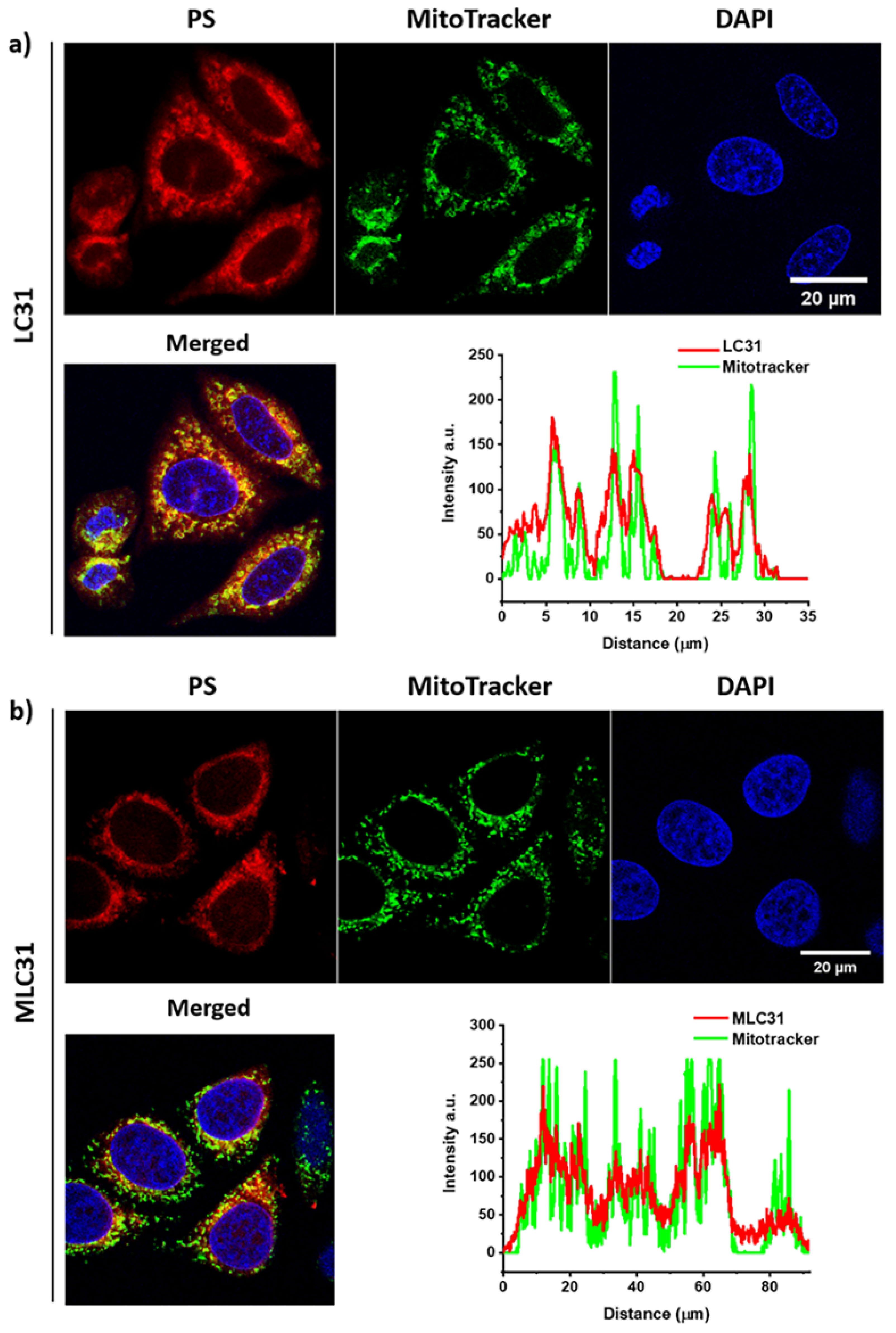
2.5. Cell Localization via In Vitro Fluorescence Evaluation
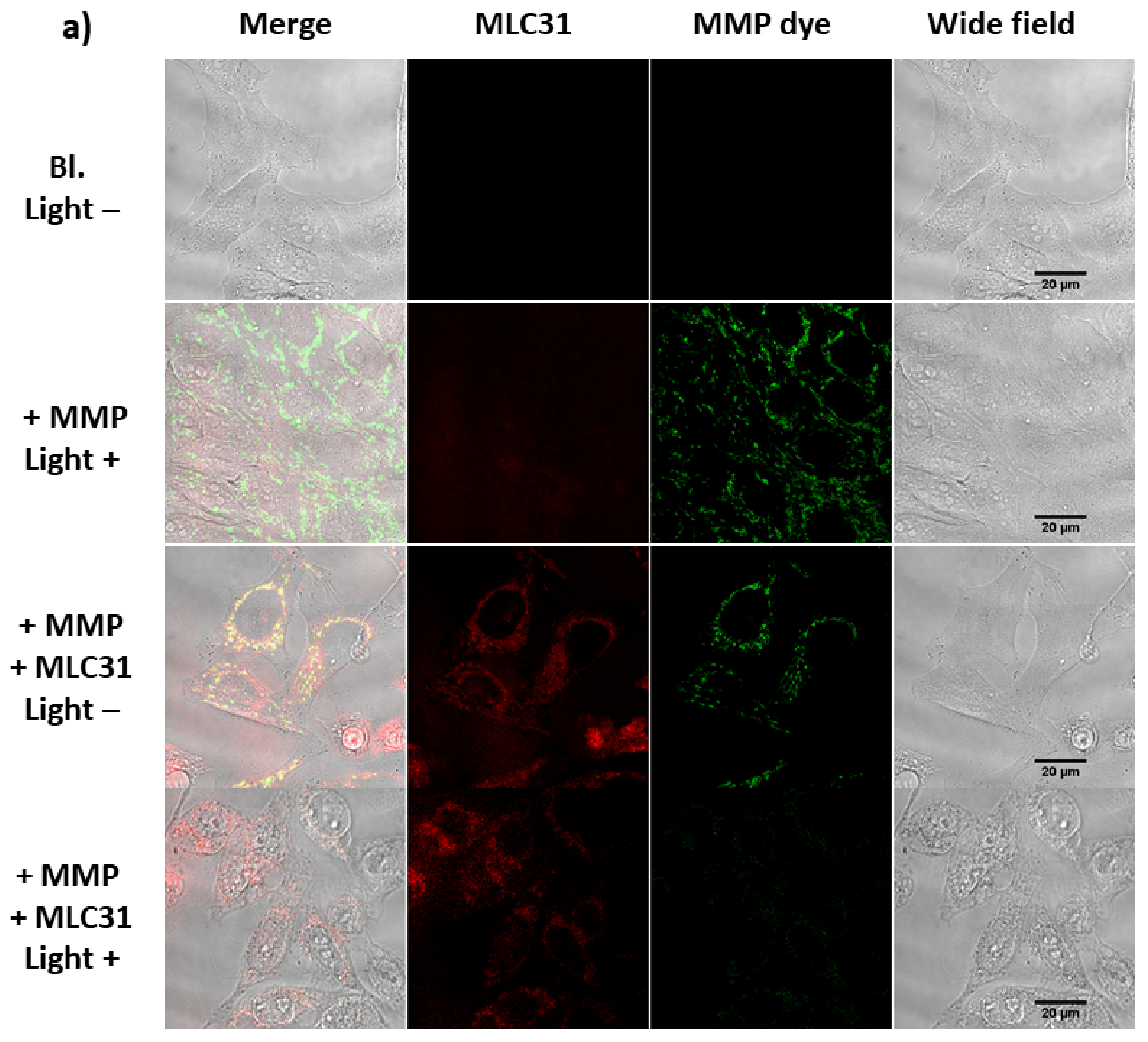
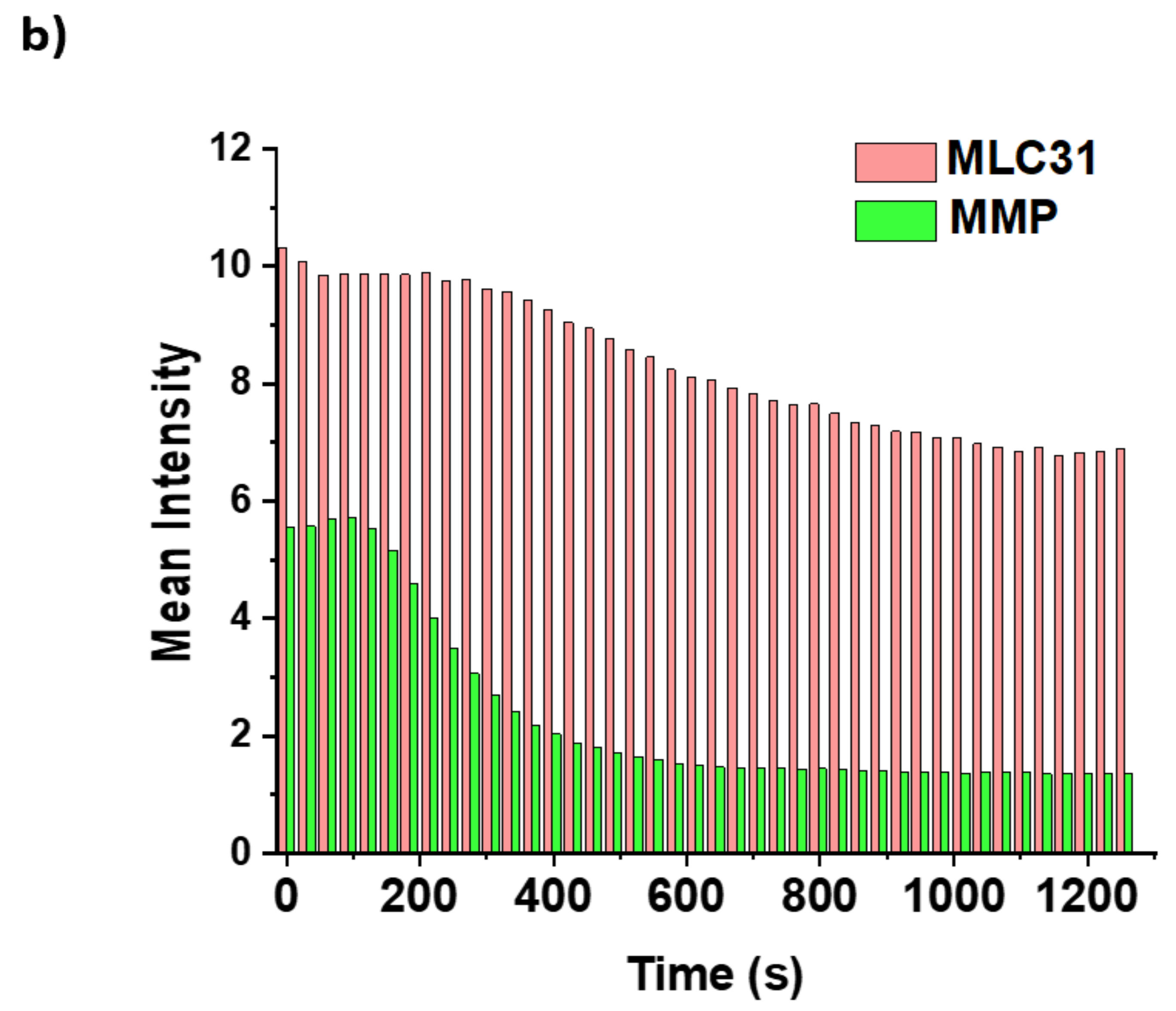
2.6. Detection of the Loss of the Mitochondrial Membrane Permeabilization
2.7. Effects under Hypoxia
3. Materials and Methods
3.1. General Materials and Instrumentation
3.2. Syntheses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Antoni, P.M.; Naik, A.; Albert, I.; Rubbiani, R.; Gupta, S.; Ruiz-Sanchez, P.; Munikorn, P.; Mateos, J.M.; Luginbuehl, V.; Thamyongkit, P.; et al. (Metallo)porphyrins as Potent Phototoxic Anti-Cancer Agents after Irradiation with Red Light. Chem. Eur. J. 2015, 21, 1179–1183. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef]
- Wagner, A.; Denzer, U.W.; Neureiter, D.; Kiesslich, T.; Puespoeck, A.; Rauws, E.A.J.; Emmanuel, K.; Degenhardt, N.; Frick, U.; Beuers, U.; et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: A multicenter prospective phase II study. Hepatology 2015, 62, 1456–1465. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.S.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Jiang, X.-J.; Fong, W.-P.; Ng, D.K.P. Highly photocytotoxic 1,4-dipegylated zinc(II) phthalocyanines. Effects of the chain length on the in vitro photodynamic activities. Org. Biomol. Chem. 2008, 6, 4560–4566. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Durmus, M.; Atilla, D.; Gürek, A.G.; Ahsen, V.; Nyokong, T. Synthesis, photophysical and photochemical studies on long chain zinc phthalocyanine derivatives. Synth. Met. 2008, 158, 839–847. [Google Scholar] [CrossRef]
- Moreira, L.M.; dos Santos, F.V.; Lyon, J.P.; Maftoum-Costa, M.; Pacheco-Soares, C.; da Silva, N.S. Photodynamic therapy: Porphyrins and Phthalocyanines as Photosensitizers. Aust. J. Chem. 2008, 61, 741–754. [Google Scholar] [CrossRef]
- McRae, E.K.S.; Nevonen, D.E.; McKenna, S.A.; Nemykin, V.N. Binding and photodynamic action of the cationic zinc phthalocyanines with different types of DNA toward understanding of their cancer therapy activity. J. Inorg. Biochem. 2019, 199, 110793. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, T.; Miyaji, Y.; Maeda, K.; Maeda, H.; Segi, M. Extremely Photostable Electron-Deficient Phthalocyanines that Generate High Levels of Singlet Oxygen. Chem. Eur. J. 2019, 25, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Huang, Y. Imaging in Photodynamic Therapy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 479. [Google Scholar]
- Sen, P.; Managa, M.; Nyokong, T. New type of metal-free and Zinc(II), In(III), Ga(III) phthalocyanines carrying biologically active substituents: Synthesis and photophysicochemical properties and photodynamic therapy activity. Inorg. Chim. Acta 2019, 491, 1–8. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodriguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Safar Sajadi, S.M.; Khoee, S. The simultaneous role of porphyrins’ H- and J-aggregates and host–guest chemistry on the fabrication of reversible Dextran-PMMA polymersome. Sci. Rep. 2021, 11, 2832. [Google Scholar] [CrossRef]
- Naik, A.; Rubbiani, R.; Gasser, G.; Spingler, B. Visible-Light-Induced Annihilation of Tumor Cells with Platinum–Porphyrin Conjugates. Angew. Chem. Int. Ed. 2014, 53, 6938–6941. [Google Scholar] [CrossRef]
- Schneider, L.; Larocca, M.; Wu, W.; Babu, V.; Padrutt, R.; Slyshkina, E.; König, C.; Ferrari, S.; Spingler, B. Exocyclically metallated tetrapyridinoporphyrazine as a potential photosensitizer for photodynamic therapy. Photochem. Photobiol. Sci. 2019, 18, 2792–2803. [Google Scholar] [CrossRef]
- Rubbiani, R.; Wu, W.; Naik, A.; Larocca, M.; Schneider, L.; Padrutt, R.; Babu, V.; König, C.; Hinger, D.; Maake, C.; et al. Studying the cellular distribution of highly phototoxic platinated metalloporphyrins by isotope labelling. Chem. Commun. 2020, 56, 14373–14376. [Google Scholar] [CrossRef]
- Dingiswayo, S.; Babu, B.; Prinsloo, E.; Mack, J.; Nyokong, T. A comparative study of the photophysicochemical and photodynamic activity properties of meso-4-methylthiophenyl functionalized Sn(IV) tetraarylporphyrins and triarylcorroles. J. Porphyr. Phthalocyanines 2020, 24, 1138–1145. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Yang, M.; Wu, F. Palladium porphyrin complexes for photodynamic cancer therapy: Effect of porphyrin units and metal. Photochem. Photobiol. Sci. 2020, 19, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Padrutt, R.; Babu, V.; Klingler, S.; Kalt, M.; Schumer, F.; Anania, M.I.; Schneider, L.; Spingler, B. Distyryl-BODIPY-Transplatin Conjugates as Highly Phototoxic Photosensitizers for Photodynamic Therapy. ChemMedChem 2021, 16, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Gandosio, A.; Purkait, K.; Gasser, G. Recent Approaches towards the Development of Ru(II) Polypyridyl Complexes for Anticancer Photodynamic Therapy. Chimia 2021, 75, 845–855. [Google Scholar] [CrossRef]
- Le, N.A.; Babu, V.; Kalt, M.; Schneider, L.; Schumer, F.; Spingler, B. Photo-stable platinated bacteriochlorins as potent photodynamic agents. J. Med. Chem. 2021, 64, 6792–6801. [Google Scholar] [CrossRef]
- Schneider, L.; Kalt, M.; Larocca, M.; Babu, V.; Spingler, B. Potent PBS-Soluble Transplatin Derived Porphyrin-Based Photosensitizer for Photodynamic Therapy. Inorg. Chem. 2021, 60, 9416–9426. [Google Scholar] [CrossRef]
- Li, H.; Fronczek, F.R.; Vicente, M.G.H. Pegylated phthalocyanines: Synthesis and spectroscopic properties. Tetrahedron Lett. 2011, 52, 6675–6678. [Google Scholar] [CrossRef]
- Tuncel, S.; Dumoulin, F.; Gailer, J.; Sooriyaarachchi, M.; Atilla, D.; Durmuş, M.; Bouchu, D.; Savoie, H.; Boyle, R.W.; Ahsen, V. A set of highly water-soluble tetraethyleneglycol-substituted Zn(ii) phthalocyanines: Synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011, 40, 4067–4079. [Google Scholar] [CrossRef]
- Akkuş, F.; Kabay, N.; Gök, Y. The first synthesis and characterization of new metal-free and metallophthalocyanine containing 33-membered crown ether moieties. J. Porphyr. Phthalocyanines 2013, 17, 473–479. [Google Scholar] [CrossRef]
- Wierzchowski, M.; Sobotta, L.; Skupin-Mrugalska, P.; Kruk, J.; Jusiak, W.; Yee, M.; Konopka, K.; Düzgüneş, N.; Tykarska, E.; Gdaniec, M.; et al. Phthalocyanines functionalized with 2-methyl-5-nitro-1H-imidazolylethoxy and 1,4,7-trioxanonyl moieties and the effect of metronidazole substitution on photocytotoxicity. J. Inorg. Biochem. 2013, 127, 62–72. [Google Scholar] [CrossRef]
- Jia, X.; Yang, F.-F.; Li, J.; Liu, J.-Y.; Xue, J.-P. Synthesis and in Vitro Photodynamic Activity of Oligomeric Ethylene Glycol–Quinoline Substituted Zinc(II) Phthalocyanine Derivatives. J. Med. Chem. 2013, 56, 5797–5805. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, S.; Trivella, A.; Atilla, D.; Bennis, K.; Savoie, H.; Albrieux, F.; Delort, L.; Billard, H.; Dubois, V.; Ahsen, V.; et al. Assessing the Dual Activity of a Chalcone-Phthalocyanine Conjugate: Design, Synthesis, and Antivascular and Photodynamic Properties. Mol. Pharm. 2013, 10, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Wierzchowski, M.; Mierzwicki, M.; Gdaniec, Z.; Mielcarek, J.; Persoons, L.; Goslinski, T.; Balzarini, J. Photochemical studies and nanomolar photodynamic activities of phthalocyanines functionalized with 1,4,7-trioxanonyl moieties at their non-peripheral positions. J. Inorg. Biochem. 2016, 155, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, D.; Lafont, D.; Poyer, F.; Garcia, G.; Albrieux, F.; Maillard, P.; Bretonniere, Y.; Dumoulin, F. Design of an amphiphilic porphyrin exhibiting high in vitro photocytotoxicity. New J. Chem. 2016, 40, 2044–2050. [Google Scholar] [CrossRef]
- Aribi, F.; Vey, C.; Topkaya, D.; Kostakoglu, S.T.; Fournier-dit-Chabert, J.; Buyukeksi, S.I.; Taskin, G.C.; Alpugan, S.; Albrieux, F.; Gurek, A.G.; et al. Phthalocyanine-chalcone conjugates. J. Porphyr. Phthalocyanines 2016, 20, 497–504. [Google Scholar] [CrossRef]
- Kasprzycki, P.; Sobotta, L.; Lijewski, S.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J.; Radzewicz, C.; Fita, P. Unusual cis-diprotonated forms and fluorescent aggregates of non-peripherally alkoxy-substituted metallophthalocyanines. Phys. Chem. Chem. Phys. 2017, 19, 21390–21400. [Google Scholar] [CrossRef]
- Wierzchowski, M.; Sobotta, L.; Łażewski, D.; Kasprzycki, P.; Fita, P.; Goslinski, T. Spectroscopic and quantum chemical study of phthalocyanines with 1,4,7-trioxanonyl moieties. J. Mol. Struct. 2020, 1203, 127371. [Google Scholar] [CrossRef]
- Aykota, M.R.; Yılmaz, S.; Erbiş, H.; Kabay, N.; Tuncel Kostakoğlu, S.; Ahsen, V.; Dumoulin, F.; Yenisey, Ç.; Kabay, B. In vivo phototoxic effects of a tetraethyleneglycol-substituted Zn phthalocyanine in tumor bearing rats at an enzymatic level. J. Porphyr. Phthalocyanines 2021, 25, 120–127. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Liang, Z.; Wang, J.; Pang, M.; Huang, W.; Wu, W.; Hong, Z. Synthesis and biological evaluation of peptide-conjugated phthalocyanine photosensitizers with highly hydrophilic modifications. Org. Biomol. Chem. 2016, 14, 3409–3422. [Google Scholar] [CrossRef]
- Luby, B.M.; Walsh, C.D.; Zheng, G. Advanced Photosensitizer Activation Strategies for Smarter Photodynamic Therapy Beacons. Angew. Chem. Int. Ed. 2019, 58, 2558–2569. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, N.; Avirah, R.R.; Ramaiah, D. Tuning Photosensitized Singlet Oxygen Generation Efficiency of Novel Aza-BODIPY Dyes. Org. Lett. 2010, 12, 5720–5723. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Kokubun, H.; Koizumi, M. Determination of the S*–T Transition Probabilities of Some Xanthene and Thiazine Dyes on the Basis of the T-Energy Transfer. I. Experiment in Ethanol Solutions. Bull. Chem. Soc. Jpn. 1969, 42, 1223–1230. [Google Scholar] [CrossRef]
- Jailaubekov, A.E.; Willard, A.P.; Tritsch, J.R.; Chan, W.L.; Sai, N.; Gearba, R.; Kaake, L.G.; Williams, K.J.; Leung, K.; Rossky, P.J.; et al. Hot charge-transfer excitons set the time limit for charge separation at donor/acceptor interfaces in organic photovoltaics. Nat. Mater. 2013, 12, 66–73. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, F.; Li, Z.; Huang, L.; Tang, Y.; Zhang, F.; Tung, C.-H. A novel self-aggregates of phthalocyanine based on Zn-O coordination. Chem. Lett. 2007, 36, 108–109. [Google Scholar] [CrossRef]
- Honda, T.; Nakanishi, T.; Ohkubo, K.; Kojima, T.; Fukuzumi, S. Structure and Photoinduced Electron Transfer Dynamics of a Series of Hydrogen-Bonded Supramolecular Complexes Composed of Electron Donors and a Saddle-Distorted Diprotonated Porphyrin. J. Am. Chem. Soc. 2010, 132, 10155–10163. [Google Scholar] [CrossRef]
- Honda, T.; Kojima, T.; Kobayashi, N.; Fukuzumi, S. Crystal Structures and Electronic Properties of Saddle-Distorted and Protonated Phthalocyanines. Angew. Chem. Int. Ed. 2011, 50, 2725–2728. [Google Scholar] [CrossRef]
- Ogunsipe, A.O.; Idowu, M.A.; Ogunbayo, T.B.; Akinbulu, I.A. Protonation of some non-transition metal phthalocyanines - spectral and photophysicochemical consequences. J. Porphyr. Phthalocyanines 2012, 16, 885–894. [Google Scholar] [CrossRef]
- Hirose, K. A practical guide for the determination of binding constants. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 193–209. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhao, F.; Zhang, F.; Tang, Y.; Song, X.; Zhou, F.-T. Effects of Protonation and Deprotonation on Phthalocyanine’s Spectra. Acta Phys. Chim. Sin. 1996, 12, 202–207. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.-X. γ-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]
- Pierroz, V.; Rubbiani, R.; Gentili, C.; Patra, M.; Mari, C.; Gasser, G.; Ferrari, S. Dual mode of cell death upon the photo-irradiation of a Ru-II polypyridyl complex in interphase or mitosis. Chem. Sci. 2016, 7, 6115–6124. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Carobeli, L.R.; Meirelles, L.E.d.F.; Damke, G.M.Z.F.; Damke, E.; Souza, M.V.F.d.; Mari, N.L.; Mashiba, K.H.; Shinobu-Mesquita, C.S.; Souza, R.P.; Silva, V.R.S.d.; et al. Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy. Pharmaceutics 2021, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Uslan, C.; İşleyen, N.D.; Öztürk, Y.; Yıldız, B.T.; Çakar, Z.P.; Göksel, M.; Durmuş, M.; Gürsel, Y.H.; Sesalan, B.Ş. A novel of PEG-conjugated phthalocyanine and evaluation of its photocytotoxicity and antibacterial properties for photodynamic therapy. J. Porphyr. Phthalocyanines 2018, 22, 10–24. [Google Scholar] [CrossRef]
- Gülmez, A.D.; Göksel, M.; Durmuş, M. Silicon(IV) phthalocyanine-biotin conjugates: Synthesis, photophysicochemical properties and in vitro biological activity for photodynamic therapy. J. Porphyr. Phthalocyanines 2017, 21, 547–554. [Google Scholar] [CrossRef]
- Balçik-Erçin, P.; Çetin, M.; Göksel, M.; Durmuş, M. Improved targeting for photodynamic therapy via a biotin–phthalocyanine conjugate: Synthesis, photophysical and photochemical measurements, and in vitro cytotoxicity assay. New J. Chem. 2020, 44, 3392–3401. [Google Scholar] [CrossRef]
- Göksel, M. Synthesis of asymmetric zinc(II) phthalocyanines with two different functional groups & spectroscopic properties and photodynamic activity for photodynamic therapy. Bioorg. Med. Chem. 2016, 24, 4152–4164. [Google Scholar] [CrossRef]
- Göksel, M.; Durmuş, M.; Biyiklioglu, Z. Synthesis and photodynamic activities of novel silicon(iv) phthalocyanines axially substituted with water soluble groups against HeLa cancer cell line. Dalton Trans. 2021, 50, 2570–2584. [Google Scholar] [CrossRef]
- Manisova, B.; Binder, S.; Malina, L.; Jiravova, J.; Langova, K.; Kolarova, H. Phthalocyanine-mediated Photodynamic Treatment of Tumoural and Non-tumoural cell lines. Anticancer Res. 2015, 35, 3943–3952. [Google Scholar]
- Halaskova, M.; Rahali, A.; Almeida-Marrero, V.; Machacek, M.; Kucera, R.; Jamoussi, B.; Torres, T.; Novakova, V.; de la Escosura, A.; Zimcik, P. Peripherally Crowded Cationic Phthalocyanines as Efficient Photosensitizers for Photodynamic Therapy. ACS Med. Chem. Lett. 2021, 12, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Makhseed, S.; Machacek, M.; Alfadly, W.; Tuhl, A.; Vinodh, M.; Simunek, T.; Novakova, V.; Kubat, P.; Rudolf, E.; Zimcik, P. Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem. Commun. 2013, 49, 11149–11151. [Google Scholar] [CrossRef] [PubMed]
- Puckett, C.A.; Ernst, R.J.; Barton, J.K. Exploring the cellular accumulation of metal complexes. Dalton Trans. 2010, 39, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Zhang, L.Y.; Chen, H.X.; Gu, Y.; Zhao, J.Q. A novel hypocrellin B derivative designed and synthesized by taking consideration to both drug delivery and biological photodynamic activity. J. Photochem. Photobiol. B 2009, 94, 171–178. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Drozd, A.; Stochel, G.; Pereira, M.M.; Pinto, S.M.A.; Arnaut, L.G.; Dąbrowski, J.M. Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 2786. [Google Scholar] [CrossRef]
- Boyle, R.W.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef]
- Soriano, J.; Mora-Espi, I.; Alea-Reyes, M.E.; Perez-Garcia, L.; Barrios, L.; Ibanez, E.; Nogues, C. Cell Death Mechanisms in Tumoral and Non-Tumoral Human Cell Lines Triggered by Photodynamic Treatments: Apoptosis, Necrosis and Parthanatos. Sci. Rep. 2017, 7, 41340. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Lane, J.D.; Allan, V.J.; Woodman, P.G. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J. Cell Sci. 2005, 118, 4059–4071. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Ann. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Hu, Q.L.; Gao, M.; Feng, G.X.; Liu, B. Mitochondria-Targeted Cancer Therapy Using a Light-Up Probe with Aggregation-Induced-Emission Characteristics. Angew. Chem. Int. Ed. 2014, 53, 14225–14229. [Google Scholar] [CrossRef] [PubMed]
- Oleinick, N.L.; Morris, R.L.; Belichenko, T. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Kurtcuoglu, V.; Scholz, C.C.; Marti, H.H.; Hoogewijs, D. Frequently asked questions in hypoxia research. Hypoxia 2015, 3, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; William, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.J.; Mata, E.G.; Mascaretti, O.A. Scope and Mechanism of Deprotection of Carboxylic Esters by Bis(Tributyltin) Oxide. J. Org. Chem. 1994, 59, 7259–7266. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, 1.171.41; Rigaku Corporation: Tokyo, Japan, 2021. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, L.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Higashi, R.; Ishii, K.; Hatsusaka, K.; Ohta, K. Aggregation, Complexation with Guest Molecules, and Mesomorphism of Amphiphilic Phthalocyanines Having Four- or Eight Tri(ethylene oxide) Chains. Bull. Chem. Soc. Jpn. 1999, 72, 1263–1271. [Google Scholar] [CrossRef]
- Chidawanyika, W.; Nyokong, T. The synthesis and photophysicochemical properties of low-symmetry zinc phthalocyanine analogues. J. Photochem. Photobiol. A 2009, 206, 169–176. [Google Scholar] [CrossRef]
- Caron, G.; Ermondi, G.; Gariboldi, M.B.; Monti, E.; Gabano, E.; Ravera, M.; Osella, D. The Relevance of Polar Surface Area (PSA) in Rationalizing Biological Properties of Several cis-Diamminemalonatoplatinum(II) Derivatives. ChemMedChem 2009, 4, 1677–1685. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Zhao, M.; Qiao, Q.-L.; Lang, H.-J.; Xu, J.-Z.; Xu, Z.-C. Fluorescein-derived fluorescent probe for cellular hydrogen sulfide imaging. Chin. Chem. Lett. 2014, 25, 1060–1064. [Google Scholar] [CrossRef]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring Singlet Oxygen and Hydroxyl Radical Formation with Fluorescent Probes During Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef]
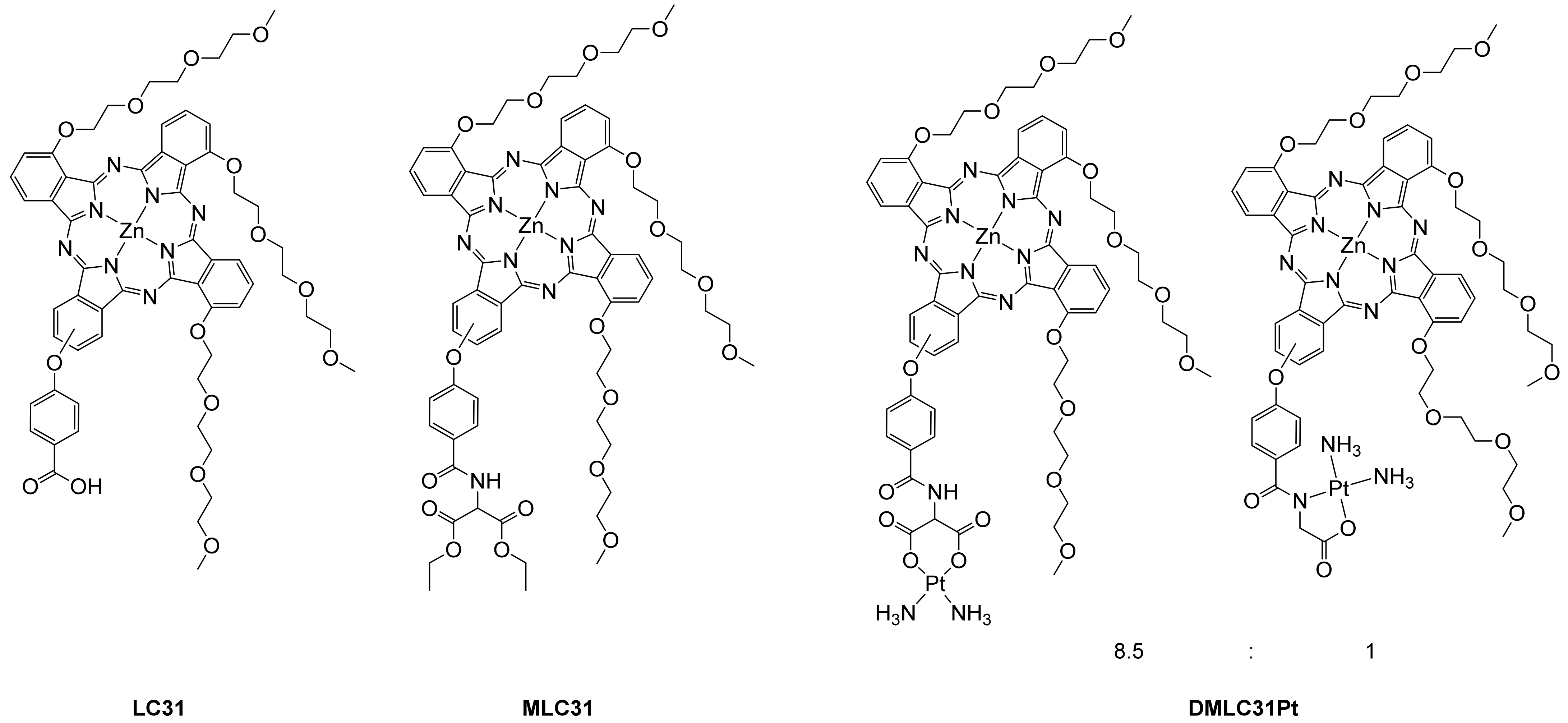
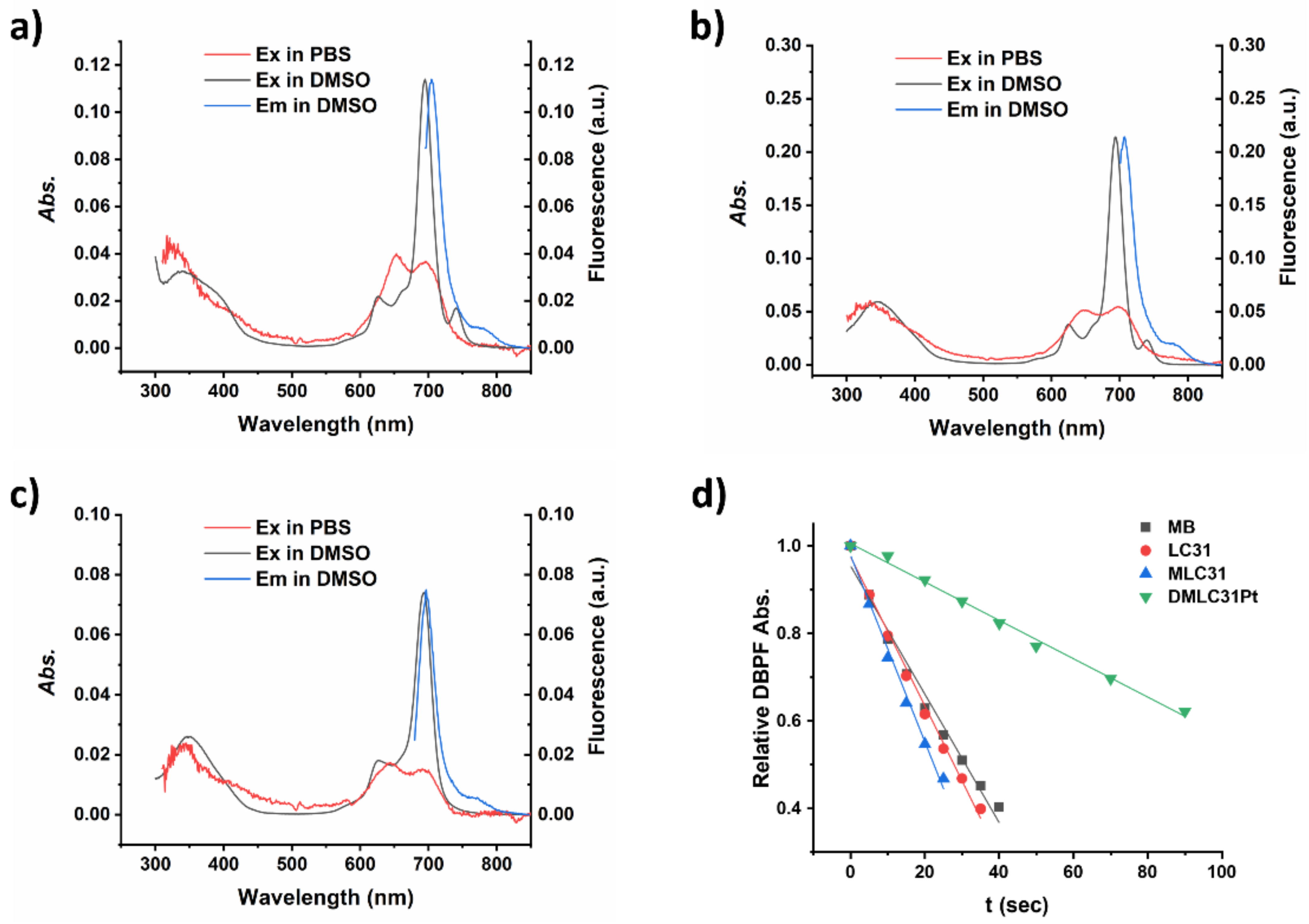


| B-Band (nm) | Q-Band (nm) | ϵ of the Q-Band (M−1 cm−1) | λem (nm) | τF (ns) a | Stokes shift (nm) a | Φ∆ b | |||
|---|---|---|---|---|---|---|---|---|---|
| DMSO | DMSO | PBS | DMSO | MeOH | DMSO | ||||
| LC31 | 338 | 696 | 37,400 | 114,800 | 105,000 | 704 | 2 | 8 | 0.49 |
| MLC31 | 345 | 694 | 57,900 | 206,900 | 145,800 | 707 | 3 | 13 | 0.84 |
| DMLC31Pt | 350 | 693 | 19,500 | 79,000 | 31,000 | 697 | - | 4 | 0.39 |
| Comp. | HeLa (μM) | A2780 (μM) | A2780/CP70 (μM) | MRC-5 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dark a | Light b | p.i. c | Dark a | Light b | p.i. c | Dark a | Light b | p.i. c | Dark a | Light b | p.i. c | |
| LC31 | 14.7 | 0.426 | 35 | 31.0 | 0.211 | 147 | 69.5 | 1.2 | 58 | 48.1 | 1.1 | 44 |
| MLC31 | 5.2 | 0.009 | 578 | 44.0 | 0.018 | 2444 | 117.5 | 0.157 | 748 | 12.8 | 0.019 | 674 |
| DMLC31Pt | 8.0 | 0.775 | 10 | 25.0 | 0.199 | 126 | 88.7 | 0.773 | 115 | 61.5 | 0.845 | 73 |
| cisplatin | 1.3 | 1.7 | 0.76 | 2.8 | 4.0 | 0.7 | 102.3 | 128.8 | 0.79 | 5.4 | 4.5 | 1.2 |
| Comp. | Dark Toxicity [μM] | Light Toxicity [μM] | p.i. | Wavelength [nm] | Fluence [J cm−2] | Ref. |
|---|---|---|---|---|---|---|
| MLC31 | 5.2 | 0.009 | 578 | λ > 600 a | 6.96 | This work |
| (SiPc)((OC2H4)22OH)2 | >3 | 0.3 | >10 | 690 b | 2 | [56] |
| SiPc(R-Biotin)2 | >10 | 0.4 | >25 | 690 b | 2 | [57] |
| ZnPc(branchedPEG)3 biotin | >5 | 1.5 | >3.3 | 690 b | 2 | [58] |
| ZnPc(I3)R-Biotin | >10 | 2.2 | >4.6 | 690 b | 2 | [59] |
| [(SiPc)(RNEt2Me)2]2+ | >10 | 1.5 | >6.7 | 690 b | 2 | [60] |
| [ZnPc]8+ | >100 | 0.04 | 2500 | 740 c | 15 | [61] |
| [ZnPc-(R(pyMe)4)4]16+ | 675 | 0.48 | 1409 | λ > 570 d | 11.2 | [62] |
| [ZnPc-(R’imidazolium4)4]16+ | 395 | 0.037 | 10675 | λ > 570 d | 11.2 | [63] |
| 18.6% O2 | 0.2% O2 | |||||
|---|---|---|---|---|---|---|
| Dark | Light a | p.i. | Dark | Light a | p.i. | |
| LC31 | >20 | 0.169 | >118 | >20 | 0.177 | >113 |
| MLC31 | 14.8 | 0.051 | 290 | >20 | 0.061 | 328 |
| DMLC31Pt | 16.7 | 7.0 | 2.4 | >20 | 8.1 | >2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu Klingler, W.; Giger, N.; Schneider, L.; Babu, V.; König, C.; Spielmann, P.; Wenger, R.H.; Ferrari, S.; Spingler, B. Low-Dose Near-Infrared Light-Activated Mitochondria-Targeting Photosensitizers for PDT Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 9525. https://doi.org/10.3390/ijms23179525
Wu Klingler W, Giger N, Schneider L, Babu V, König C, Spielmann P, Wenger RH, Ferrari S, Spingler B. Low-Dose Near-Infrared Light-Activated Mitochondria-Targeting Photosensitizers for PDT Cancer Therapy. International Journal of Molecular Sciences. 2022; 23(17):9525. https://doi.org/10.3390/ijms23179525
Chicago/Turabian StyleWu Klingler, Wenyu, Nadine Giger, Lukas Schneider, Vipin Babu, Christiane König, Patrick Spielmann, Roland H. Wenger, Stefano Ferrari, and Bernhard Spingler. 2022. "Low-Dose Near-Infrared Light-Activated Mitochondria-Targeting Photosensitizers for PDT Cancer Therapy" International Journal of Molecular Sciences 23, no. 17: 9525. https://doi.org/10.3390/ijms23179525
APA StyleWu Klingler, W., Giger, N., Schneider, L., Babu, V., König, C., Spielmann, P., Wenger, R. H., Ferrari, S., & Spingler, B. (2022). Low-Dose Near-Infrared Light-Activated Mitochondria-Targeting Photosensitizers for PDT Cancer Therapy. International Journal of Molecular Sciences, 23(17), 9525. https://doi.org/10.3390/ijms23179525







