Dysregulated Metabolic Pathways in Subjects with Obesity and Metabolic Syndrome
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
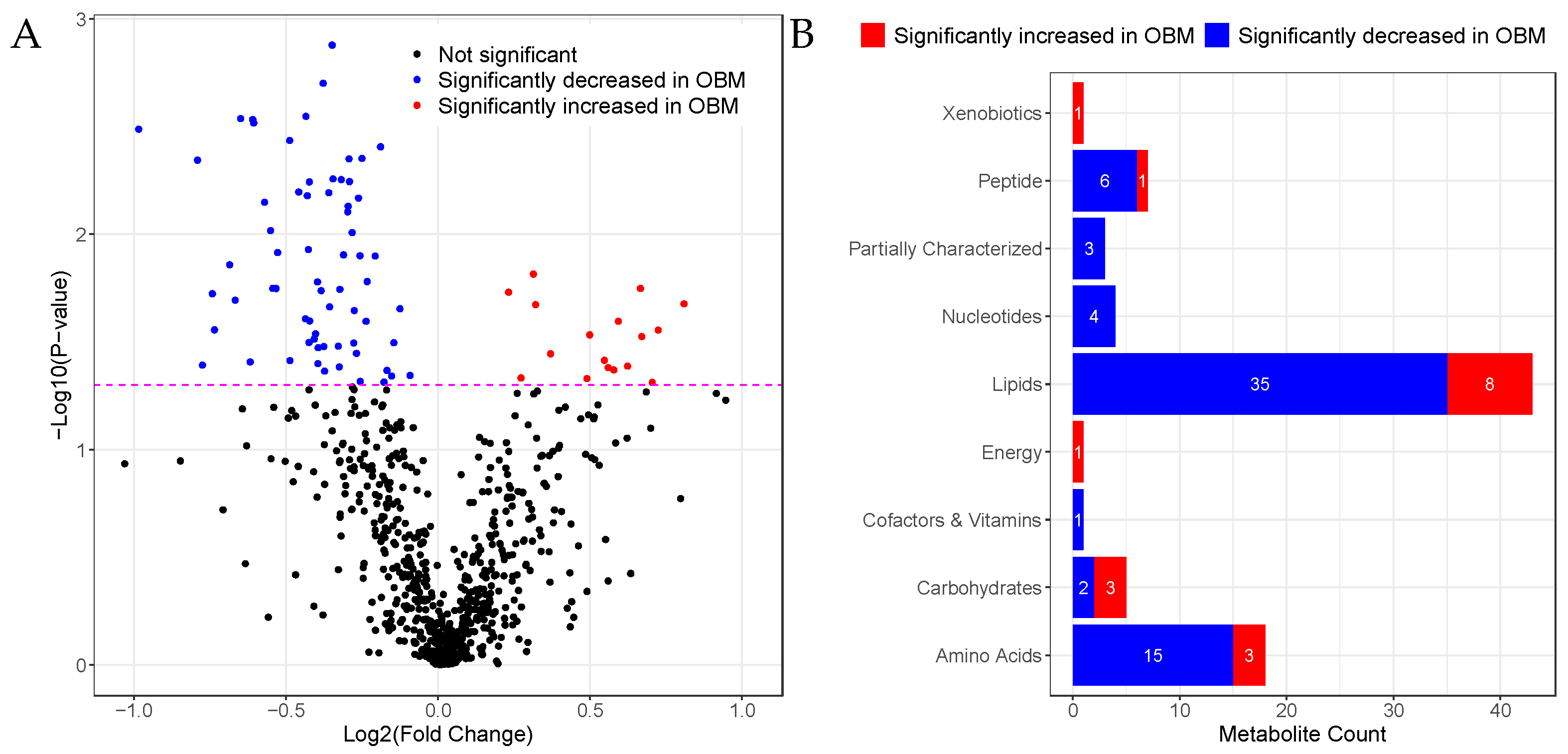
2.2. Univariate Analysis
2.2.1. Sphingomyelins Are Significantly Decreased in OBM
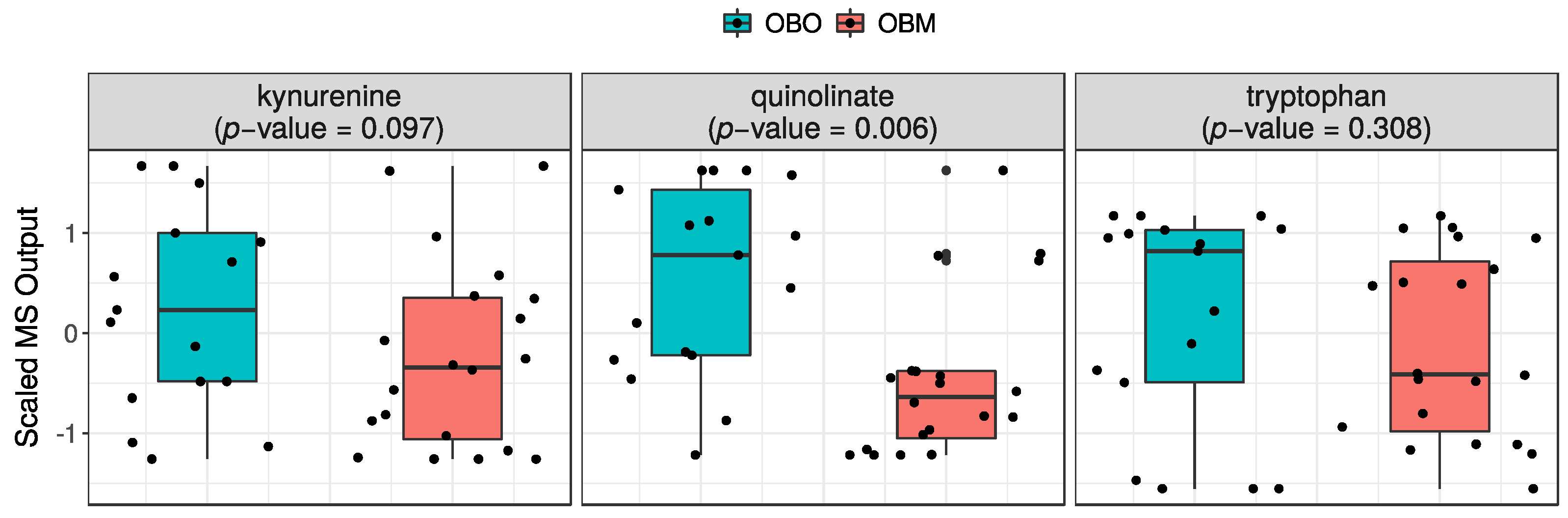
2.2.2. Quinolinate Is Significantly Decreased in OBM
2.3. Pathway Enrichment Analysis
2.4. Association of Metabolite Concentration with Clinical Parameters
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Baseline Statistical Analysis
4.3. Metabolomics Profiling and Quality Control
4.4. Univariate Statistical Analysis
4.5. Pathway Enrichment Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Obesity and Overweight Report. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 June 2022).
- World Health Organisation. Obesity and Overwieght Report. 2016. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 14 June 2022).
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J.; Sipilä, P.N.; et al. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef]
- Isomaa, B.O.; Almgren, P.; Tuomi, T.; Forsen, B.; Lahti, K.; Nissen, M.; Taskinen, M.R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef]
- Alexander, C.M.; Landsman, P.B.; Teutsch, S.M.; Haffner, S.M. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003, 52, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama 2002, 288, 2709–2716. [Google Scholar] [CrossRef]
- Grundy, S.M.; Hansen, B.; Smith, S.C., Jr.; Cleeman, J.I.; Kahn, R.A.; Conference Participants. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 2004, 109, 551–556. [Google Scholar] [CrossRef]
- Karelis, A.D.; Brochu, M.; Rabasa-Lhoret, R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004, 30, 569–572. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Meigs, J.B.; Wilson, P.W.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D’Agostino, R.B. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef]
- Arnlov, J.; Ingelsson, E.; Sundstrom, J.; Lind, L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010, 121, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ullah, E.; El-Menyar, A.; Kunji, K.; Elsousy, R.; Mokhtar, H.R.B.; Ahmad, E.; Al-Nesf, M.; Beotra, A.; Al-Maadheed, M.; Mohamed-Ali, V.; et al. Untargeted Metabolomics Profiling Reveals Perturbations in Arginine-NO Metabolism in Middle Eastern Patients with Coronary Heart Disease. Metabolites 2022, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Leandro, J.; Houten, S.M. The lysine degradation pathway: Subcellular compartmentalization and enzyme deficiencies. Mol. Genet. Metab. 2020, 131, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pitkanen, E. Mannose, mannitol, fructose and 1,5-anhydroglucitol concentrations measured by gas chromatography/mass spectrometry in blood plasma of diabetic patients. Clin. Chim. Acta 1996, 251, 91–103. [Google Scholar] [CrossRef]
- Helsley, R.N.; Moreau, F.; Gupta, M.K.; Radulescu, A.; DeBosch, B.; Softic, S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr. Diabetes Rep. 2020, 20, 64. [Google Scholar] [CrossRef]
- Ercan, N.; Nuttall, F.Q.; Gannon, M.C.; Redmon, J.B.; Sheridan, K.J. Effects of glucose, galactose, and lactose ingestion on the plasma glucose and insulin response in persons with non-insulin-dependent diabetes mellitus. Metabolism 1993, 42, 1560–1567. [Google Scholar] [CrossRef]
- Voet, D.J.; Voet, J.G.; Pratt, C.W. Lipids, Bilayers and Membranes, 3rd ed.; Wileypp: Hoboken, NJ, USA, 2008. [Google Scholar]
- Testi, R. Sphingomyelin breakdown and cell fate. Trends Biochem. Sci. 1996, 21, 468–471. [Google Scholar] [CrossRef]
- Schlitt, A.; Blankenberg, S.; Yan, D.; von Gizycki, H.; Buerke, M.; Werdan, K.; Bickel, C.; Lackner, K.J.; Meyer, J.; Rupprecht, H.J.; et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr. Metab. 2006, 3, 5. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Gerl, M.J.; Klose, C.; Surma, M.A.; Fernandez, C.; Melander, O.; Mannisto, S.; Borodulin, K.; Havulinna, A.S.; Salomaa, V.; Ikonen, E.; et al. Machine learning of human plasma lipidomes for obesity estimation in a large population cohort. PLoS Biol. 2019, 17, e3000443. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; Thomas, M.K.; et al. Muscle sphingolipids during rest and exercise: A C18:0 signature for insulin resistance in humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.A.; Mall, R.; Iskandarani, A.N.; Ullah, E.; Samra, T.A.; Cyprian, F.S.; Parray, A.S.; Meis, A.; Abdalhakam, I.; Farooq, F.; et al. Characteristic MicroRNAs linked to dysregulated metabolic pathways in Qatari adult subjects with obesity and metabolic syndrome. Front. Endocrinol. 2022, 1599. [Google Scholar] [CrossRef] [PubMed]
- Im, S.S.; Park, H.Y.; Shon, J.C.; Chung, I.S.; Cho, H.C.; Liu, K.H.; Song, D.K. Plasma sphingomyelins increase in pre-diabetic Korean men with abdominal obesity. PLoS ONE 2019, 14, e0213285. [Google Scholar] [CrossRef]
- Khan, S.R.; Manialawy, Y.; Obersterescu, A.; Cox, B.J.; Gunderson, E.P.; Wheeler, M.B. Diminished Sphingolipid Metabolism, a Hallmark of Future Type 2 Diabetes Pathogenesis, Is Linked to Pancreatic beta Cell Dysfunction. iScience 2020, 23, 101566. [Google Scholar] [CrossRef]
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD(+) Synthesis During Inflammation and Infection. Front. Immunol. 2020, 11, 31. [Google Scholar] [CrossRef]
- Slotte, J.P. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem. Phys. Lipids 1999, 102, 13–27. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Christensen, J.J.; Formo, G.A.; Holven, K.B.; Ulven, S.M. A comprehensive metabolic profiling of the metabolically healthy obesity phenotype. Lipids Health Dis. 2020, 19, 90. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MSEA: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G., Jr.; Brouwer, C. Pathview Web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Friedman, M.S.; Shedden, K.; Hankenson, K.D.; Woolf, P.J. GAGE: Generally applicable gene set enrichment for pathway analysis. BMC Bioinform. 2009, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| OBO | OBM | OBO vs. OBM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M n = 7 | F n = 11 | All n = 18 | pg | M n = 12 | F n = 9 | All n = 21 | pg | pm | pf | pa | |
| ALT | 28.1 (12.1) | 15.9 (8.8) | 20.7 (11.6) | 0.026 | 48.8 (42.5) | 20.1 (8.8) | 36.5 (35.1) | 0.042 | 0.137 | 0.310 | 0.063 |
| AST | 25.8 (12.6) | 14.3 (2.5) | 18.8 (9.6) | 0.006 | 28.8 (18.0) | 16.7 (4.8) | 23.6 (15.0) | 0.044 | 0.712 | 0.337 | 0.251 |
| Age | 39.9 (3.0) | 36.9 (4.6) | 38.1 (4.2) | 0.153 | 41.3 (7.8) | 39.6 (6.8) | 40.5 (7.3) | 0.497 | 0.588 | 0.402 | 0.283 |
| Albumin | 40.8 (3.7) | 35.8 (3.9) | 37.7 (4.5) | 0.030 | 40.7 (2.8) | 36.9 (2.3) | 39.1 (3.2) | 0.008 | 0.866 | 0.939 | 0.367 |
| BMI | 38.6 (4.0) | 40.9 (4.7) | 40.0 (4.5) | 0.238 | 40.4 (2.4) | 38.7 (3.3) | 39.6 (2.9) | 0.201 | 0.271 | 0.196 | 0.746 |
| C-Peptide | 4.9 (5.2) | 3.2 (1.4) | 3.9 (3.4) | 0.429 | 4.5 (1.7) | 3.5 (1.0) | 4.1 (1.5) | 0.111 | 0.847 | 0.605 | 0.814 |
| CRP | 9.0 (6.5) | 14.0 (13.1) | 12.0 (11.0) | 0.364 | 5.3 (3.1) | 10.6 (2.8) | 7.6 (4.0) | 0.003 | 0.218 | 0.428 | 0.121 |
| Cholesterol | 5.7 (1.2) | 4.5 (0.8) | 4.9 (1.1) | 0.025 | 4.8 (1.3) | 4.8 (0.9) | 4.8 (1.1) | 0.887 | 0.154 | 0.437 | 0.665 |
| Creatinine | 80.9 (7.7) | 59.0 (9.8) | 67.5 (14.1) | <0.001 | 70.8 (14.0) | 58.1 (11.2) | 65.3 (14.1) | 0.059 | 0.057 | 0.820 | 0.563 |
| Glucose | 5.1 (0.7) | 5.2 (0.5) | 5.2 (0.6) | 0.819 | 6.5 (1.4) | 8.5 (4.9) | 7.3 (3.4) | 0.268 | 0.031 | 0.081 | 0.009 |
| HDL | 1.6 (1.0) | 1.4 (0.4) | 1.5 (0.7) | 0.766 | 0.9 (0.4) | 1.1 (0.1) | 1.0 (0.3) | 0.275 | 0.166 | 0.006 | 0.008 |
| HbA1C | 5.6 (0.3) | 5.5 (0.3) | 5.5 (0.3) | 0.383 | 6.8 (1.2) | 7.3 (2.6) | 7.0 (1.9) | 0.618 | 0.016 | 0.068 | 0.002 |
| Insulin | 16.2 (7.1) | 21.0 (16.1) | 19.1 (13.3) | 0.479 | 30.4 (15.1) | 22.9 (8.3) | 27.2 (12.9) | 0.277 | 0.056 | 0.743 | 0.062 |
| LDL | 3.4 (1.8) | 2.4 (0.7) | 2.8 (1.3) | 0.179 | 2.6 (1.3) | 2.6 (0.9) | 2.6 (1.1) | 0.915 | 0.397 | 0.492 | 0.728 |
| Triglycerides | 1.5 (0.4) | 1.3 (0.5) | 1.4 (0.5) | 0.318 | 2.8 (1.8) | 2.4 (1.1) | 2.7 (1.5) | 0.523 | 0.031 | 0.013 | 0.001 |
| Pathway | Total | Hits | Statistic Q | Expected Q | p |
|---|---|---|---|---|---|
| Lysine degradation | 25 | 5 | 11.615 | 2.778 | 0.003 |
| Amino sugar and nucleotide sugar metabolism | 37 | 3 | 14.145 | 2.778 | 0.005 |
| Arginine and proline metabolism | 38 | 12 | 6.149 | 2.778 | 0.015 |
| Fructose and mannose metabolism | 20 | 3 | 10.852 | 2.778 | 0.017 |
| Galactose metabolism | 27 | 6 | 8.527 | 2.778 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, F.A.; Ullah, E.; Mall, R.; Iskandarani, A.; Samra, T.A.; Cyprian, F.; Parray, A.; Alkasem, M.; Abdalhakam, I.; Farooq, F.; et al. Dysregulated Metabolic Pathways in Subjects with Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 9821. https://doi.org/10.3390/ijms23179821
Mir FA, Ullah E, Mall R, Iskandarani A, Samra TA, Cyprian F, Parray A, Alkasem M, Abdalhakam I, Farooq F, et al. Dysregulated Metabolic Pathways in Subjects with Obesity and Metabolic Syndrome. International Journal of Molecular Sciences. 2022; 23(17):9821. https://doi.org/10.3390/ijms23179821
Chicago/Turabian StyleMir, Fayaz Ahmad, Ehsan Ullah, Raghvendra Mall, Ahmad Iskandarani, Tareq A. Samra, Farhan Cyprian, Aijaz Parray, Meis Alkasem, Ibrahem Abdalhakam, Faisal Farooq, and et al. 2022. "Dysregulated Metabolic Pathways in Subjects with Obesity and Metabolic Syndrome" International Journal of Molecular Sciences 23, no. 17: 9821. https://doi.org/10.3390/ijms23179821
APA StyleMir, F. A., Ullah, E., Mall, R., Iskandarani, A., Samra, T. A., Cyprian, F., Parray, A., Alkasem, M., Abdalhakam, I., Farooq, F., & Abou-Samra, A.-B. (2022). Dysregulated Metabolic Pathways in Subjects with Obesity and Metabolic Syndrome. International Journal of Molecular Sciences, 23(17), 9821. https://doi.org/10.3390/ijms23179821






