Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase
Abstract
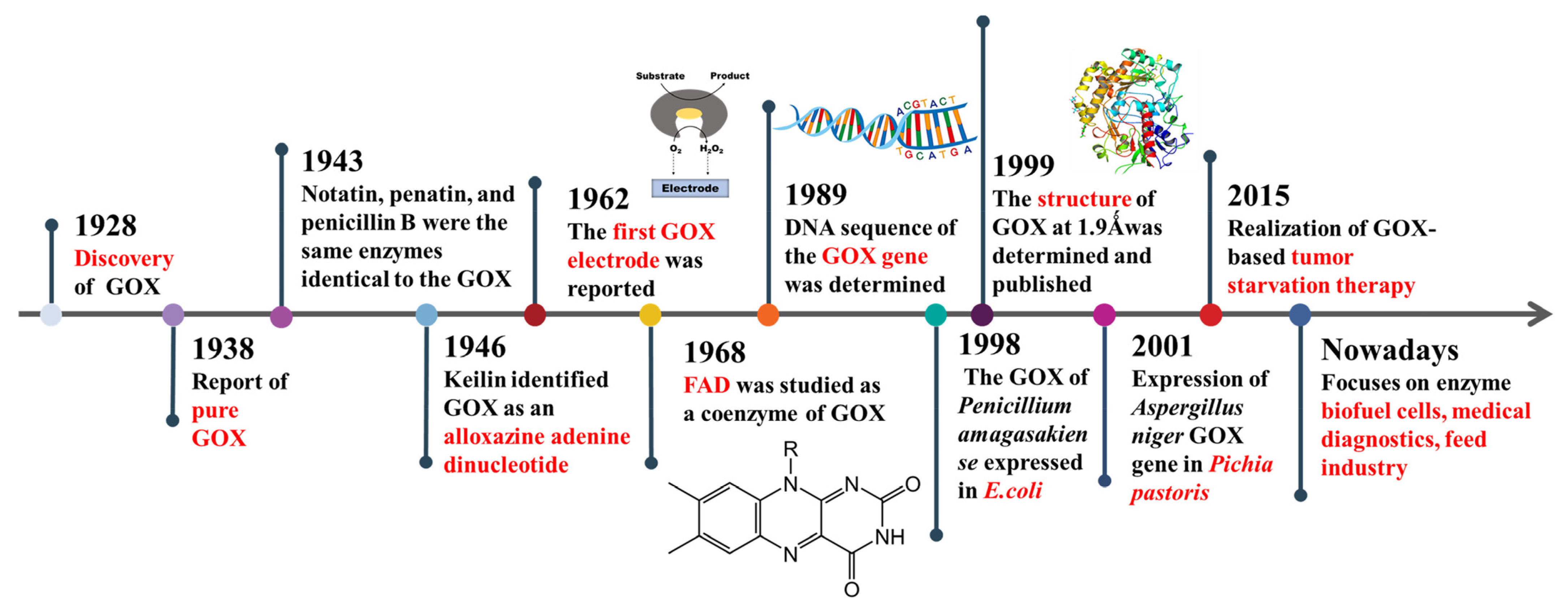
:1. Introduction
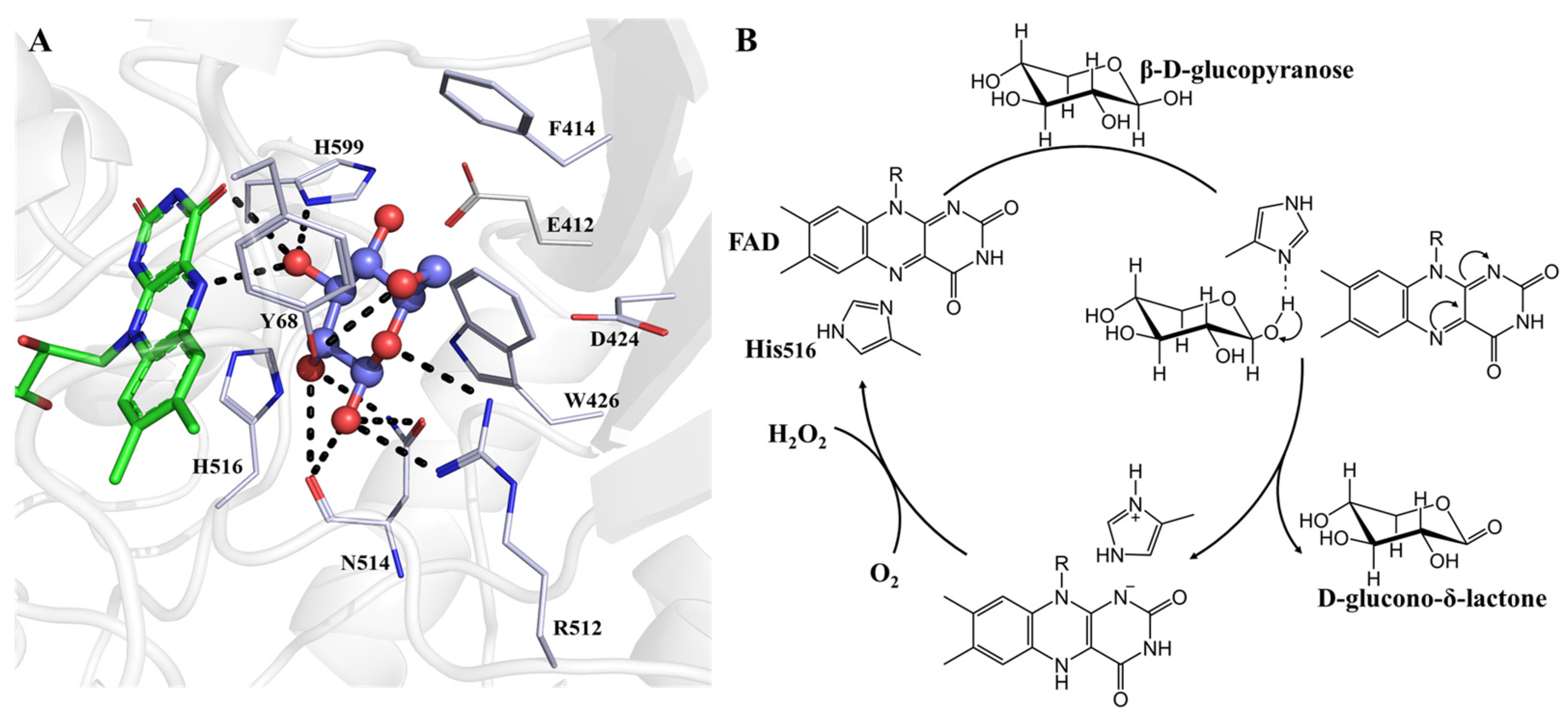
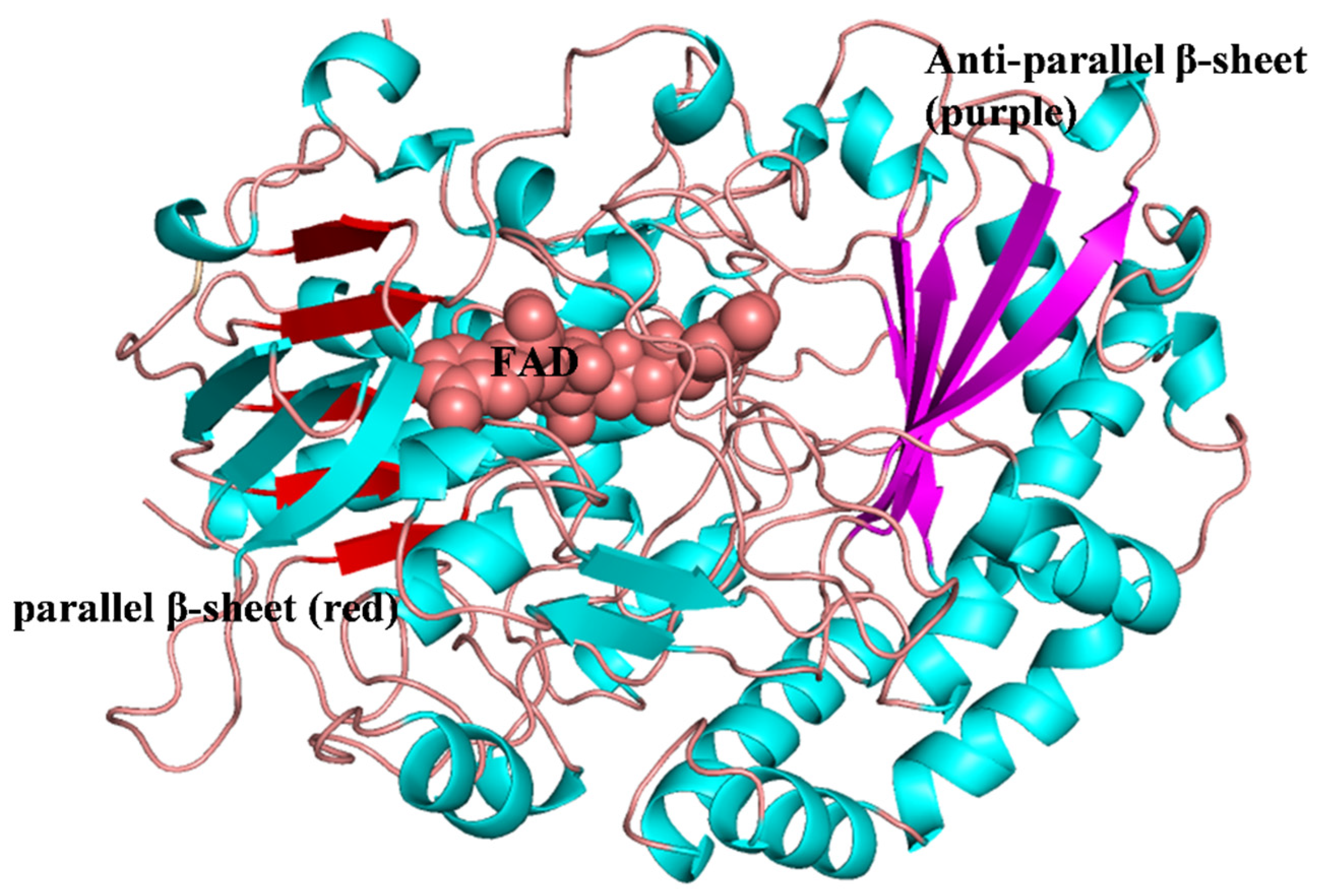
2. The Structure of Glucose Oxidase
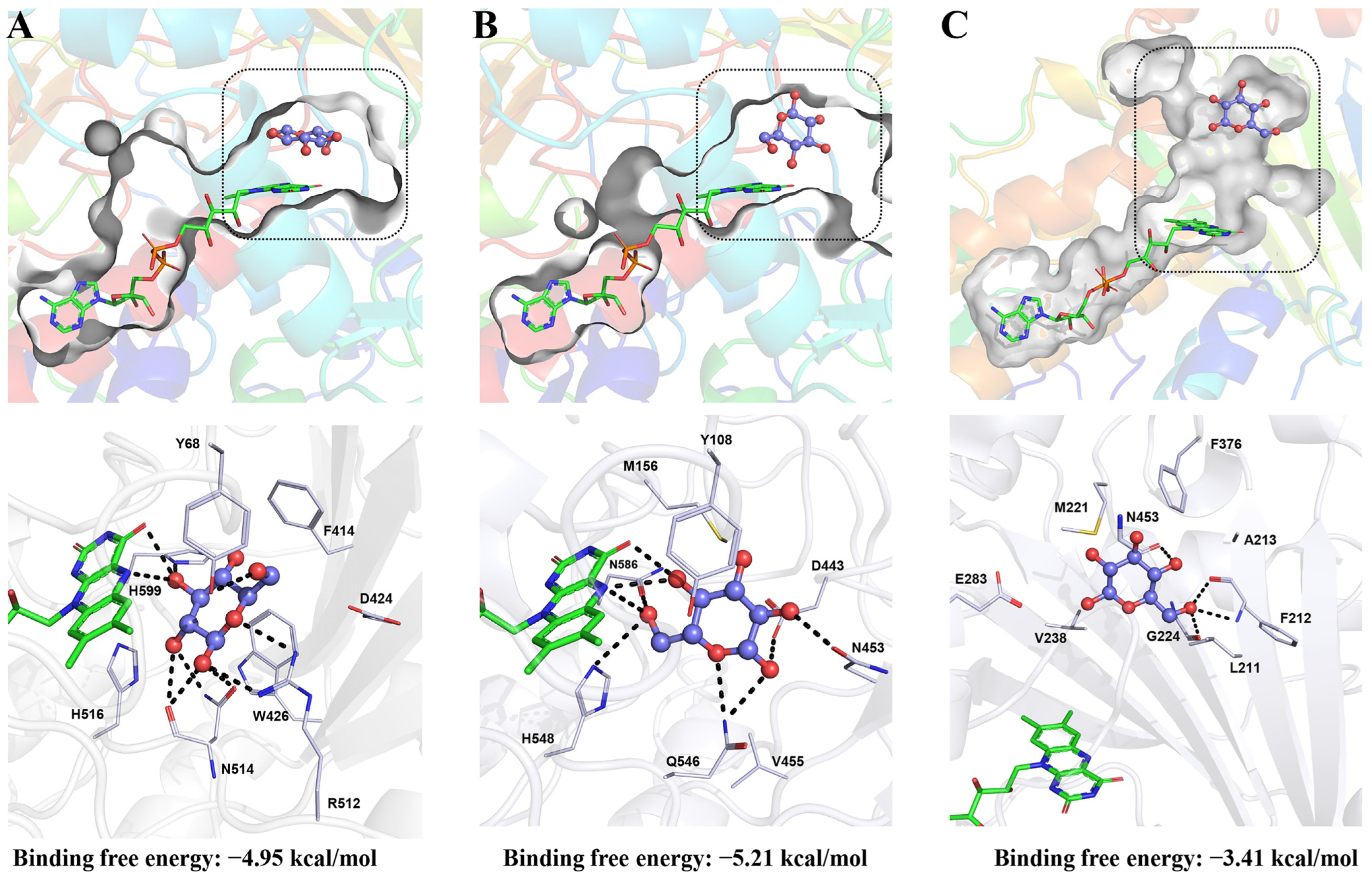
3. Inhibitors of Glucose Oxidase
4. Strategies for Improving Glucose Oxidase Yields
4.1. Optimization of Culture Conditions
4.2. Mutation Technology
4.3. Heterologous Expression of Glucose Oxidase
4.3.1. Heterologous Expression of Glucose Oxidase by Yeast
4.3.2. Heterologous Expression of Glucose Oxidase by E. coli
4.3.3. Heterologous Expression of GOX Using Other Technologies
| Source | Host | Vector | Molecular Weight (kDa) | Characterization | Activity (U/mL) | Reference |
|---|---|---|---|---|---|---|
| Aspergillus niger A9 | Pichia pastoris GS115 | pPIC9 | 75 | pH 6.0, temperature = 40 °C Km = 21.06 mM Vmax = 359 μmol/min/mg | 99 | [3] |
| Aspergillus niger ATCC 9202 | Yarrowia lipolytica | pINA1296 | 80 | pH 5.5, temperature = 37 °C | 0.37 | [87] |
| Aspergillus niger | Saccharomyces cerevisiae | pGal | 78–105 | pH 6.0 specific activity 194 U/mg | 10 | [106] |
| Aspergillus niger NRRL-3 | Trichoderma reesei | pRS424 | 70–90 | NA | NA | [107] |
| Aspergillus niger Z-25 | Pichia Pastoris SMD1168 | pPICZαA | 94 | pH 6.0, temperature = 40 °C Km = 16.95 mM Kcat = 484.26 s−1 specific activity 153.46 U/mg | 40 | [108] |
| Aspergillus niger | Hansenula polymorpha | YEpl3 | 71 | NA | NA | [109] |
| Aspergillums niger ATCC 9029 | Pichia pastoris GS115 | pPIC9 | 69 | pH 7, temperature = 50 °C | NA | [76] |
| Aspergillums niger ATCC 9029 | Pichia pastoris X33 | pGAPZαC | 78 | pH 6, temperature = 37 °C | NA | [86] |
| Aspergillums niger | Penicillium nalgiovense | pSK + 3.Sma | NA | specific activity 2.8 U/mL | NA | [110] |
| Aspergillus heteromophus CBS 117.55 | Pichia pastoris GS115 | pPIC9k | 75 | pH 6.0, temperature = 35 °C Km = 187 mM Vmax = 185.6 µmol/min/mg | NA | [84] |
| Penicillium amagasakiense ATCC 332245 | Pichia pastoris GS115 | pPICZαA | 72 | pH 6.0, temperature = 50 °C Km = 18.2 ± 1.2 mM specific activity 365 U/mg | 4 | [83] |
| Penicillium notatum F4 | Pichia pastoris GS115 | pPIC9 | 72–95 | pH 6.2, temperature = 35 °C Km = 83 mM Vmax = 2170 µmol/min/mg | 148 | [89] |
| Penicillium amagasakiense ATCC 28686 | Escherichia coli | pCYTEXP1 | 60 | pH 5.2–6.2, temperature = 28–40 °C Km = 5.2–6.2 mM | NA | [93] |
| Penicillium sp MX3343 | Pichia pastoris X33 | pPICZαA | NA | pH 5.5, temperature = 30 °C Km = 65.7 mM | 458.6 | [111] |
| Cladosporium neopsychrotolerans SL16 | Pichia pastoris GS115 | pPIC9 | 68 | pH 7.0, temperature = 20 °C Km = 103 mM | 2.9 | [112] |
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, M.K.; Zehra, A.; Aamir, M.; Meena, M.; Ahirwal, L.; Singh, S.; Shukla, S.; Upadhyay, R.S.; Bueno-Mari, R.; Bajpai, V.K. Improvement strategies, cost effective production, and potential applications of fungal glucose oxidase (GOD): Current Updates. Front. Microbiol. 2017, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.A.; Ghoneimy, E.A.; El-Khawaga, M.A.; Negm-Eldein, A.; Awad, G.E.A. Statistical optimization of glucose oxidase production from Aspergillus niger NRC9 under submerged fermentation using response surface methodology. Ann. Microbiol. 2013, 63, 523–531. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, M.; Yang, M.; Zhang, Q.; Hao, J.; Meng, Y. Production and characterization of recombinant glucose oxidase from Aspergillus niger expressed in Pichia pastoris. Lett. Appl. Microbiol. 2014, 58, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.A.; Zamocka, M.; Majtan, J.; Bauerova-Hlinkova, V. Glucose oxidase, an enzyme “Ferrari”: Its structure, function, production and properties in the light of various industrial and biotechnological applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Hatzinikolaou, D.G.; Hansen, O.C.; Macris, B.J.; Tingey, A.; Kekos, D.; Goodenough, P.; Stougaard, P. A new glucose oxidase from Aspergillus niger: Characterization and regulation studies of enzyme and gene. Appl. Microbiol. Biotechnol. 1996, 46, 371–381. [Google Scholar] [CrossRef]
- Bhat, S.V.; Swathi, B.R.; Rosy, M.; Govindappa, M. Isolation and charecterization of glucose oxidase (GOD) from Aspergillus flavus and Penicillium sp. J. Curr. Microbiol. Appl. Sci. 2013, 2, 153–161. [Google Scholar]
- Kriaa, M.; Hammami, I.; Sahnoun, M.; Azebou, M.C.; Triki, M.A.; Kammoun, R. Purification, biochemical characterization and antifungal activity of a novel Aspergillus tubingensis glucose oxidase steady on broad range of pH and temperatures. Bioprocess Biosyst. Eng. 2015, 38, 2155–2166. [Google Scholar] [CrossRef]
- Ahmad Anas, N.G.; Arbain, D.; Ahmad, M.S. Effects of selected medium components for production of glucose oxidase by a local isolate Aspergillus Terreus UniMAP AA-1. APCBEE Procedia 2012, 2, 125–128. [Google Scholar] [CrossRef]
- Leiter, E.; Marx, F.; Pusztahelyi, T.; Haas, H.; Pocsi, I. Penicillium chrysogenum glucose oxidase—A study on its antifungal effects. J. Appl. Microbiol. 2004, 97, 1201–1209. [Google Scholar] [CrossRef]
- Sukhacheva, M.V.; Davydova, M.E.; Netrusov, A.I. Production of Penicillium funiculosum 433 glucose oxidase and its properties. Appl. Biochem. Microbiol. 2004, 40, 25–29. [Google Scholar] [CrossRef]
- Rando, D.; Kohring, G.W.; Giffhorn, F. Production, purification and characterization of glucose oxidase from a newly isolated strain of Penicillium pinophilum. Appl. Microbiol. Biotechnol. 1997, 48, 34–40. [Google Scholar] [CrossRef]
- Mikhailova, R.; Semashko, T.; Demeshko, O.; Ramanaviciene, A.; Ramanavicius, A. Effect of some redox mediators on FAD fluorescence of glucose oxidase from Penicillium adametzii LF F-2044.1. Enzym. Microb. Technol. 2015, 72, 10–15. [Google Scholar] [CrossRef]
- Kim, H.W.; Kimura, S.; Ohno, N.; Okadome, M.; Fujii, T. Purification of glucose oxidase and catalase produced by the apple blue mold, Penicillium expansum O-385-10, and their characteristics including the browning of apple fruit. Jpn. J. Food Microbiol. 2005, 22, 10–16. [Google Scholar] [CrossRef]
- Santos, K.S.; dos Santos, L.D.; Mendes, M.A.; de Souza, B.M.; Malaspina, O.; Palma, M.S. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.). Insect Biochem. Mol. Biol. 2005, 35, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Afshar, K.; Dufresne, P.J.; Pan, L.; Merkx-Jacques, M.; Bede, J.C. Diet-specific salivary gene expression and glucose oxidase activity in Spodoptera exigua (Lepidoptera: Noctuidae) larvae. J. Insect Physiol. 2010, 56, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, Y.; Kang, L.; Wang, C.Z. Characterization of glucose-induced glucose oxidase gene and protein expression in Helicoverpa armigera larvae. Arch. Insect Biochem. Physiol. 2012, 79, 104–119. [Google Scholar] [CrossRef]
- Candy, D.J. Glucose oxidase and other enzymes of hydrogen peroxide metabolism from cuticle of Schistocerca americana gregaria. Insect Biochem. 1979, 9, 661–665. [Google Scholar] [CrossRef]
- Kang, S.O.; Shin, K.S.; Han, Y.H.; Youn, H.D.; Hah, Y.C. Purification and characterization of D-glucose oxidase from white-rot fungus Pleurotus Ostreatus. Eur. J. Biochem. 1993, 215, 747–752. [Google Scholar] [CrossRef]
- Yuivar, Y.; Barahona, S.; Alcaino, J.; Cifuentes, V.; Baeza, M. Biochemical and thermodynamical characterization of glucose oxidase, invertase, and alkaline phosphatase secreted by antarctic yeasts. Front. Mol. Biosci. 2017, 4, 86. [Google Scholar] [CrossRef]
- Kim, K.K.; Fravel, D.R.; Papavizas, G.C. Production, purification, and properties of glucose oxidase from the biocontrol fungus Talaromyces flavus. Can. J. Microbiol. 2011, 36, 199–205. [Google Scholar] [CrossRef]
- Zhao, S.F.; Jiang, H.; Chi, Z.; Liu, G.L.; Chi, Z.M.; Chen, T.J.; Yang, G.; Hu, Z. Genome sequencing of Aureobasidium pullulans P25 and overexpression of a glucose oxidase gene for hyper-production of Ca2+ gluconic acid. Antonie Van Leeuwenhoek 2018, 112, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Oeljeklaus, S.; Gerhardt, B.; Tudzynski, B. Purification and characterization of glucose oxidase of Botrytis cinerea. Physiol. Mol. Plant Pathol. 1998, 53, 123–132. [Google Scholar] [CrossRef]
- Ma, Y.; Chi, Z.; Li, Y.F.; Jiang, H.; Liu, G.L.; Hu, Z.; Chi, Z.M. Cloning, deletion, and overexpression of a glucose oxidase gene in Aureobasidium sp. P6 for Ca2+ gluconic acid overproduction. Ann. Microbiol. 2018, 68, 871–879. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Zia, M.A.; Riaz, A.; Rasul, S.; Abbas, R.Z. Evaluation of antimicrobial activity of glucose oxidase from Aspergillus niger EBL-A and Penicillium notatum. Braz. Arch. Biol. Technol. 2013, 56, 956–961. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme production of D-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Witt, S.; Wohlfahrt, G.; Schomburg, D.; Hecht, H.J.; Kalisz, H.M. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of beta-D-glucose. Biochem. J. 2000, 347, 553–559. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivic, S.; Wohlfahrt, G.; Kandrac, J.; Pericin, D. Glucose oxidase from Aspergillus niger: The mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int. J. Biochem. Cell Biol. 2005, 37, 731–750. [Google Scholar] [CrossRef]
- Karyakin, A.A. Glucose biosensors for clinical and personal use. Electrochem. Commun. 2021, 125, 106973. [Google Scholar] [CrossRef]
- Mandpe, P.; Prabhakar, B.; Gupta, H.; Shende, P. Glucose oxidase-based biosensor for glucose detection from biological fluids. Sens. Rev. 2020, 40, 497–511. [Google Scholar] [CrossRef]
- Mano, N. Engineering glucose oxidase for bioelectrochemical applications. Bioelectrochemistry 2019, 128, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Dagdelen, A.F.; Gocmen, D. Effects of glucose oxidase, hemicellulase and ascorbic acid on dough and bread quality. J. Food Qual. 2007, 30, 1009–1022. [Google Scholar] [CrossRef]
- Sisak, C.; Csanádi, Z.; Rónay, E.; Szajáni, B. Elimination of glucose in egg white using immobilized glucose oxidase. Enzyme Microb. Technol. 2006, 39, 1002–1007. [Google Scholar] [CrossRef]
- Zia, M.A.; Ain, Q.; Iftikhar, T.; Abbas, R.Z.; Rahman, K. Production of rabbit antibodies against purified glucose oxidase. Braz. Arch. Biol. Technol. 2012, 55, 69–74. [Google Scholar] [CrossRef]
- Cruz, A.G.; Castro, W.F.; Faria, J.A.F.; Bogusz, S.; Granato, D.; Celeguini, R.M.S.; Lima-Pallone, J.; Godoy, H.T. Glucose oxidase: A potential option to decrease the oxidative stress in stirred probiotic yogurt. LWT 2012, 47, 512–515. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Dong, C.; Shi, S. Glucose oxidase-related cancer therapies. Adv. Ther. 2020, 3, 2000110. [Google Scholar] [CrossRef]
- Liang, Z.; Yan, Y.; Zhang, W.; Luo, H.; Yao, B.; Huang, H.; Tu, T. Review of glucose oxidase as a feed additive: Production, engineering, applications, growth-promoting mechanisms, and outlook. Crit. Rev. Biotechnol. 2022, 1–18. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Soltani Fard, E.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2021, 69, 939–950. [Google Scholar] [CrossRef]
- Haouz, A.; Twist, C.; Zentz, C.; Tauc, P.; Alpert, B. Dynamic and structural properties of glucose oxidase enzyme. Eur. Biophys. J. 1998, 27, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sriwaiyaphram, K.; Punthong, P.; Sucharitakul, J.; Wongnate, T. Structure and function relationships of sugar oxidases and their potential use in biocatalysis. Enzymes 2020, 47, 193–230. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, H.M.; Hecht, H.J.; Schomburg, D.; Schmid, R.D. Effects of carbohydrate depletion on the structure, stability and activity of glucose oxidase from Aspergillus niger. Biochim. Biophys. Acta Proteins Proteom. 1991, 1080, 138–142. [Google Scholar] [CrossRef]
- Wohlfahrt, G.; Witt, S.; Hendle, J.; Schomburg, D.; Hecht, H. 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Hu, S.S.; Zhang, Q.F.; Li, M.Y.; Yu, S.; Chi, N.Y. Cloning of low-temperature glucose oxidase gene from marine bacteria and its expression in Escherichia coli. Food Ferment. Ind. 2019, 45, 34–39. (In Chinese) [Google Scholar] [CrossRef]
- Qu, G.; Bi, Y.; Liu, B.; Li, J.; Han, X.; Liu, W.; Jiang, Y.; Qin, Z.; Sun, Z. Unlocking the stereoselectivity and substrate acceptance of enzymes: Proline-induced loop engineering test. Angew. Chem. Int. Ed. Engl. 2022, 61, e202110793. [Google Scholar] [CrossRef]
- AlphaFold Reveals the Structure of the Protein Universe. Available online: https://www.deepmind.com/blog/alphafold-reveals-the-structure-of-the-protein-universe (accessed on 28 July 2022).
- Jithendar, T.; Sairam, K.V.S.S.; Verma, V. Purification, characterization, thermostability and shelf life studies of glucose oxidase from Aspergillus niger PIL7. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1666–1678. [Google Scholar]
- Jiang, Z.B.; Song, H.T.; Xiao, W.J.; Yang, Y.M.; Li, N.N. Improving the anti-oxidation of glucose oxidase with computer-aided structure optimization. J. Adv. Biotechnol. 2015, 5, 736–740. [Google Scholar] [CrossRef]
- Nakamura, S.; Ogura, Y. Mode of inhibition of glucose oxidase by metal ions. J. Biochem. 1968, 64, 439. [Google Scholar] [CrossRef]
- Jie, B.; Furumoto, K.; Yoshimoto, M.; Fukunaga, K.; Nakao, K. Competitive inhibition by hydrogen peroxide produced in glucose oxidation catalyzed by glucose oxidase. Biochem. Eng. J. 2003, 13, 69–72. [Google Scholar] [CrossRef]
- Vuong, T.V.; Foumani, M.; MacCormick, B.; Kwan, R.; Master, E.R. Direct comparison of gluco-oligosaccharide oxidase variants and glucose oxidase: Substrate range and H2O2 stability. Sci. Rep. 2016, 6, 37356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Wu, P.; Xu, W.; Shao, Q.; An, L.; Zhang, H.; Cai, C.; Zhao, B. Effects of guanidinium ions on the conformational structure of glucose oxidase studied by electrochemistry, spectroscopy, and theoretical calculations: Towards developing a chemical-induced protein conformation assay. Phys. Chem. Chem. Phys. 2012, 14, 5824–5836. [Google Scholar] [CrossRef] [PubMed]
- Hatzinikolaou, D.G.; Macris, B.J. Factors regulating production of glucose oxidase by Aspergillus niger. Enzyme Microb. Technol. 1995, 17, 530–534. [Google Scholar] [CrossRef]
- Bodade, R.G.; Khobragade, C.N.; Arfeen, S. Optimization of culture conditions for glucose oxidase production by a Penicillium chrysogenum SRT 19 strain. Eng. Life Sci. 2010, 10, 35–39. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Optimization of Aspergillus niger fermentation for the production of glucose oxidase. Food Bioproc. Technol. 2008, 2, 344–352. [Google Scholar] [CrossRef]
- Zetelaki, K.; Vas, K. The role of aeration and agitation in the production of glucose oxidase in submerged culture. Biotechnol. Bioeng. 2010, 10, 45–59. [Google Scholar] [CrossRef]
- Li, T.H.; Chen, T.L. Enhancement of glucose oxidase fermentation by addition of hydrocarbons. J. Biosci. Bioeng. 1994, 78, 298–303. [Google Scholar] [CrossRef]
- Ramzan, M.; Mehmood, T. Enhanced production of glucose oxidase from UV-mutant of Aspergillus niger. Afr. J. Biotechnol. 2009, 8, 288–290. [Google Scholar] [CrossRef]
- Zia, M.A.; Rasul, S.; Iftikhar, T. Effect of gamma irradiation on Aspergillus niger for enhanced production of glucose oxidase. Pak. J. Bot. 2012, 44, 1575–1580. [Google Scholar]
- Zia, M.A.; Sheikh, M.A.; Khan, I.A. Chemically treated strain improvement of Aspergillus niger for enhanced production of glucose oxidase. Int. J. Agric. Biol. 2010, 12, 964–966. [Google Scholar] [CrossRef]
- Khattab, A.A.; Bazaraa, W.A. Screening, mutagenesis and protoplast fusion of Aspergillus niger for the enhancement of extracellular glucose oxidase production. J. Ind. Microbiol. Biotechnol. 2005, 32, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kodym, A.; Afza, R. Physical and chemical mutagenesis. Methods Mol. Biol. 2003, 236, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Caspary, T.; Anderson, K.V. Uncovering the uncharacterized and unexpected: Unbiased phenotype-driven screens in the mouse. Dev. Dyn. 2006, 235, 2412–2423. [Google Scholar] [CrossRef]
- Kriaa, M.; Boukedi, H.; Ben Rhouma, M.; Ben Nasr, Y.; Tounsi, S.; Mellouli, L.; Kammoun, R. Overproduction of glucose oxidase by Aspergillus tubingensis CTM 507 randomly obtained mutants and study of its insecticidal activity against Ephestia kuehniella. Biomed. Res. Int. 2020, 2020, 9716581. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. Chapter 2 Mutagenesis. Methods Cell Biol. 1995, 48, 31–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, M.; Gautam, A.; Nazor, J.; Momeu, C.; Prodanovic, R.; Schwaneberg, U. Directed evolution of glucose oxidase from Aspergillus niger for ferrocenemethanol-mediated electron transfer. Biotechnol. J. 2007, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Horaguchi, Y.; Saito, S.; Kojima, K.; Tsugawa, W.; Ferri, S.; Sode, K. Engineering glucose oxidase to minimize the influence of oxygen on sensor response. Electrochim. Acta 2014, 126, 158–161. [Google Scholar] [CrossRef]
- Tu, T.; Wang, Y.; Huang, H.Q.; Wang, Y.R.; Jiang, X.; Wang, Z.X.; Yao, B.; Luo, H.Y. Improving the thermostability and catalytic efficiency of glucose oxidase from Aspergillus niger by molecular evolution. Food Chem. 2019, 281, 163–170. [Google Scholar] [CrossRef]
- Marin-Navarro, J.; Roupain, N.; Talens-Perales, D.; Polaina, J. Identification and structural analysis of amino acid substitutions that increase the stability and activity of Aspergillus niger glucose oxidase. PLoS ONE 2015, 10, e0144289. [Google Scholar] [CrossRef]
- Tobin, M.B.; Gustafsson, C.; Huisman, G.W. Directed evolution: The ‘rational’ basis for ‘irrational’ design. Curr. Opin. Struct. Biol. 2000, 10, 421–427. [Google Scholar] [CrossRef]
- Mu, Q.; Cui, Y.; Tian, Y.; Hu, M.; Tao, Y.; Wu, B. Thermostability improvement of the glucose oxidase from Aspergillus niger for efficient gluconic acid production via computational design. Int. J. Biol. Macromol. 2019, 136, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Zhang, Y.; Yuan, T.; Li, Q.; Tian, J.; Guan, W.; Liu, B.; Zhang, W.; Xu, X.; Zhang, Y. Enhanced thermostability of glucose oxidase through computer-aided molecular design. Int. J. Mol. Sci. 2018, 19, 425. [Google Scholar] [CrossRef] [Green Version]
- Ittisoponpisan, S.; Jeerapan, I. In silico analysis of glucose oxidase from Aspergillus niger: Potential cysteine mutation sites for enhancing protein stability. Bioengineering 2021, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Belyad, F.; Karkhanei, A.A.; Raheb, J. Expression, characterization and one step purification of heterologous glucose oxidase gene from Aspergillus niger ATCC 9029 in Pichia pastoris. EuPA Open Proteom. 2018, 19, 1–5. [Google Scholar] [CrossRef]
- Fiedurek, J.; Gromada, A. Screening and mutagenesis of molds for improvement of the simultaneous production of catalase and glucose oxidase. Enzyme Microb. Technol. 1997, 20, 344–347. [Google Scholar] [CrossRef]
- Rocha, S.; Abrahão-Neto, J.; Cerdán, M.; González-Siso, M.; Gombert, A.J. Heterologous expression of glucose oxidase in the yeast Kluyveromyces marxianus. Microb. Cell Factories 2010, 9, 4. [Google Scholar] [CrossRef]
- Malherbe, D.F.; du Toit, M.; Cordero Otero, R.R.; van Rensburg, P.; Pretorius, I.S. Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Appl. Microbiol. Biotechnol. 2003, 61, 502–511. [Google Scholar] [CrossRef]
- Yu, S.; Miao, L.; Huang, H.; Li, Y.; Zhu, T. High-level production of glucose oxidase in Pichia pastoris: Effects of Hac1p overexpression on cell physiology and enzyme expression. Enzyme Microb. Technol. 2020, 141, 109671. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast expression systems: Overview and recent advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Park, E.H.; Shin, Y.M.; Lim, Y.Y.; Kwon, T.H.; Kim, D.H.; Yang, M.S. Expression of glucose oxidase by using recombinant yeast. J. Biotechnol. 2000, 81, 35–44. [Google Scholar] [CrossRef]
- Courjean, O.; Mano, N. Recombinant glucose oxidase from Penicillium amagasakiense for efficient bioelectrochemical applications in physiological conditions. J. Biotechnol. 2011, 151, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, M.; Zhang, X.; Liang, Q.; Yang, M.; Mou, H.; Zhu, C. A thermostable glucose oxidase from Aspergillus heteromophus CBS 117.55 with broad pH stability and digestive enzyme resistance. Protein Expr. Purif. 2020, 176, 105717. [Google Scholar] [CrossRef] [PubMed]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tahara, Y.; Nakano, A.; Taniyama, T. Secretory and continuous expression of Aspergillus niger glucose oxidase gene in Pichia pastoris. Protein Expr. Purif. 2007, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Khadivi Derakshan, F.; Darvishi, F.; Dezfulian, M.; Madzak, C. Expression and characterization of glucose oxidase from Aspergillus niger in Yarrowia lipolytica. Mol. Biotechnol. 2017, 59, 307–314. [Google Scholar] [CrossRef]
- Xiong, A.S.; Yao, Q.H.; Peng, R.H.; Zhang, Z.; Xu, F.; Liu, J.G.; Han, P.L.; Chen, J.M. High level expression of a synthetic gene encoding Peniophora lycii phytase in methylotrophic yeast Pichia pastoris. Appl. Microbiol. Biotechnol. 2006, 72, 1039–1047. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Z.; Zhang, Y.; Huang, H.; Li, M.; Zhou, L.; Tang, Y.; Yao, B.; Zhang, W. High-level expression of the Penicillium notatum glucose oxidase gene in Pichia pastoris using codon optimization. Biotechnol. Lett. 2012, 34, 507–514. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Du, G.; Chen, J. Multivariate modular engineering of the protein secretory pathway for production of heterologous glucose oxidase in Pichia pastoris. Enzyme Microb. Technol. 2015, 68, 33–42. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.I.; Hong, E.; Ryu, Y. Strategies for increasing heterologous expression of a thermostable esterase from Archaeoglobus fulgidus in Escherichia coli. Protein Expr. Purif. 2016, 127, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.; Singh, M.; Kalisz, H.M. Structural and kinetic properties of nonglycosylated recombinant Penicillium amagasakiense glucose oxidase expressed in Escherichia coli. Appl. Environ. Microbiol. 1998, 64, 1405. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Panda, A.K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, G.J.; Kumar, A. Strategies for the production of recombinant protein in Escherichia coli. Protein J. 2013, 32, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; An, Y.J.; Kang, M.H.; Lee, Y.H.; Cha, S.S. Cultivation at 6-10 degrees C is an effective strategy to overcome the insolubility of recombinant proteins in Escherichia coli. Protein Expr. Purif. 2012, 82, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kyratsous, C.A.; Panagiotidis, C.A. Heat-shock protein fusion vectors for improved expression of soluble recombinant proteins in Escherichia coli. Methods Mol. Biol. 2012, 824, 109–129. [Google Scholar] [CrossRef]
- Esposito, D.; Chatterjee, D.K. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 2006, 17, 353–358. [Google Scholar] [CrossRef]
- Urbar-Ulloa, J.; Montano-Silva, P.; Ramirez-Pelayo, A.S.; Fernandez-Castillo, E.; Amaya-Delgado, L.; Rodriguez-Garay, B.; Verdin, J. Cell surface display of proteins on filamentous fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6949–6972. [Google Scholar] [CrossRef]
- Park, M. Surface display technology for biosensor applications: A review. Sensors 2020, 20, 2775. [Google Scholar] [CrossRef]
- Wang, H.; Lang, Q.; Li, L.; Liang, B.; Tang, X.; Kong, L.; Mascini, M.; Liu, A. Yeast surface displaying glucose oxidase as whole-cell biocatalyst: Construction, characterization, and its electrochemical glucose sensing application. Anal. Chem. 2013, 85, 6107–6112. [Google Scholar] [CrossRef]
- Wang, H.; Lang, Q.; Liang, B.; Liu, A. Electrochemical glucose biosensor based on glucose oxidase displayed on yeast surface. Methods Mol. Biol. 2015, 1319, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.D.; Karim, A.S.; Jewett, M.C. Cell-free gene expression: An expanded repertoire of applications. Nat. Rev. Genet. 2020, 21, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2012, 30, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buntru, M.; Vogel, S.; Stoff, K.; Spiegel, H.; Schillberg, S. A versatile coupled cell-free transcription-translation system based on tobacco BY-2 cell lysates. Biotechnol. Bioeng. 2015, 112, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Hahm, M.S.; Kang, H.A.; Nam, S.W.; Chung, B.H. Secretory expression and purification of Aspergillus niger glucose oxidase in Saccharomyces cerevisiae mutant deficient in PMR1 gene. Protein Expr. Purif. 2002, 25, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, X.; Xue, X.; Luo, H.; Yao, B.; Xie, X.; Su, X. Overexpressing key component genes of the secretion pathway for enhanced secretion of an Aspergillus niger glucose oxidase in Trichoderma reesei. Enzyme Microb. Technol. 2017, 106, 83–87. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, F.; Zhao, H.; Tang, Y.; Lu, Z. Cloning and heterologous expression of glucose oxidase gene from Aspergillus niger Z-25 in Pichia pastoris. Appl. Biochem. Biotechnol. 2010, 162, 498–509. [Google Scholar] [CrossRef]
- Hodgkins, M.; Mead, D.; Ballance, D.J.; Goodey, A.; Sudbery, P. Expression of the glucose oxidase gene from Aspergillus niger in Hansenula polymorpha and its use as a reporter gene to isolate regulatory mutations. Yeast 2010, 9, 625–635. [Google Scholar] [CrossRef]
- Geisen, R. Expression of the Aspergillus niger glucose oxidase gene in Penicillium nalgiovense. World J. Microbiol. Biotechnol. 1995, 11, 322–325. [Google Scholar] [CrossRef]
- Yuan, M.; Ning, C.; Yang, S.; Liang, Q.; Mou, H.; Liu, Z. A new cold-active glucose oxidase from Penicillium: High-level expression and application in fish preservation. Front. Microbiol. 2020, 11, 606007. [Google Scholar] [CrossRef]
- Ge, J.; Jiang, X.; Liu, W.; Wang, Y.; Huang, H.; Bai, Y.; Su, X.; Yao, B.; Luo, H. Characterization, stability improvement, and bread baking applications of a novel cold-adapted glucose oxidase from Cladosporium neopsychrotolerans SL16. Food Chem. 2020, 310, 125970. [Google Scholar] [CrossRef] [PubMed]
| Species | Genus Name | Molecular Weight (kDa) | Characterization | Source | References |
|---|---|---|---|---|---|
| Aspergillus | A. niger | 75 | Km = 23.7 ± 0.3 mM PI = 3.7 | Corn cobs | [5] |
| A. flavus | NA | NA | Soil samples | [6] | |
| A. tubingensis | 60 (three subunits) | pH 4.5 temperature = 60 °C specific activity 3435 U/mg | Contaminated cereal sample | [7] | |
| A.terreus | NA | NA | Soil sample | [8] | |
| Penicillium | P chrysogenum | 76 | PI = 5.4 | NA | [9] |
| P. funiculosum | 70 | pH 6.0–8.6 PI = 4.40–4.55 specific activity 3730 U/mg | NA | [10] | |
| P. pinophilum | 77.7 | Km = 6.2 mM specific activity 113.5 U/mg | Soil sample | [11] | |
| P. adametzii | NA | specific activity 75.8 U/mg | NA | [12] | |
| P. expansum | 72 | temperature = 60 °C specific activity 178 U/mg | NA | [13] | |
| Insect | Honeybee | 70 | NA | Apiary of institute | [14] |
| Spodoptera exigua | 70 | NA | Insect rearing facility | [15] | |
| Helicoverpa armigera larvae | 67 | NA | Cotton fields | [16] | |
| Locust cuticle | NA | pH 6.5 Km = 28 mM | Locusts | [17] | |
| Other | Pleurotus ostreatus | 70 (four subunits) | pH 5.5–6.0 temperature = 50 °C Km = 1.34 mM | NA | [18] |
| Goffeauzyma gastrica | NA | NA | Soil samples | [19] | |
| Talaromyces flavus | 71 | pH 5.6 temperature = 37 °C Km = 1.6 mM | NA | [20] | |
| Aureobasidium pullulans | NA | specific activity 1766.1 U/mg | The leaf of the mangrove plant | [21] | |
| Botrytis cinerea | 35 (four subunits) | pH 7.5 PI = 4.2 | Vine (Vitis vinifera) | [22] | |
| Aureobasidium sp | 65.1 | PI = 4.9 | A mangrove ecosystem | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chen, X.; Wang, Y.; Li, X.; Wan, M.; Zhang, G.; Leng, F.; Zhang, H. Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase. Int. J. Mol. Sci. 2022, 23, 9841. https://doi.org/10.3390/ijms23179841
Wang F, Chen X, Wang Y, Li X, Wan M, Zhang G, Leng F, Zhang H. Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase. International Journal of Molecular Sciences. 2022; 23(17):9841. https://doi.org/10.3390/ijms23179841
Chicago/Turabian StyleWang, Fan, Xiaona Chen, Yonggang Wang, Xing Li, Minglai Wan, Ge Zhang, Feifan Leng, and Haibo Zhang. 2022. "Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase" International Journal of Molecular Sciences 23, no. 17: 9841. https://doi.org/10.3390/ijms23179841
APA StyleWang, F., Chen, X., Wang, Y., Li, X., Wan, M., Zhang, G., Leng, F., & Zhang, H. (2022). Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase. International Journal of Molecular Sciences, 23(17), 9841. https://doi.org/10.3390/ijms23179841


_Kim.png)




