An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective
Abstract
1. Introduction
2. Efficacy of ALA-PDT Therapy
3. Efficacy of Non-ALA-PDT Therapy
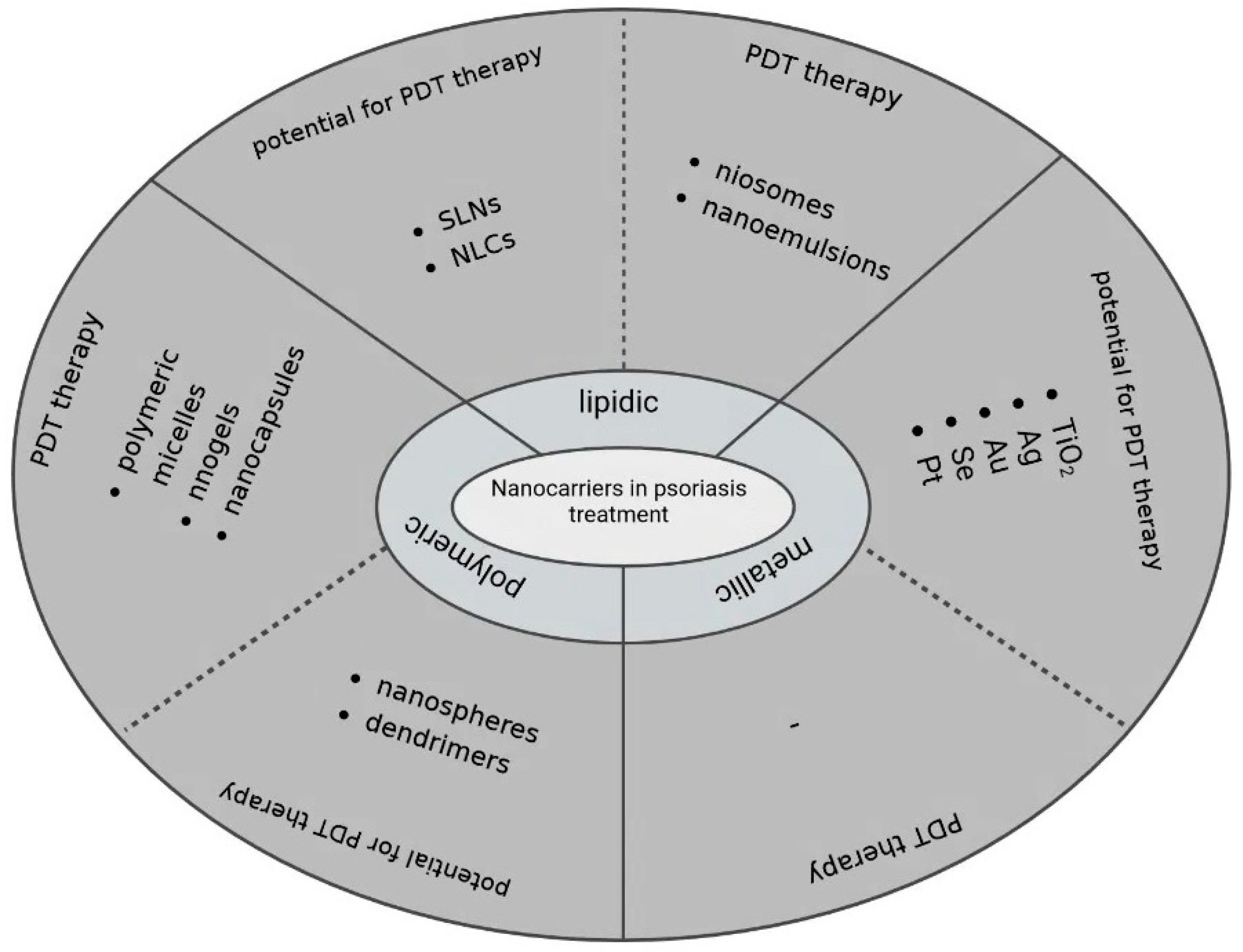
4. Nanotechnology Combined with PDT in Psoriasis Treatment—Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J. Safe and effective use of phototherapy and photochemotherapy in the treatment of psoriasis. Br. J. Nurs. 2020, 29, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Fitch, E.; Harper, E.; Skorcheva, I.; Kurtz, S.E.; Blauvelt, A. Pathophysiology of psoriasis: Recent advances on IL-23 and TH17 cytokines. Curr. Rheumatol. Rep. 2007, 9, 461–467. [Google Scholar] [CrossRef]
- Wang, A.; Bai, Y.P. Dendritic cells: The driver of psoriasis. J. Dermatol. 2020, 47, 104–113. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.M.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J. Am. Acad. Dermatol. 2010, 62, 114–135. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Mehta, D.; Lim, H.W. Ultraviolet B Phototherapy for Psoriasis: Review of Practical Guidelines. Am. J. Clin. Dermatol. 2016, 17, 125–133. [Google Scholar] [CrossRef]
- Stern, R.S. Psoralen and Ultraviolet A Light Therapy for Psoriasis. N. Engl. J. Med. 2007, 357, 682–690. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment-an update review. J. Cancer Metastasis Treat. 2019, 2019, 25. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef]
- Agostinis, P.; Buytaert, E.; Breyssens, H.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. Photodynamic therapy for psoriasis. J. Dermatolog. Treat. 2015, 26, 202–207. [Google Scholar] [CrossRef]
- Almutawa, F.; Thalib, L.; Hekman, D.; Sun, Q.; Hamzavi, I.; Lim, H.W. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: A systematic review and meta-analysis. Photodermatol. Photoimmunol. Photomed. 2015, 31, 5–14. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Rossi, M.T.; Aronson, E.; Sala, R.; Arpaia, N.; Burtica, E.C.; Amerio, P.; Virgili, A.; Rossi, R.; Buggiani, G.; et al. A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 1: Inflammatory and aesthetic indications. Photochem. Photobiol. Sci. 2013, 12, 148–157. [Google Scholar] [CrossRef]
- Byun, J.Y.; Lee, G.Y.; Choi, H.Y.; Myung, K.B.; Choi, Y.W. The expressions of TGF-β1and IL-10 in cultured fibroblasts after ALA-IPL photodynamic treatment. Ann. Dermatol. 2011, 23, 19–22. [Google Scholar] [CrossRef]
- Tandon, Y.K.; Yang, M.F.; Baron, E.D. Role of photodynamic therapy in psoriasis: A brief review. Photodermatol. Photoimmunol. Photomed. 2008, 24, 222–230. [Google Scholar] [CrossRef]
- Lee, Y.; Baron, E.D. Photodynamic therapy: Current evidence and applications in dermatology. Semin. Cutan. Med. Surg. 2011, 30, 199–209. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic acid (ala) as a prodrug in photodynamic therapy of cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Malik, Z. Fundamentals of 5-aminolevulinic acid photodynamic therapy and diagnosis: An overview. Transl. Biophotonics 2020, 2, e201900022. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Sterry, W.; Kaufmann, R. Treatment of psoriasis by topical photodynamic therapy with polychromatic light. Lancet 1994, 343, 801. [Google Scholar] [CrossRef]
- Collins, P.; Robinson, D.J.; Stringer, M.R.; Stables, G.I.; Sheehan-Dare, R.A. The variable response of plaque psoriasis after a single treatment with topical 5-aminolaevulinic acid photodynamic therapy. Br. J. Dermatol. 1997, 137, 743–749. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, Q. Photodynamic therapy with 5-aminolevulinic acid suppresses IFN-γ-induced K17 expression in HaCaT cells via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4694–4702. [Google Scholar]
- Yi, F.; Zheng, X.; Fang, F.; Zhang, J.; Zhou, B.; Chen, X. ALA-PDT alleviates the psoriasis by inhibiting JAK signalling pathway. Exp. Dermatol. 2019, 28, 1227–1236. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, L.W.; Fu, L.X.; Wu, Y.B.; Liu, X.Y.; Guo, Z.P. Systemic ALA-PDT effectively blocks the development of psoriasis-like lesions and alleviates leucocyte infiltration in the K14-VEGF transgenic mouse. Clin. Exp. Dermatol. 2017, 42, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, B.R.; Yi, F.; Wu, H.J.; Zhang, J.A.; Luo, D. Pyogenic granuloma in a patient with psoriasis successfully treated by 5-aminolevulinic acid photodynamic therapy: A case report. Exp. Ther. Med. 2016, 11, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Le Pillouer-Prost, A.; Cartier, H. Photodynamic photorejuvenation: A review. Dermatol. Surg. 2016, 42, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and late onset side effects of photodynamic therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef]
- Warren, C.B.; Karai, L.J.; Vidimos, A.; Maytin, E.V. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J. Am. Acad. Dermatol. 2009, 61, 1033–1043. [Google Scholar] [CrossRef]
- Maytin, E.V.; Honari, G.; Khachemoune, A.; Taylor, C.R.; Ortel, B.; Pogue, B.W.; Sznycer-Taub, N.; Hasan, T. The vitamin d analog calcipotriol combined with aminolevulinate-mediated photodynamic therapy for human psoriasis: A proof-of-principle study. Isr. J. Chem. 2012, 52, 767–775. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.M.; Ma, H.; Gu, W.T.; Chen, Z.Q. Photodynamic Therapy Combined with Dermabrasion in Cutaneous Squamous Cell Carcinoma Concomitant with Psoriasis. Photobiomodulation Photomed. Laser Surg. 2019, 37, 191–193. [Google Scholar] [CrossRef]
- Dogra, A.; Arora, A.K. Nail psoriasis: The journey so far. Indian J. Dermatol. 2014, 59, 319–333. [Google Scholar] [CrossRef]
- Tehranchinia, Z.; Barzkar, N.; Mohammad Riahi, S.; Khazan, M. A comparison of the effects of clobetasol 0.05% and photodynamic therapy using aminolevulinic acid with red light in the treatment of severe nail psoriasis. J. Lasers Med. Sci. 2020, 11, 3–7. [Google Scholar] [CrossRef]
- De Annunzio, S.R.; Costa, N.C.S.; Graminha, M.A.S.; Fontana, C.R.; Mezzina, R.D. Chlorin, phthalocyanine, and porphyrin types derivatives in phototreatment of cutaneous manifestations: A review. Int. J. Mol. Sci. 2019, 20, 3861. [Google Scholar] [CrossRef]
- Merchat, M.; Spikes, J.D.; Bertoloni, G.; Jori, G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B Biol. 1996, 35, 149–157. [Google Scholar] [CrossRef]
- Slomp, A.M.; Barreira, S.M.W.; Carrenho, L.Z.B.; Vandresen, C.C.; Zattoni, I.F.; Ló, S.M.S.; Dallagnol, J.C.C.; Ducatti, D.R.B.; Orsato, A.; Duarte, M.E.R.; et al. Photodynamic effect of meso-(aryl)porphyrins and meso-(1-methyl-4-pyridinium)porphyrins on HaCaT keratinocytes. Bioorganic Med. Chem. Lett. 2017, 27, 156–161. [Google Scholar] [CrossRef]
- Carrenho, L.Z.B.; Moreira, C.G.; Vandresen, C.C.; Gomes, R.; Gonçalves, A.G.; Barreira, S.M.W.; Noseda, M.D.; Duarte, M.E.R.; Ducatti, D.R.B.; Dietrich, M.; et al. Investigation of anti-inflammatory and anti-proliferative activities promoted by photoactivated cationic porphyrin. Photodiagnosis Photodyn. Ther. 2015, 12, 444–458. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wang, Y.M.; Li, W.F.; Li, C.; Jiang, Z.H.; Bao, J.; Wei, J.F.; Jin, H.T.; Wang, A.P. Anti-Psoriasis Effects and Mechanisms of A-(8-Quinolinoxy) Zinc Phthalocyanine-Mediated Photodynamic Therapy. Cell. Physiol. Biochem. 2017, 44, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Soler, D.C.; Ohtola, J.; Sugiyama, H.; Rodriguez, M.E.; Han, L.; Oleinick, N.L.; Lam, M.; Baron, E.D.; Cooper, K.D.; McCormick, T.S. Activated T cells exhibit increased uptake of silicon phthalocyanine Pc 4 and increased susceptibility to Pc 4-photodynamic therapy-mediated cell death. Photochem. Photobiol. Sci. 2016, 15, 822–831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, R.K.; Venkatesan, P.; Yeh, C.H.; Chien, C.M.; Lin, T.S.; Lin, C.C.; Lin, C.C.; Lai, P.S. Effective topical treatments using innovative NNO-tridentate vanadium(iv) complexes-mediated photodynamic therapy in a psoriasis-like mouse model. J. Mater. Chem. B 2022, 10, 4759–4770. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; Collins, P.; Stringer, M.R.; Vernon, D.I.; Stables, G.I.; Brown, S.B.; Sheehan-Dare, R.A. Improved response of plaque psoriasis after multiple treatments with topical 5-aminolaevulinic acid photodynamic therapy. Acta Derm. Venereol. 1999, 79, 451–455. [Google Scholar] [CrossRef]
- Bissonnette, R.; Tremblay, J.F.; Juzenas, P.; Boushira, M.; Lui, H. Systemic photodynamic therapy with aminolevulinic acid induces apoptosis in lesional T lymphocytes of psoriatic plaques. J. Investig. Dermatol. 2002, 119, 77–83. [Google Scholar] [CrossRef]
- Beattie, P.E.; Dawe, R.S.; Ferguson, J.; Ibbotson, S.H. Lack of efficacy and tolerability of topical PDT for psoriasis in comparison with narrowband UVB phototherapy. Clin. Exp. Dermatol. 2004, 29, 560–562. [Google Scholar] [CrossRef]
- Radakovic-Fijan, S.; Blecha-Thalhammer, U.; Schleyer, V.; Szeimies, R.M.; Zwingers, T.; Hönigsmann, H.; Tanew, A. Topical aminolaevulinic acid-based photodynamic therapy as a treatment option for psoriasis? Results of a randomized, observer-blinded study. Br. J. Dermatol. 2005, 152, 279–283. [Google Scholar] [CrossRef]
- Fransson, J.; Ros, A.M. Clinical and immunohistochemical evaluation of psoriatic plaques treated with topical 5-aminolaevulinic acid photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2005, 21, 326–332. [Google Scholar] [CrossRef]
- Schleyer, V.; Radakovic-Fijan, S.; Karrer, S.; Zwingers, T.; Tanew, A.; Landthaler, M.; Szeimies, R.M. Disappointing results and low tolerability of photodynamic therapy with topical 5-aminolaevulinic acid in psoriasis. A randomized, double-blind phase I/II study. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 823–828. [Google Scholar] [CrossRef]
- Smits, T.; Kleinpenning, M.M.; Van Erp, P.E.J.; Van De Kerkhof, P.C.M.; Gerritsen, M.J.P. A placebo-controlled randomized study on the clinical effectiveness, immunohistochemical changes and protoporphyrin IX accumulation in fractionated 5-aminolaevulinic acid-photodynamic therapy in patients with psoriasis. Br. J. Dermatol. 2006, 155, 429–436. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, H.Y.; Lee, E.S.; Kim, Y.C. Topical 5-aminolaevulinic acid photodynamic therapy for intractable palmoplantar psoriasis. J. Dermatol. 2007, 34, 37–40. [Google Scholar] [CrossRef]
- Salah, M.; Samy, N.; Fadel, M. Methylene blue mediated photodynamic therapy for resistant plaque psoriasis. J. Drugs Dermatol. 2009, 8, 42–49. [Google Scholar]
- Fernández-Guarino, M.; Harto, A.; Sánchez-Ronco, M.; García-Morales, I.; Jaén, P. Pulsed dye laser vs. photodynamic therapy in the treatment of refractory nail psoriasis: A comparative pilot study. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 891–895. [Google Scholar] [CrossRef]
- Rook, A.H.; Wood, G.S.; Duvic, M.; Vonderheid, E.C.; Tobia, A.; Cabana, B. A phase II placebo-controlled study of photodynamic therapy with topical hypericin and visible light irradiation in the treatment of cutaneous T-cell lymphoma and psoriasis. J. Am. Acad. Dermatol. 2010, 63, 984–990. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current advances of nanocarrier technology-based active cosmetic ingredients for beauty applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef]
- Gungor, S.; Rezigue, M. Nanocarriers Mediated Topical Drug Delivery for Psoriasis Treatment. Curr. Drug Metab. 2017, 18, 454–468. [Google Scholar] [CrossRef]
- Li, N.; Qin, Y.; Dai, D.; Wang, P.; Shi, M.; Gao, J.; Yang, J.; Xiao, W.; Song, P.; Xu, R. Transdermal Delivery of Therapeutic Compounds With Nanotechnological Approaches in Psoriasis. Front. Bioeng. Biotechnol. 2022, 9, 1446. [Google Scholar] [CrossRef]
- Battah, S.H.; Chee, C.E.; Nakanishi, H.; Gerscher, S.; MacRobert, A.J.; Edwards, C. Synthesis and biological studies of 5-aminolevulinic acid-containing dendrimers for photodynamic therapy. Bioconjug. Chem. 2001, 12, 980–988. [Google Scholar] [CrossRef]
- Casas, A.; Battah, S.; Di Venosa, G.; Dobbin, P.; Rodriguez, L.; Fukuda, H.; Batlle, A.; MacRobert, A.J. Sustained and efficient porphyrin generation in vivo using dendrimer conjugates of 5-ALA for photodynamic therapy. J. Control. Release 2009, 135, 136–143. [Google Scholar] [CrossRef]
- Zhou, T.; Battah, S.; Mazzacuva, F.; Hider, R.C.; Dobbin, P.; Macrobert, A.J. Design of Bifunctional Dendritic 5-Aminolevulinic Acid and Hydroxypyridinone Conjugates for Photodynamic Therapy. Bioconjug. Chem. 2018, 29, 3411–3428. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.S.; Almokdad, A.A.; Shaluf, S.I.M.; Debe, M.S. Polymer-Based Nanomaterials for Drug-Delivery Carriers. Nanocarriers Drug Deliv. Nanosci. Nanotechnol. Drug Deliv. 2018, 531–556. [Google Scholar] [CrossRef]
- Yin, X.; Ran, S.; Cheng, H.; Zhang, M.; Sun, W.; Wan, Y.; Shao, C.; Zhu, Z. Polydopamine-modified ZIF-8 nanoparticles as a drug carrier for combined chemo-photothermal osteosarcoma therapy. Colloids Surf. B Biointerfaces 2022, 216, 112507. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.D.; Ziembowicz, F.I.; Müller, D.F.; Lauermann, S.C.; Kloster, C.L.; Santos, R.C.V.; Lopes, L.Q.S.; Ourique, A.F.; MacHado, G.; Villetti, M.A. Evaluation of photodynamic activity, photostability and in vitro drug release of zinc phthalocyanine-loaded nanocapsules. Eur. J. Pharm. Sci. 2016, 83, 88–98. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, S.; Lu, Y.; Lai, R.; Liu, Z.; Luo, W.; Xu, Y. Chitosan/hyaluronan nanogels co-delivering methotrexate and 5-aminolevulinic acid: A combined chemo-photodynamic therapy for psoriasis. Carbohydr. Polym. 2022, 277, 118819. [Google Scholar] [CrossRef]
- Freag, M.S.; Torky, A.S.; Nasra, M.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Liquid crystalline nanoreservoir releasing a highly skin-penetrating berberine oleate complex for psoriasis management. Nanomedicine 2019, 14, 931–954. [Google Scholar] [CrossRef]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat. Drug Deliv. Formul. 2019, 12, 252–266. [Google Scholar] [CrossRef]
- Nagaich, U. Polymeric nanocapsules: An emerging drug delivery system. J. Adv. Pharm. Technol. Res. 2018, 9, 73–79. [Google Scholar] [CrossRef]
- Shi, L.; Hu, F.; Duan, Y.; Wu, W.; Dong, J.; Meng, X.; Zhu, X.; Liu, B. Hybrid Nanospheres to Overcome Hypoxia and Intrinsic Oxidative Resistance for Enhanced Photodynamic Therapy. ACS Nano 2020, 14, 2183–2190. [Google Scholar] [CrossRef]
- Liu, C.; Wang, D.; Zhang, S.; Cheng, Y.; Yang, F.; Xing, Y.; Xu, T.; Dong, H.; Zhang, X. Biodegradable Biomimic Copper/Manganese Silicate Nanospheres for Chemodynamic/Photodynamic Synergistic Therapy with Simultaneous Glutathione Depletion and Hypoxia Relief. ACS Nano 2019, 13, 4267–4277. [Google Scholar] [CrossRef]
- Amantino, C.F.; de Baptista-Neto, Á.; Badino, A.C.; Siqueira-Moura, M.P.; Tedesco, A.C.; Primo, F.L. Anthraquinone encapsulation into polymeric nanocapsules as a new drug from biotechnological origin designed for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 31, 1018152. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Oliveira, H.; Moreiras, A.; Fernandes, J.C.; Bronze-da-Rocha, E.; Figueiredo, A.; Custódio, J.B.A.; Rocha-Pereira, P.; Santos-Silva, A. Cytotoxic and genotoxic effects of acitretin, alone or in combination with psoralen-ultraviolet A or narrow-band ultraviolet B-therapy in psoriatic patients. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 753, 42–47. [Google Scholar] [CrossRef]
- Parnami, N.; Garg, T.; Rath, G.; Goyal, A.K. Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2014, 42, 406–412. [Google Scholar] [CrossRef]
- Hashim, I.I.A.; El-Magd, N.F.A.; El-Sheakh, A.R.; Hamed, M.F.; El-Gawad, A.E.G.H.A. Pivotal role of acitretin nanovesicular gel for effective treatment of psoriasis: Ex vivo–in vivo evaluation study. Int. J. Nanomed. 2018, 13, 1059–1079. [Google Scholar] [CrossRef]
- Viegas, J.S.R.; Praça, F.G.; Caron, A.L.; Suzuki, I.; Silvestrini, A.V.P.; Medina, W.S.G.; Del Ciampo, J.O.; Kravicz, M.; Bentley, M.V.L.B. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv. Transl. Res. 2020, 10, 646–660. [Google Scholar] [CrossRef]
- Depieri, L.V.; Borgheti-Cardoso, L.N.; Campos, P.M.; Otaguiri, K.K.; De Carvalho Vicentini, F.T.M.; Lopes, L.B.; Fonseca, M.J.V.; Bentley, M.V.L.B. RNAi mediated IL-6 in vitro knockdown in psoriasis skin model with topical siRNA delivery system based on liquid crystalline phase. Eur. J. Pharm. Biopharm. 2016, 105, 50–58. [Google Scholar] [CrossRef]
- Suzuki, I.L.; de Araujo, M.M.; Bagnato, V.S.; Bentley, M.V.L.B. TNFα siRNA delivery by nanoparticles and photochemical internalization for psoriasis topical therapy. J. Control. Release 2021, 338, 316–329. [Google Scholar] [CrossRef]
- Wang, H.; Su, D.; Huang, R.; Shu, F.; Cheng, F.; Zheng, G. Cellular nanovesicles with bioorthogonal targeting enhance photodynamic/photothermal therapy in psoriasis. Acta Biomater. 2021, 134, 674–685. [Google Scholar] [CrossRef]
- Goto, P.L.; Siqueira-Moura, M.P.; Tedesco, A.C. Application of aluminum chloride phthalocyanine-loaded solid lipid nanoparticles for photodynamic inactivation of melanoma cells. Int. J. Pharm. 2017, 518, 228–241. [Google Scholar] [CrossRef]
- Anand, K.; Ray, S.; Rahman, M.; Shaharyar, A.; Bhowmik, R.; Bera, R.; Karmakar, S. Nano-emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 16–35. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-delivery of imiquimod and curcumin by nanoemugel for improved topical delivery and reduced psoriasis-like skin lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The comparison of in vitro photosensitizing efficacy of curcumin-loaded liposomes following photodynamic therapy on melanoma mug-mel2, squamous cell carcinoma scc-25, and normal keratinocyte hacat cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xie, Y.; Wu, J.; Wang, J.; Petković, M.; Stepić, M.; Zhao, J.; Ma, J.; Mi, L. Functional titanium dioxide nanoparticle conjugated with phthalocyanine and folic acid as a promising photosensitizer for targeted photodynamic therapy in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2021, 215, 112122. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Boosting the anti-inflammatory effect of self-assembled hybrid lecithin–chitosan nanoparticles via hybridization with gold nanoparticles for the treatment of psoriasis: Elemental mapping and in vivo modeling. Drug Deliv. 2022, 29, 1726–1742. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ho, L.W.C.; Bai, Q.; Chan, C.K.W.; Lee, L.K.C.; Choi, P.C.L.; Choi, C.H.J. Alkyl-Terminated Gold Nanoparticles as a Self-Therapeutic Treatment for Psoriasis. Nano Lett. 2021, 21, 8723–8733. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, G.R.; Lin, Z.C.; Tsai, M.J.; Yang, S.C.; Alalaiwe, A.; Fang, J.Y. Photothermal treatment by PLGA–gold nanorod–isatin nanocomplexes under near-infrared irradiation for alleviating psoriasiform hyperproliferation. J. Control. Release 2021, 333, 487–499. [Google Scholar] [CrossRef]
- Crisan, D.; Scharffetter-Kochanek, K.; Crisan, M.; Schatz, S.; Hainzl, A.; Olenic, L.; Filip, A.; Schneider, L.A.; Sindrilaru, A. Topical silver and gold nanoparticles complexed with Cornus mas suppress inflammation in human psoriasis plaques by inhibiting NF-κB activity. Exp. Dermatol. 2018, 27, 1166–1169. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef]

| Study | Photosensitizer | Way of Delivery | Wavelength | Treatment Parameters | Pre-Treatment with Drug | Number of Patients | Total Number of Treatment Sessions | Results | Side Effects | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment of psoriasis by topical photodynamic therapy with polychromatic light [24]. | 5-aminolevulinic acid (ALA, 10%) | Topical | 600–700 nm | Light dose: 25 J/cm2 Dose rate: 70 mW/cm2 | 5 h | 3 | Max: - 3 times per week | Dithranol and topical PDT were comparable. | Burning sensations during irradiation | Boehncke et al. (1994) |
| The variable response of plaque psoriasis after a single treatment with topical 5-aminolaevulinic acid photodynamic therapy [25]. | ALA (20%) | Topical | 400–650 nm | Light dose: 2–16 J/cm2, Dose rate: 10–40 mW/cm2 | 4 h | 22 | Max: 12 Once a week | Thirty-five percent of patients’ psoriasis was cleared. 80 treatment sites: 14 cleared, 6 showed a 30–50% reduction in SEI score, 60 showed little or no improvement. | Stinging, tingling, burning sensations during and after illumination | Collins et al. (1997) |
| Improved response of plaque psoriasis after multiple treatments with topical 5-aminolaevulinic acid photodynamic therapy [44]. | ALA (20%) | Topical | broad-band visible radiation | Light dose: 8 J/cm2 Dose rate: 15 mW/cm2 | 4 h | 10 | Max: 12 3 times per week | Eighty percent of patients responded to ALA-PDT. 19 treatment sites: 4 cleared, 10 responded but did not clear, 5 did not change. | Pain and discomfort (80% patients during treatment and 50% during and between treatments, respectively) | Robinson et al. (1999) |
| Systemic photodynamic therapy with aminolaevulinic acid induces apoptosis in lesional T lymphocytes of psoriatic plaques [45]. | ALA (5,10 or 15 mg/kg) | Oral | Blue light, maximum at 417 nm | Light dose: for 5 or 10 mg/kg ALA: 1, 3, 6, 12, or 20 J/cm2 for 15 mg/kg ALA: 1, 2, 4, 8 or 10 J/cm2 Dose rate: 9–11 mW/cm2 | 1, 3 or 6 h | 12 | Max: 1 1 time per week | A 5 or 10 mg/kg ALA dose did not show improvement. 15 mg/kg ALA dose showed improvement. | Mild burning during light exposure | Bissonnette et al. (2002) |
| Lack of efficacy and tolerability of topical PDT for psoriasis in comparison with narrowband UVB phototherapy [46]. | ALA (20%) | Topical | 630 nm | Light dose: 10 J/cm2 Dose rate: 120 mW/cm2 | 4 h | 4 | Max: 12 1–3 times per week | SEI score reduced by 5% in 2 patients, 17% in 1 patient and was unchanged in one patient. These results were lower than the NB-UVB values. | Pain during treatment | Beattie et al. (2004) |
| Topical aminolaevulinic acid-based photodynamic therapy as a treatment option for psoriasis? Results of a randomized, observer-blinded study [47]. | ALA (1%) | Topical | 600–740 nm | Light dose: 5, 10, 20 J/cm2 Dose rate: 60 mW/cm2 | 4–6 h | 29 | Max: 12 2 times per week | Eight patients were excluded. Sixty-three treatment sites: 8 cleared, 53 from substantial to minimal improvement, 2 with no improvement. | Pain, stinging, burning during irradiation, lasting up to several hours | Radakovic-Fijan et al. (2005) |
| Clinical and immunohistochemical evaluation of psoriatic plaques treated with topical 5-aminolaevulinic acid photodynamic therapy [48]. | Δ -ALA hydrochloride (20%) | Topical | 630 nm | Light dose: 10–30 J/cm2 Dose rate: 20–315 mW/cm2 | 4–5 h | 12 | Max: 5 Once a week | Psoriatic plaques improved. The SEI score decreased. | Pain and discomfort during treatment | Fransson et al. (2005) |
| Disappointing results and low tolerability of photodynamic therapy with topical 5-aminolaevulinic acid in psoriasis. A randomized, double-blind phase I/II study [49]. | ALA (0.1%, 1%, or 5%) | Topical | 600–740 nm | Light dose: 20 J/cm2 Dose rate: 60 mW/cm2 | 4–6 h | 12 | Max: 12 2 times per week | Three patients were excluded. In the 0.1%, 1%, and 5% ALA-treated groups, the mean percentage improvement was 37.5%, 45.6%, and 51.2%, accordingly. | Pain and burning during and after irradiation | Schleyer et al. (2006) |
| A placebo-controlled randomized study on the clinical effectiveness, immunohistochemical changes and protoporphyrin IX accumulation in fractionated 5-aminolaevulinic acid-photodynamic therapy in patients with psoriasis [50]. | ALA (10%) | Topical | 600–750 nm | Light dose: 2 and 8 J/cm2 Dose rate: 40 mW/cm2 | 4 h + 2 h of dark interval | 8 | Max: 4 Once a week | Psoriatic lesions and plaques cleared, and plaque severity score decreased. | Burning and stinging during irradiation | Smits et al. (2006) |
| Topical 5-aminolaevulinic acid photodynamic therapy for intractable palmoplantar psoriasis [51]. | ALA (20%) | Topical | 630 nm | Light dose: 15 J/cm2 Dose rate: 30 mW/cm2 | 4 h | 3 | Max: 10 Once a week | The patients showed partial improvement in psoriatic lesions and plaques. | - | Kim et al. (2007) |
| Methylene blue mediated photodynamic therapy for resistant plaque psoriasis [52]. | MB (0.1%) | Topical | 670 nm | Light dose: 5 J/cm2 Dose rate: 565 mW/cm2 | - | 16 | - | Sixteen patients showed improvement. Sixty-eight percent of the patients achieved a seventy-five percent reduction in severity score. | - | Salah et al. (2009) |
| Pulsed dye laser vs. photodynamic therapy in the treatment of refractory nail psoriasis: a comparative pilot study [52]. | Methyl-aminolaevulinic acid (MAL) | Topical | 595 nm | Light dose: 9 J/cm2 Dose rate: - | 3 h | 14 | Max: 6 Monthly | Fourteen patients showed lower NAPSI scores. Both nail matrix and bed nail bed involvement cleared. | Slight pain during treatment | Fernandez-Guarino et al. (2009) |
| The effects of keratolytic pretreatment prior to fluorescence diagnosis and photodynamic therapy with aminolevulinic acid-induced porphyrins in psoriasis [53]. | ALA (10%) | Topical | 600–750 nm | Light dose: 10 J/cm2 Dose rate: 40 mW/cm2 | 6 h | 10 | Max: 6 Once daily | It was observed that psoriasis decreased, as well as clinical severity score. | Stinging, burning during irradiation | Kleinpenning et al. (2010) |
| A phase II placebo-controlled study of photodynamic therapy with topical hypericin and visible light irradiation in the treatment of cutaneous T-cell lymphoma and psoriasis [54]. | Hypericin (0.05%, 0.1%, 0.25%) | Topical | 590–650 nm | Light dose: 8 to 20 J/cm2 Dose rate: - | 24 h | 11 | Max: 6 2 times per week | There was an improvement in skin lesions. | Mild burning and itching during treatment | Rook et al. (2010) |
| The Vitamin D Analog Calcipotriol Combined with Aminolevulinate-Mediated Photodynamic Therapy for Human Psoriasis: A Proof-of-Principle Study [33]. | ALA (20%) | Topical | 417 nm (blue light), 635 nm (red light) | Light dose: 10, 20 or 40 J/cm2 Dose rate: 100 mW/cm2 | 2 h | 7 | Max: 7 Twice daily | No clinical improvement in psoriasis was observed. | Stinging and pain during illumination | Maytin et al. (2012) |
| A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 1: Inflammatory and aesthetic indications [17]. | MAL (160 mg/g) | Topical | 635 nm | Light dose: 37 J/cm2 Dose rate: - | 3–4 h | 17 | Max: 3.6 9.9 ± 5.6 days between treatments | Two patients experienced worsening psoriatic lesions. Three patients showed poor or no clinical improvement. Twelve patients showed a moderate or marked clinical response. | Pain or burning sensations during the treatment | Calzavara-Pinton et al. (2013) |
| Pyogenic granuloma in a patient with psoriasis successfully treated by 5-aminolevulinic acid photodynamic therapy: A case report [22] | ALA (20%) | Topical | 633 ± 10 nm | Dose rate: 90 mW/cm2 | 3 h | 1 | 1 session of ALA-PDT treatment followed-up weekly for 1 month | One week following the ALA-PDT treatment, the erosions had dried up, and the PG lesion was encrusted. No signs of recurrence were demonstrated 1 month after treatment | - | Liu et al., (2016) |
| Systemic ALA-PDT effectively blocks the development of psoriasis-like lesions and alleviates leucocyte infiltration in the K14-VEGF transgenic mouse [28]. | ALA (65 mg/kg) | Injection | 633 nm | Light dose: 108 J/cm2 Dose rate: 90 mW/cm2 | 2 h | 6 (mice) | Max: 2 Weekly | ALA-PDT blocked the development of psoriasis-like lesions. The scores lowered. | - | Chen et al. (2017) |
| Anti-Psoriasis Effects and Mechanisms of A-(8-Quinolinoxy) Zinc Phthalocyanine-Mediated Photodynamic Therapy [41]. 1) ZnPc-F7-PDT effects on propranolol-induced psoriatic lesions in cavy 2) ZnPc-F7-PDT effects on IMQ-induced psoriatic lesions in Nu/Nu mice | 1) ZnPc-F7 (1% or 5%) | Topical | 630, 670 nm | Light dose: 14.15 J/cm2 Dose rate: 300–1500 mW/cm2 | 24 h | 70 (cavies), n = 20 | Max: 1 | After 2 weeks of recovery, the ears exhibited no discernible abnormalities compared to normal animals. The histopathological traits remained, except for inflammatory cell infiltration | - | Liu et al. (2017) |
| 2) ZnPc-F7 (0.30, 0.60, 1.20 mg/kg) | Injection | Light dose: 19.10 J/cm2 Dose rate: - | 6 h | 70 (mice), n = 20 | Max: 1 | ZnPc-F7-PDT reduced the psoriatic symptoms caused by IMQ. | ||||
| ALA-PDT alleviates the psoriasis by inhibiting JAK signaling Pathway [27]. | ALA (20%) | Topical | 635 nm | Light dose: 12 J/cm2 Dose rate: 30 mW/cm2 | 4 h | Group (mice) | - | In IMQ-induced mice ALA-PDT reduced scaling, redness, erythema, scales, thickness and cumulative scores | - | Yi et al. (2019) |
| Photodynamic Therapy Combined with Dermabrasion in Cutaneous Squamous Cell Carcinoma Concomitant with Psoriasis [27] | Dermabrasion conjugated with ALA (20%) | Topical | 635 nm He–Ne laser | Light dose: 100 J/cm2 Dose rate: 0.083 W/cm2 for 1200 s | 5 h | 1 | four applications of PDT | The ulcer and plaque completely disappeared, and there were no obvious scars after treatment | - | Xu et al. (2019) |
| A Comparison of The Effects of Clobetasol 0.05% and Photodynamic Therapy Using Aminolevulinic Acid With Red Light in the Treatment of Severe Nail Psoriasis [36] | ALA (20%) and clobetasol propionate 0.05% ointment | Topical | 630 nm (range 600–730 nm); red light-PDT | Light dose: 120 J/cm2 Dose rate: 200 mW/cm2 | 3 h | 8 | Every 3 weeks for 5 cycles | Six months after the last treatment session, the mean NAPSI scores in the nails treated with ALA-PDT were greater than those in the nails treated with clobetasol 0.05% ointment. | Slight pain during irradiation | Tehranchinia et al. (2020) |
| Effective topical treatments using innovative NNO-tridentate vanadium (IV) complexes-mediated photodynamic therapy in a psoriasis-like mouse model [43] | 0.001% vanadium complex—PDT | Topical | Blue light 430 nm | Light dose: 0.1 J/cm2 Dose rate: - | 4 h | imiquimod (IMQ)-induced psoriasis mouse model | 30 min irradiation for 8 consecutive days | A higher phototoxicity index with non-toxicity under dark conditions, efficient skin morphological recovery according to the PASI score decrease in the percentage of IL-17A and IL-22 in the spleen. | - | Lin et al., 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makuch, S.; Dróżdż, M.; Makarec, A.; Ziółkowski, P.; Woźniak, M. An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective. Int. J. Mol. Sci. 2022, 23, 9845. https://doi.org/10.3390/ijms23179845
Makuch S, Dróżdż M, Makarec A, Ziółkowski P, Woźniak M. An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective. International Journal of Molecular Sciences. 2022; 23(17):9845. https://doi.org/10.3390/ijms23179845
Chicago/Turabian StyleMakuch, Sebastian, Mateusz Dróżdż, Alicja Makarec, Piotr Ziółkowski, and Marta Woźniak. 2022. "An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective" International Journal of Molecular Sciences 23, no. 17: 9845. https://doi.org/10.3390/ijms23179845
APA StyleMakuch, S., Dróżdż, M., Makarec, A., Ziółkowski, P., & Woźniak, M. (2022). An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective. International Journal of Molecular Sciences, 23(17), 9845. https://doi.org/10.3390/ijms23179845






