N-Heterocyclic Carbenes and Their Metal Complexes Based on Histidine and Histamine Derivatives of Bacteriopurpurinimide for the Combined Chemo- and Photodynamic Therapy of Cancer
Abstract
:1. Introduction
2. Results and Discussion
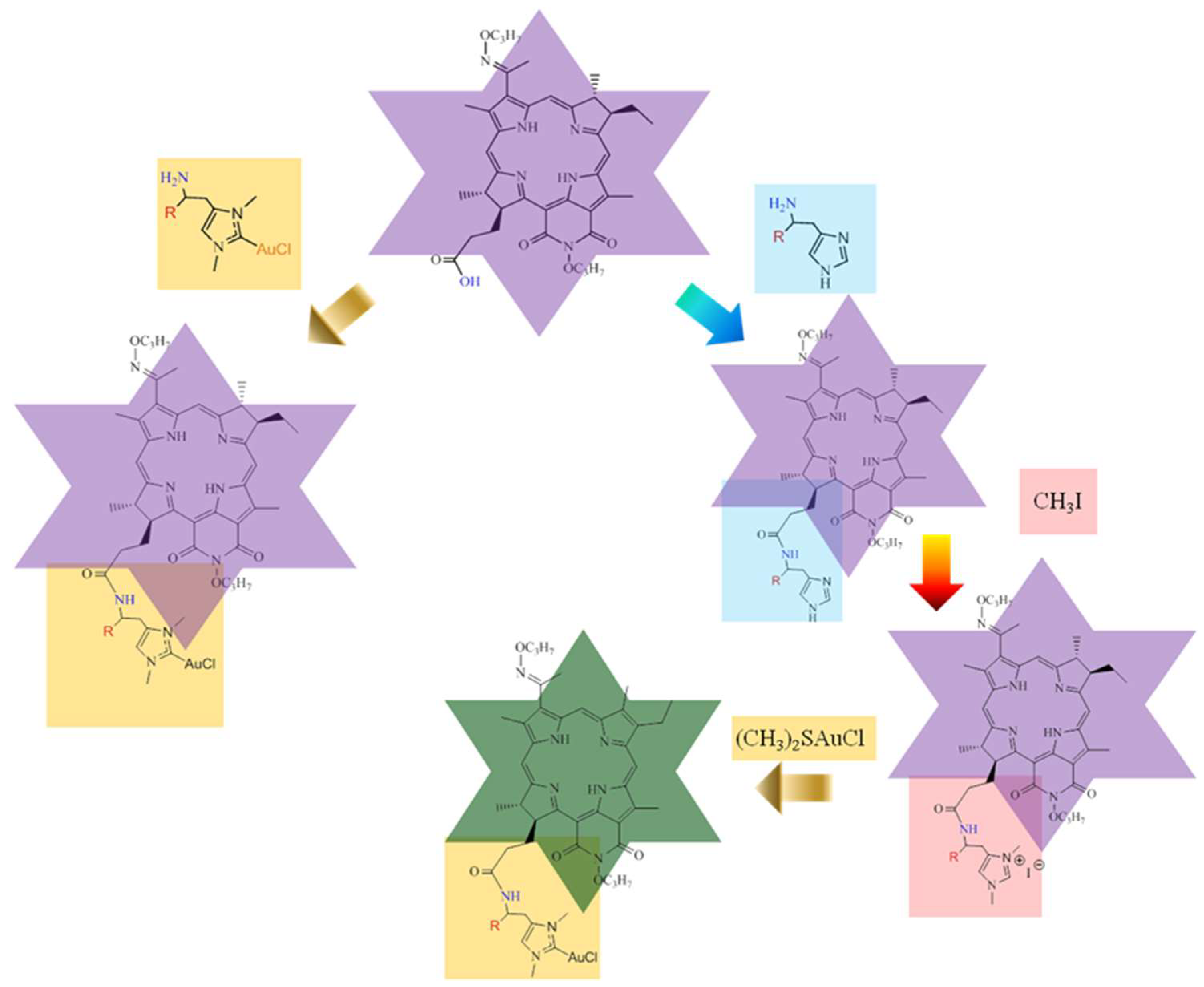
2.1. Chemistry
2.2. Biology
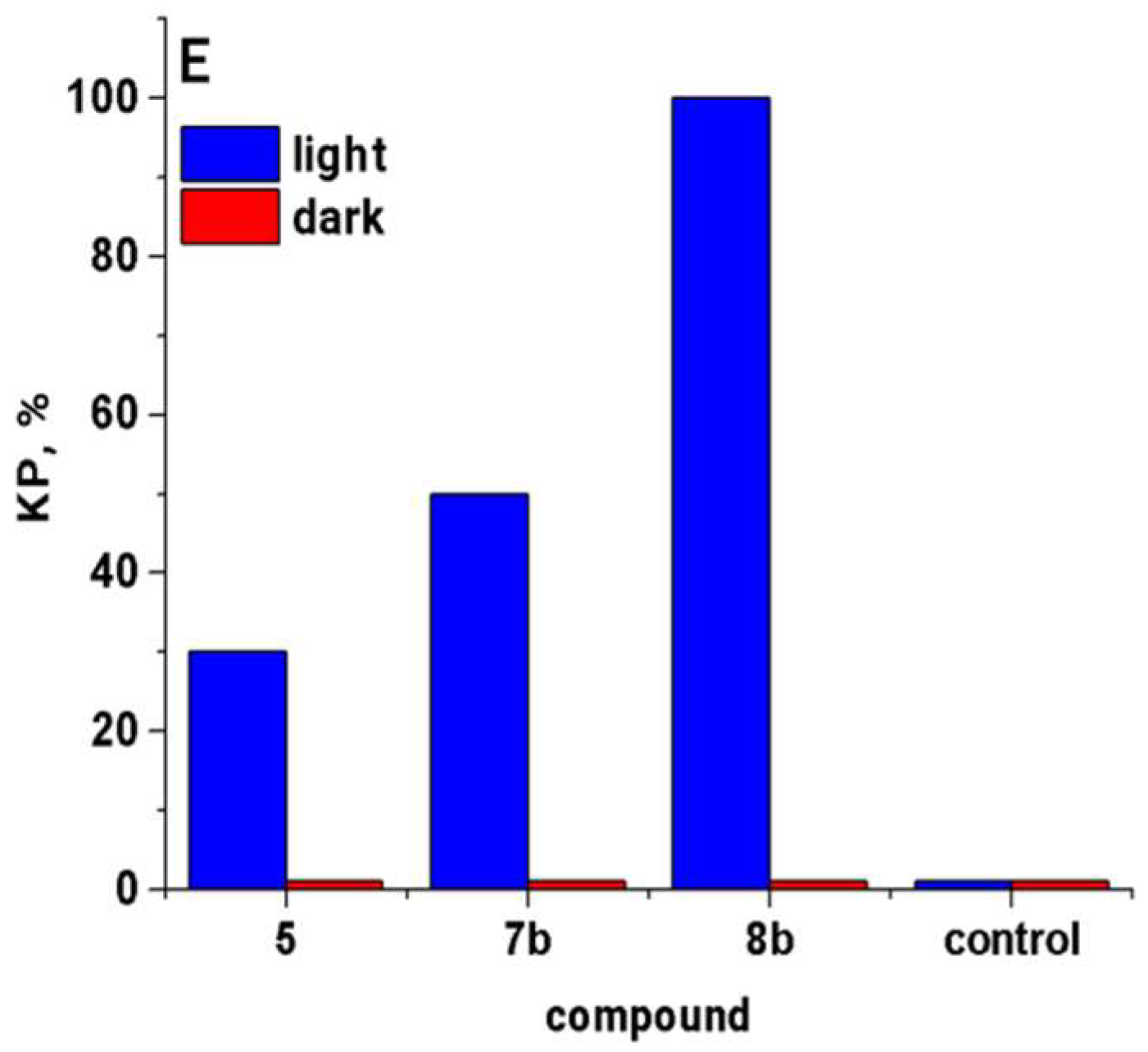
2.2.1. In Vitro
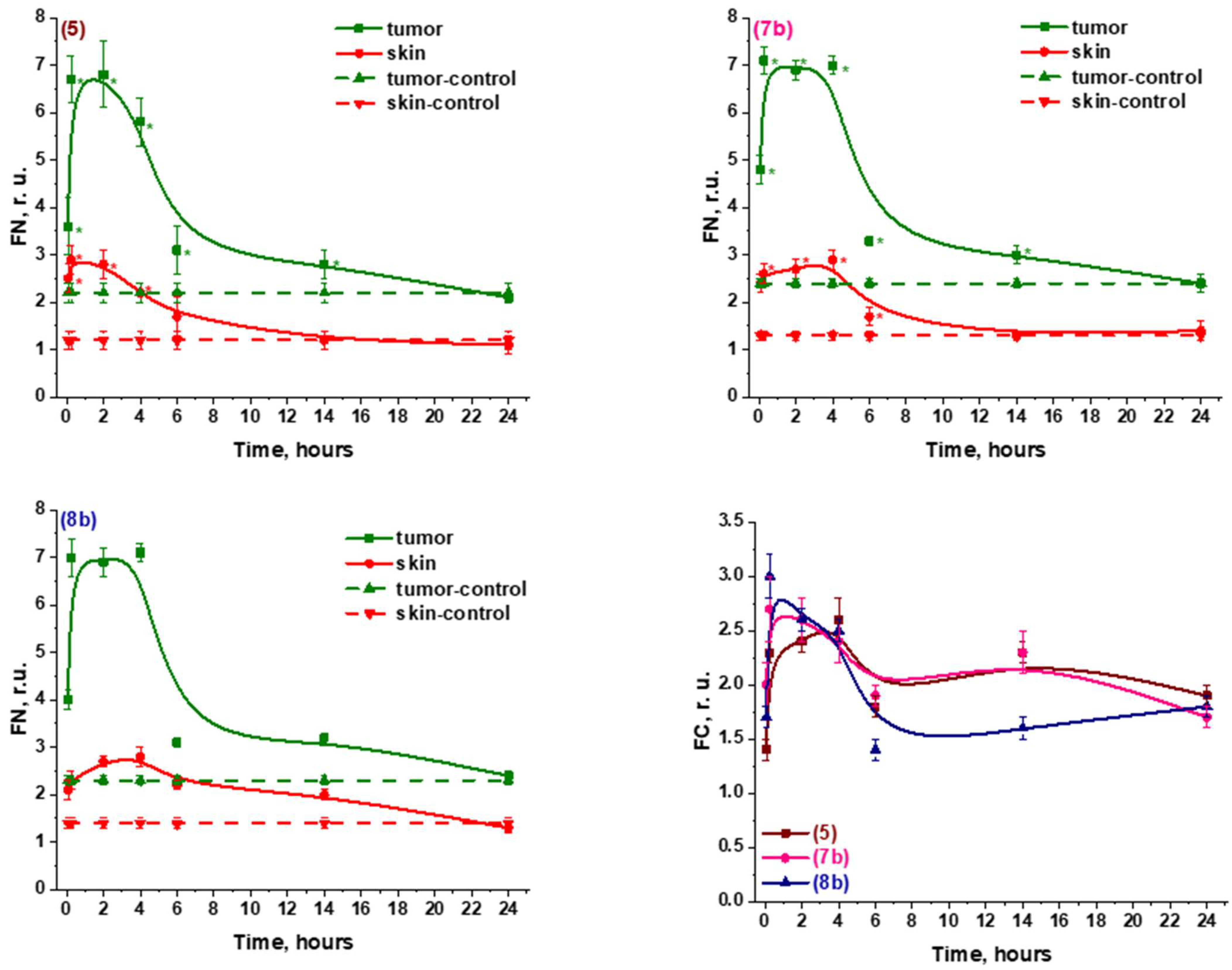
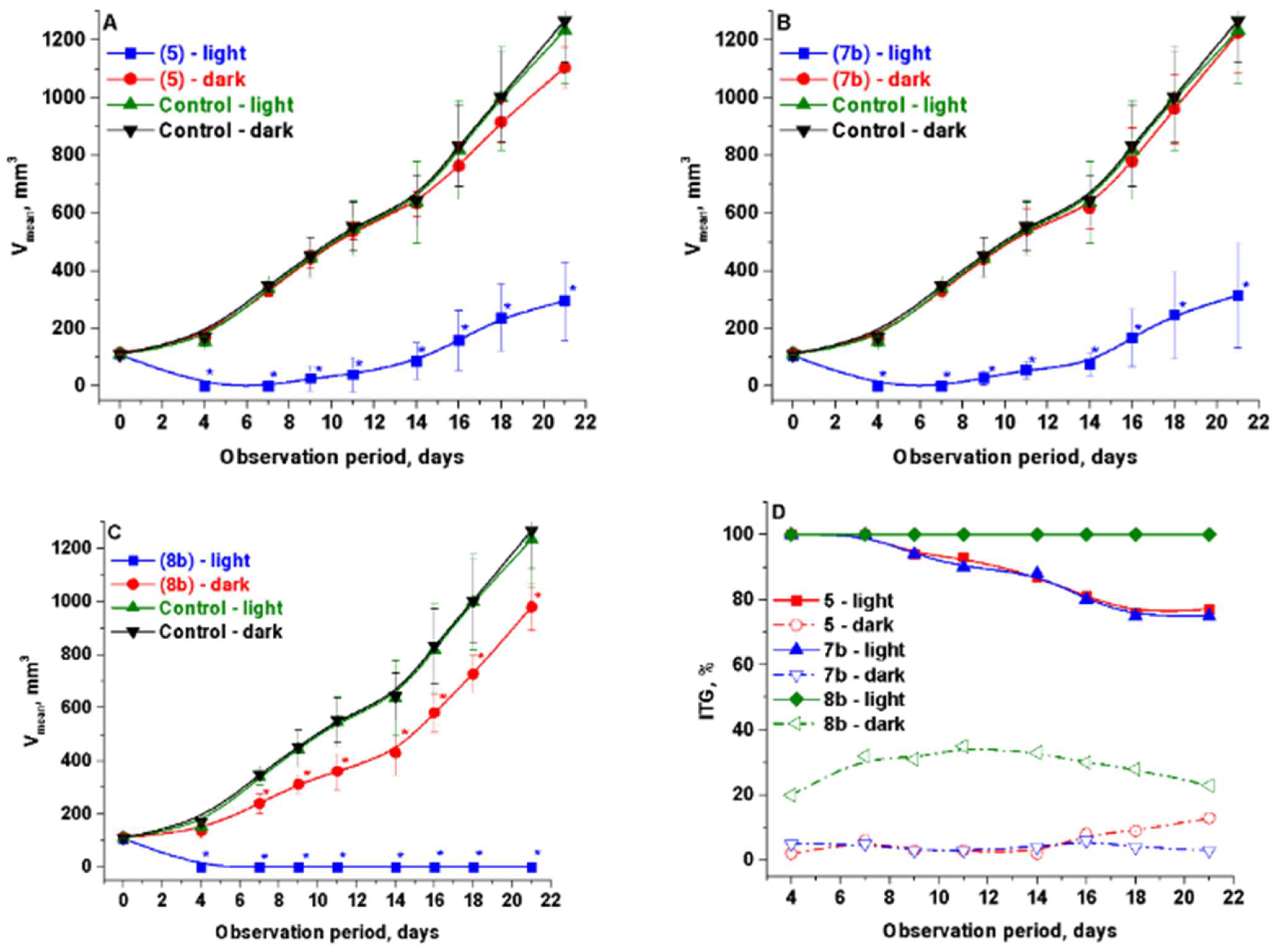
2.2.2. In Vivo
2.2.3. Specific Activity
3. Materials and Methods
3.1. Chemistry
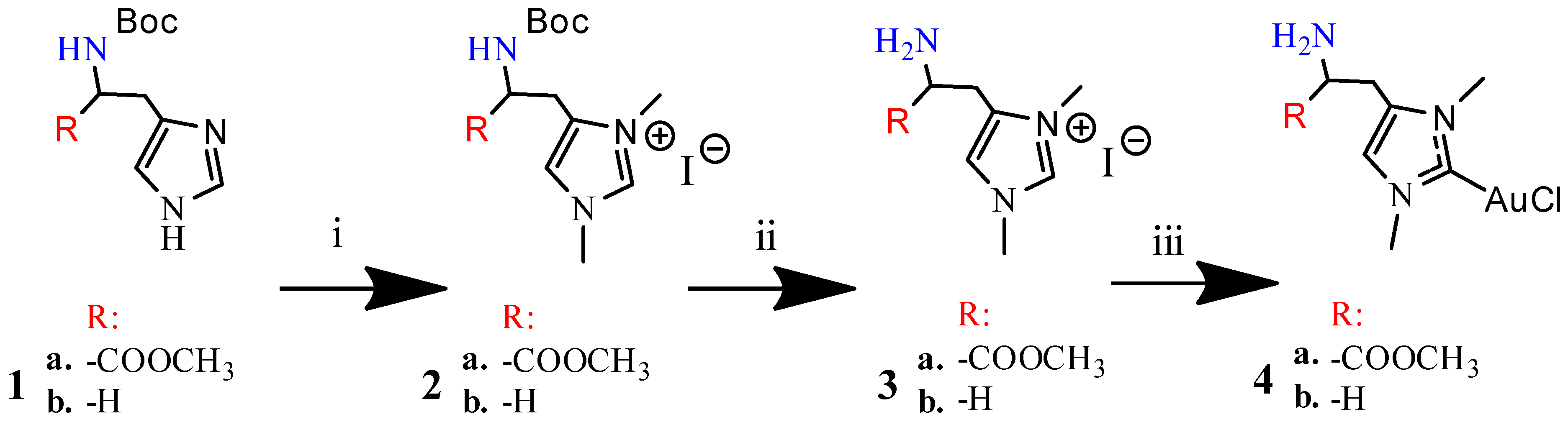
3.1.1. Synthesis of N-boc-Histidine-NHC and N-boc-Histamine-NHC (2a and 2b)
3.1.2. Synthesis of Histidine-NHC-C-AuCl complex and Histamine-NHC-C-AuCl Complex (4a and 4b)
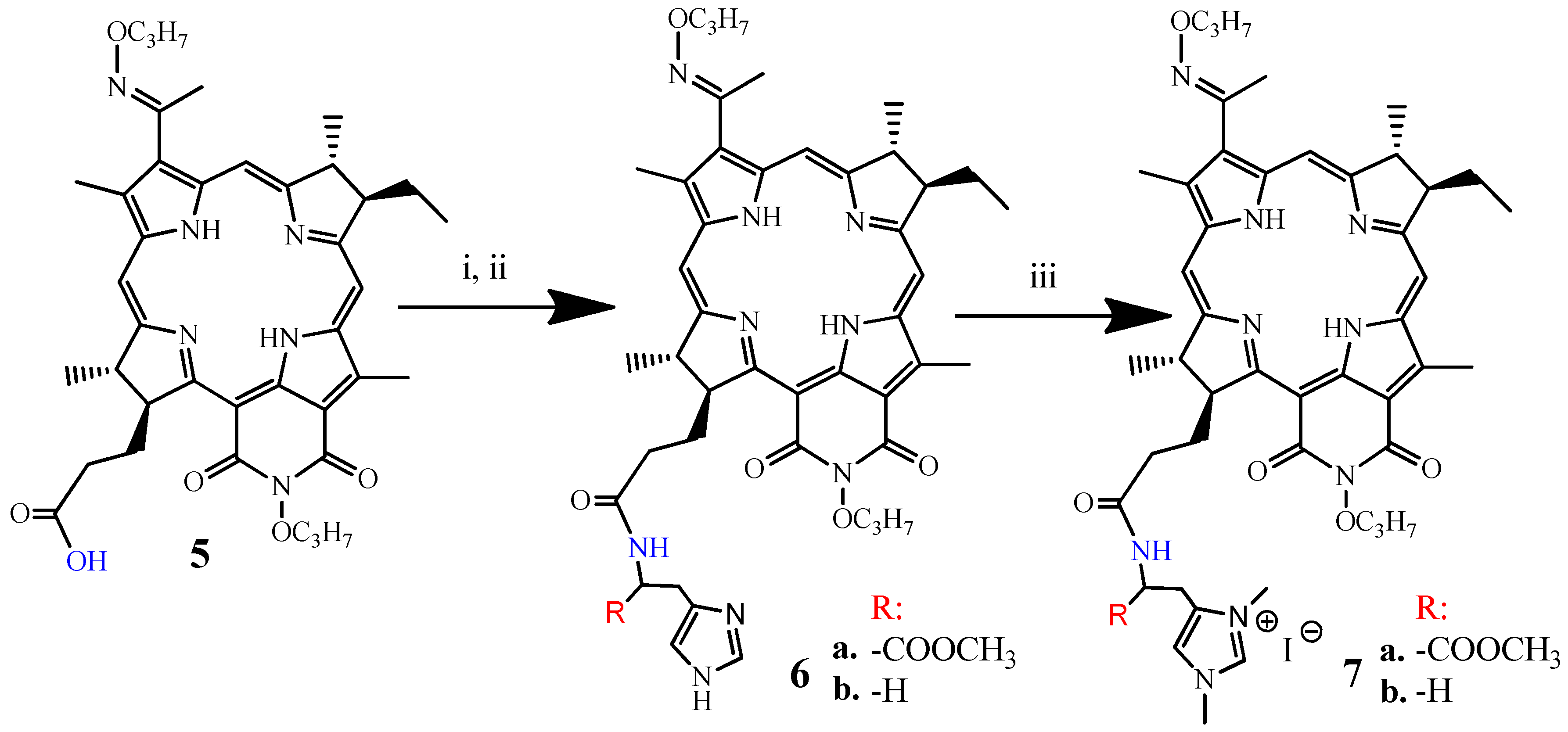
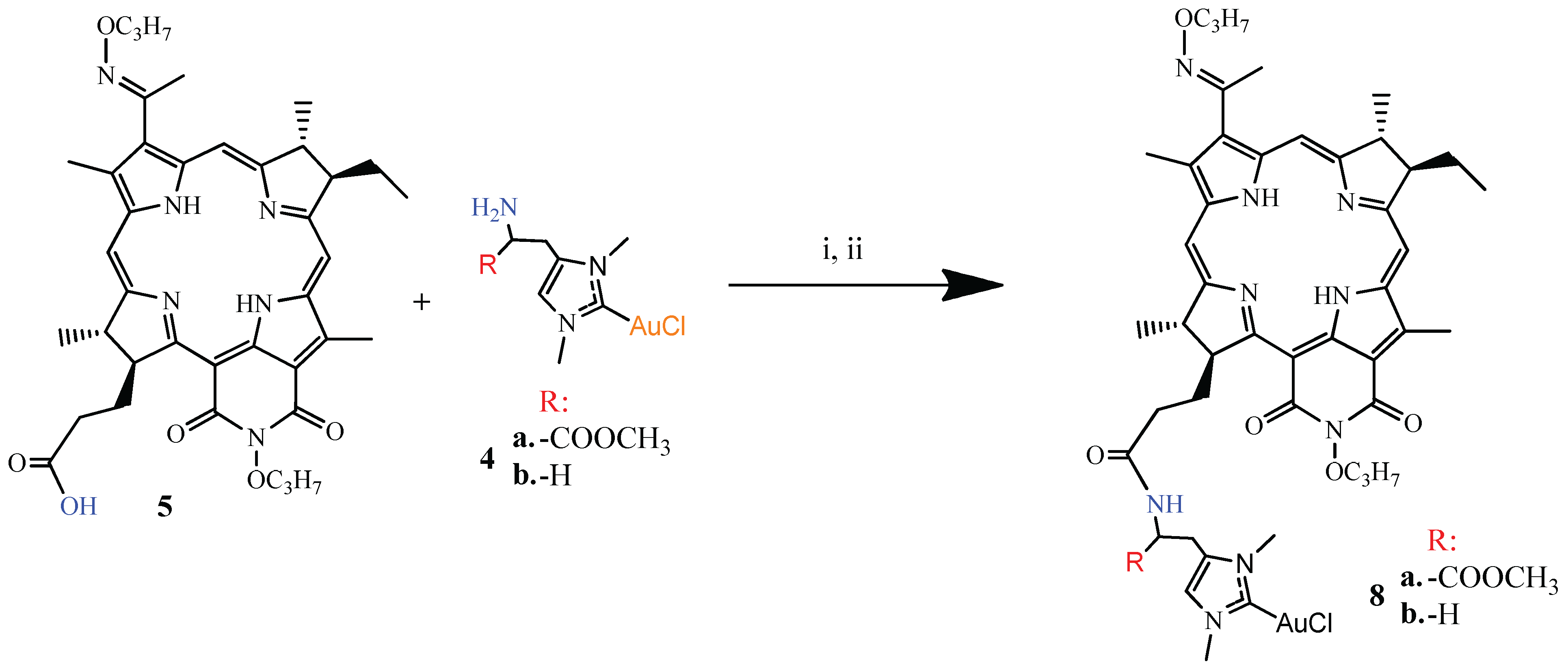
3.1.3. Synthesis of DPI-Histidine and DPI-Histamine (6a and 6b)
3.1.4. Synthesis of DPI-Histidine-NHC and DPI-Histamine-NHC (7a and 7b)
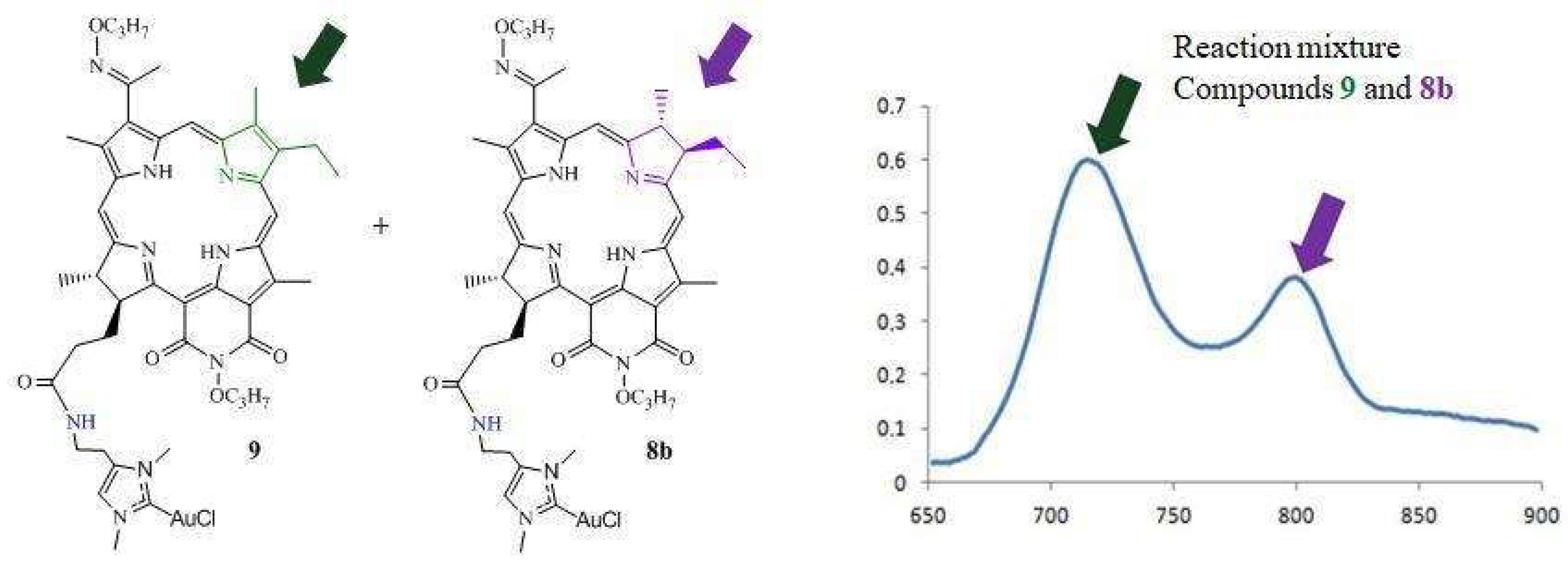
3.1.5. Synthesis of DPI-Histidine-NHC-C-AuCl and DPI-Histamine-NHC-C-AuCl Complexes (8a and 8b)
3.2. Biology
3.2.1. Photoinduced Toxicity and Cytotoxicity Studies In Vitro
3.2.2. In Vivo
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayat, M.R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar]
- Filonenko, E.V. Clinical implementation and scientific development of photodynamic therapy in Russia in 2010-2020. Biomed. Photonics 2021, 10, 4–22. [Google Scholar] [CrossRef]
- Grin, M.; Suvorov, N.; Ostroverkhov, P. Advantages of combined photodynamic therapy in the treatment of oncological diseases. Biophys Rev. 2022, 14, 1867–2469. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.G.; Jung, J.M.; Lee, E.J.; Choi, J.H. Effects of cisplatin on photosensitizer-mediated photodynamic therapy in breast tumorbearing nude mice. Obstet. Gynecol. Sci. 2019, 62, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen–gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Cao, H.; Yang, C.; Zhang, L.; Jiang, X.; Gao, X.; Yang, F.; He, G.; Song, X.; Tong, A.; et al. LHD-Modified mechanism-based liposome coencapsulation of mitoxantrone and prednisolone using novel lipid bilayer fusion for tissue-specific colocalization and synergistic antitumor effects. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Xing, R.; Jiao, T.; Shen, G.; Chen, C.H.; Li, J.; Yan, X. Injectable self-assembled dipeptide-based nanocarriers for tumor delivery and effective in vivo photodynamic therapy. ACS Appl. Mater. Interfaces 2016, 8, 30759–30767. [Google Scholar] [CrossRef]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef]
- Chow, A.L.F.; So, M.H.; Lu, W.; Zhu, N.Y.; Che, C.M. Synthesis, Photophysical Properties, and Molecular Aggregation of Gold(I) Complexes Containing Carbon-Donor Ligands. Chem. Asian J. 2011, 6, 544–553. [Google Scholar] [CrossRef]
- Lanoe, P.H.; Chan, J.; Gontard, G.; Monti, F.; Armaroli, N.; Barbieri, A.; Amouri, H. Deep-Red Phosphorescent Iridium(III) Complexes with Chromophoric N-Heterocyclic Carbene Ligands: Design, Photophysical Properties, and DFT Calculations. Eur. J. Inorg. Chem. 2016, 2016, 1631–1634. [Google Scholar] [CrossRef]
- Al Nasr, I.; Touj, N.; Koko, W.; Khan, T.; Özdemir, I.; Yaşar, S.; Hamdi, N. Biological Activities of NHC–Pd(II) Complexes Based on Benzimidazolylidene N-heterocyclic Carbene (NHC) Ligands Bearing Aryl Substituents. Catalysts 2020, 10, 1190. [Google Scholar] [CrossRef]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Franke, R.; Brönstrup, M.; Ott, I. Gold(I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects. Chem. Eur. J. 2017, 23, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.T.H.; Lok, C.N.; Chung, C.Y.S.; Fung, Y.M.E.; Chow, P.K.; Wan, P.K.; Che, C.M. Cyclometalated Palladium(II) N-Heterocyclic Carbene Complexes: Anticancer Agents for Potent In Vitro Cytotoxicity and In Vivo Tumor Growth Suppression. Angew. Chem. Int. Ed. 2016, 55, 11935–11939. [Google Scholar] [CrossRef] [PubMed]
- Maftei, C.V.; Kızrak, Ü.; Çiftçi, O.; Özdemir, I.; Gürbüz, N.; Düşünceli, S.D.; Kaloğlu, M.; Mansour, L.; Zaghrouba, F.; Hamdi, N.; et al. Amine-functionalized silver and gold N-heterocyclic carbene complexes: Synthesis, characterization and antitumor properties. J. Organomet. Chem. 2019, 882, 26–32. [Google Scholar]
- Durmus, S.; Garrison, J.C.; Panzner, M.J.; Tessier, C.A.; Youngs, W.J. Synthesis of an imidazolium-linked cyclophane from histamine. Tetrahedron 2005, 61, 97–101. [Google Scholar] [CrossRef]
- Medina, V.A.; Rivera, E.S. Histamine receptors and cancer pharmacology. Br. J. Pharmacol. 2010, 161, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Brandes, L.; Queen, G.; LaBella, F. N, N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine (DPPE), a chemopotentiating and cytoprotective agent in clinical trials: Interaction with histamine at cytochrome P450 3A4 and other isozymes that metabolize antineoplastic drugs. Cancer Chemother. Pharmacol. 2000, 45, 298–304. [Google Scholar] [CrossRef]
- Eszter, B.; Paul, J.D.; Gilles, G. Classification of Metal-Based Drugs according to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar]
- Zou, T.; Lum, C.T.; Lok, C.; Zhang, J.; Che, C. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef]
- Rigobello, M.P.; Messori, L.; Marcon, G.; Cinellu, M.A.; Marcantonio, B.; Alessandra, F.; Guido, S.; Alberto, B. Gold complexes inhibit mitochondrial thioredoxin reductase: Consequences on mitochondrial functions. J. Inorg. Biochem. 2004, 98, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Ozgencli, I.; Kilic, D.; Guller, U.; Ciftci, M.; Kufrevioglu, O.I.; Budak, H. A Comparison of the Inhibitory Effects of Anti-Cancer Drugs on Thioredoxin Reductase and Glutathione S-Transferase in Rat Liver. Anticancer Agents Med. Chem. 2018, 18, 2053–2061. [Google Scholar] [CrossRef]
- Postiglione, I.; Sunusi, Y.H.; Rosenani, A.H.; Mohd, R.R. Recent progress in silver(I)-, gold(I)/(III)- and palladium(II)-N-heterocyclic carbene complexes: A review towards biological perspectives. J. Organomet. Chem. 2019, 882, 96–111. [Google Scholar]
- Dada, O.; Sánchez-Sanz, G.; Tacke, M.; Zhu, X. Synthesis and anticancer activity of novel NHC-gold(I)-sugar complexes. Tetrahedron. Lett. 2018, 59, 2904–2908. [Google Scholar] [CrossRef]
- Dada, O.; Curran, D.; O’Beirne, C.; Müller-Bunz, H.; Zhu, X.; Tacke, M. Synthesis and cytotoxicity studies of novel NHC–Gold(I) pseudohalides and thiolates. J. Organomet. Chem. 2017, 840, 30–37. [Google Scholar] [CrossRef]
- Ostroverkhov, P.V.; Kirin, N.S.; Tikhonov, S.I.; Usachev, M.N.; Abramova, O.B.; Kaplan, M.A.; Mironov, A.F.; Grin, M.A. Guanidine and biguanidine derivatives of natural chlorins: Synthesis and biological assessment. Macroheterocycles 2021, 14, 270–279. [Google Scholar] [CrossRef]
- Mironov, A.F.; Ostroverkhov, P.V.; Tikhonov, S.I.; Pogorilyy, V.A.; Kirin, N.S.; Chudakova, O.O.; Tsygankov, A.A.; Grin, M.A. Amino acid derivatives of natural chlorins as a platform for the creation of targeted photosensitizers in oncology. FineChem. Technol. 2021, 15, 16–33. [Google Scholar] [CrossRef]
- Grin, M.A.; Tikhonov, S.I.; Petrova, A.S.; Pogorilyy, V.A.; Noev, A.N.; Tatarskiy, V.V.; Shpakovsky, D.B.; Milaeva, E.R.; Kalinina, E.V.; Chernov, N.N.; et al. New derivatives of bacteriopurpurin with thiolated Au(I) complexes: Dual dark and light activated antitumor potency. Anti-Cancer Agents Med. Chem. 2020, 20, 49–58. [Google Scholar] [CrossRef]
- Tikhonov, S.; Ostroverkhov, P.; Suvorov, N.; Mironov, A.; Efimova, Y.; Plutinskaya, A.; Pankratov, A.; Ignatova, A.; Feofanov, A.; Diachkova, E.; et al. Tin Carboxylate Complexes of Natural Bacteriochlorin for Combined Photodynamic and Chemotherapy of Cancer è. Int. J. Mol. Sci. 2021, 22, 13563. [Google Scholar] [CrossRef]
- Dubey, R.V.; Kumar, N.; Jain, R. Facile syntheses of histamine- and imidazole-4-propionic acid–derived room-temperature ionic liquids. Synth. Commun. 2012, 15, 2207–2216. [Google Scholar] [CrossRef]
- Yakubovskaya, R.I.; Chissov, V.I.; Mironov, A.F.; Grin, M.A.; Morozova, N.B.; Tsygankov, A.A.; Plotnikova, E.A. A Drug for Photodynamic Therapy and a Method of Photodynamic Therapy of Cancer with Its Use. RU Patent 2521327C1, 12 December 2012. [Google Scholar]
- Wang, W.T.; Chen, Y.H.; Hsu, J.L. Terfenadine induces anti-proliferative and apoptotic activities in human hormone-refractory prostate cancer through histamine receptor-independent Mcl-1 cleavage and Bak up-regulation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Cortesini, C.; Schiavone, N.; Perna, F.; Magnelli, L.; Fanti, E.; Bani, D.; Messerini, L.; Fabbroni, V.; Perigli, G.; et al. The role of cyclooxygenase-2 in mediating the effects of histamine on cell proliferation and vascular endothelial growth factor production in colorectal cancer. Clin. Cancer Res. 2005, 11, 6807–6815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Guide to Laboratory Animals and Alternative Models in Biomedical Research, Karkishchenko, N.N.; Grachev, S.V. (Eds.) Profil: Moscow, Russian, 2010; 358. (In Russian)
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union, 10 October 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF(accessed on 11 November 2022).
- Zharkova, N.N.; Kozlov, D.N.; Polivanov, Y.N. Laser-excited fluorescence spectrometric system for tissue diagnostics. Int. Soc. Opt. Eng. 1994, 2328, 196–202. [Google Scholar] [CrossRef]
- Loschenov, V.B.; Konov, V.I.; Photodynamic, A.M.P. Therapy and Fluorescence Diagnostics. Laser Phys. 2000, 10, 1188–1207. [Google Scholar]
- Guidelines for Conducting Preclinical Studies of Drugs. Part 1. Mironov, A.N. (Ed.) Grif and K: Moscow, Russian, 2012; 657–671. (In Russian) [Google Scholar]

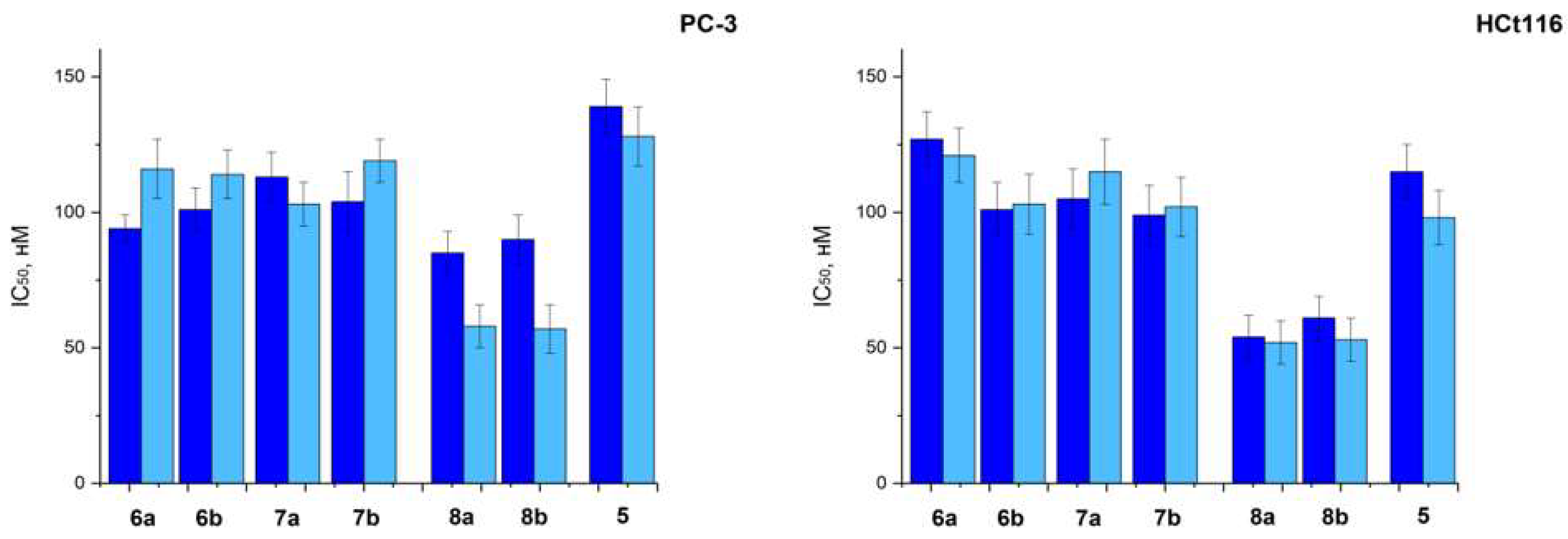
| Compounds | PC-3 | HCt116 |
|---|---|---|
| IC50, nM | ||
| 6a | 116 ± 11 | 125 ±12 |
| 6b | 95 ± 20 | 89 ± 13 |
| 7a | 98 ± 14 | 114 ± 22 |
| 7b | 92 ± 10 | 110 ± 11 |
| 8a | 140 ± 11 | 121 ± 20 |
| 8b | 101 ± 11 | 96 ± 14 |
| b | 143 ± 20 | 100 ± 15 |
| Compounds | PC-3 | HCt116 | ||||
|---|---|---|---|---|---|---|
| Incubation Time of Compounds with Cells, Hours | ||||||
| 24 | 48 | 72 | 24 | 48 | 72 | |
| IC50, nM | ||||||
| 6a | 6018 ± 301 | 5665 ± 108 | 4835 ± 105 | 6721 ± 202 | 5740 ± 311 | 4671 ± 115 |
| 6b | 5834 ± 225 | 4315 ± 212 | 4202 ± 114 | 9634 ± 312 | 7220 ± 401 | 5708 ± 325 |
| 7a | 5151 ± 157 | 4690 ± 241 | 5019 ± 228 | 6981 ± 300 | 6417 ± 321 | 5794 ± 201 |
| 7b | 7085 ± 304 | 6477 ± 301 | 5580 ± 301 | 7813 ± 228 | 6721 ± 207 | 5980 ± 100 |
| 8a | 6230 ± 165 | 5140 ± 118 | 3343 ± 298 | 5870 ± 199 | 4873 ± 210 | 2809 ± 117 |
| 8b | 5834 ± 145 | 4382 ± 214 | 2331 ± 118 | 6572 ± 203 | 5437 ± 111 | 3145 ± 110 |
| 5 | 9635 ± 209 | 7907 ± 230 | 7044 ± 314 | 5887 ± 222 | 5655 ± 112 | 4712 ± 208 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonov, S.; Morozova, N.; Plutinskaya, A.; Plotnikova, E.; Pankratov, A.; Abramova, O.; Diachkova, E.; Vasil’ev, Y.; Grin, M. N-Heterocyclic Carbenes and Their Metal Complexes Based on Histidine and Histamine Derivatives of Bacteriopurpurinimide for the Combined Chemo- and Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2022, 23, 15776. https://doi.org/10.3390/ijms232415776
Tikhonov S, Morozova N, Plutinskaya A, Plotnikova E, Pankratov A, Abramova O, Diachkova E, Vasil’ev Y, Grin M. N-Heterocyclic Carbenes and Their Metal Complexes Based on Histidine and Histamine Derivatives of Bacteriopurpurinimide for the Combined Chemo- and Photodynamic Therapy of Cancer. International Journal of Molecular Sciences. 2022; 23(24):15776. https://doi.org/10.3390/ijms232415776
Chicago/Turabian StyleTikhonov, Sergey, Natalia Morozova, Anna Plutinskaya, Ekaterina Plotnikova, Andrey Pankratov, Olga Abramova, Ekaterina Diachkova, Yuriy Vasil’ev, and Mikhail Grin. 2022. "N-Heterocyclic Carbenes and Their Metal Complexes Based on Histidine and Histamine Derivatives of Bacteriopurpurinimide for the Combined Chemo- and Photodynamic Therapy of Cancer" International Journal of Molecular Sciences 23, no. 24: 15776. https://doi.org/10.3390/ijms232415776








