Potential Application of Leelamine as a Novel Regulator of Chemokine-Induced Epithelial-to-Mesenchymal Transition in Breast Cancer Cells
Abstract
:1. Introduction
2. Results
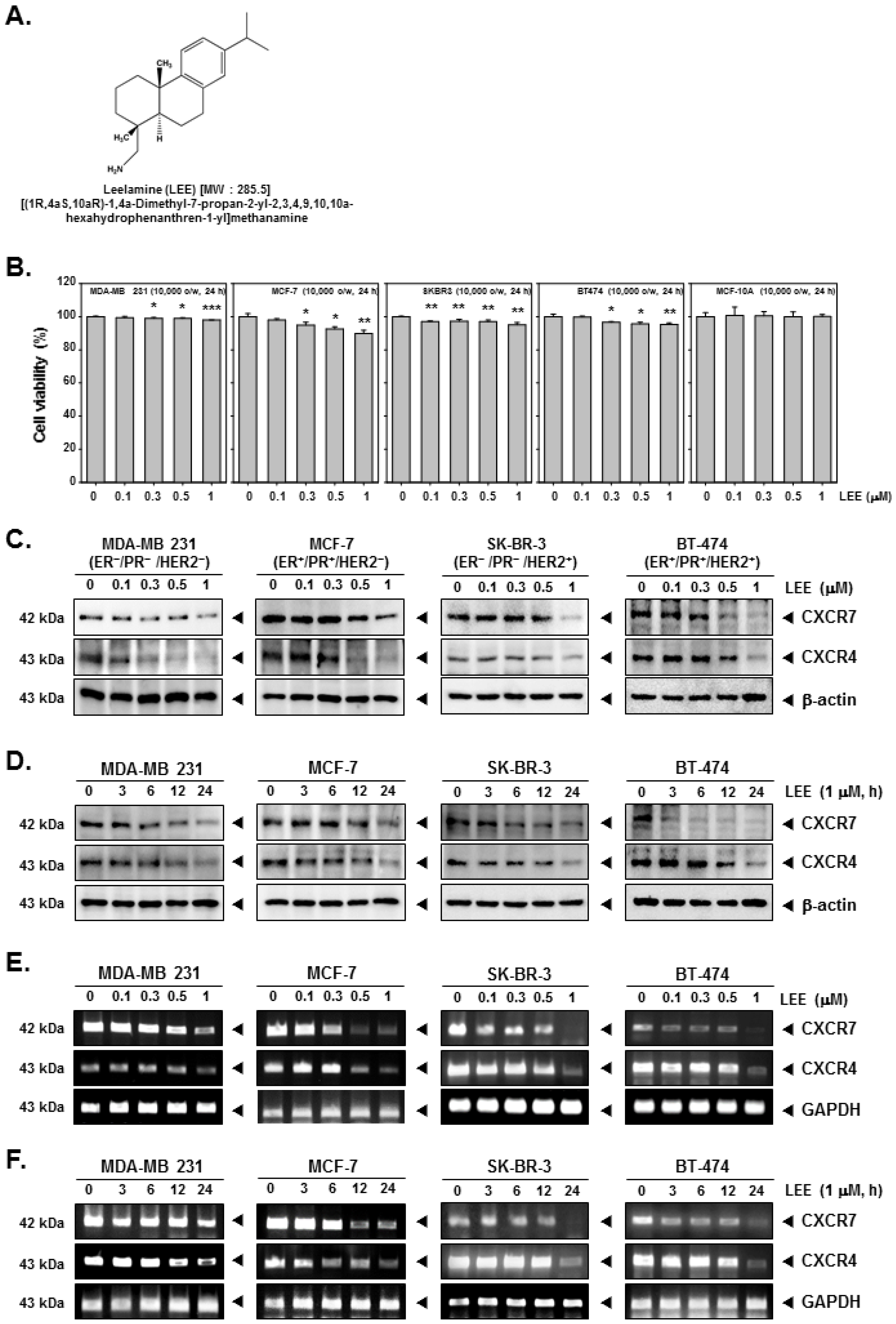
2.1. LEE Inhibited the Growth of Human Breast Cancer Cells and Affected Expression of CXCR7 and CXCR4
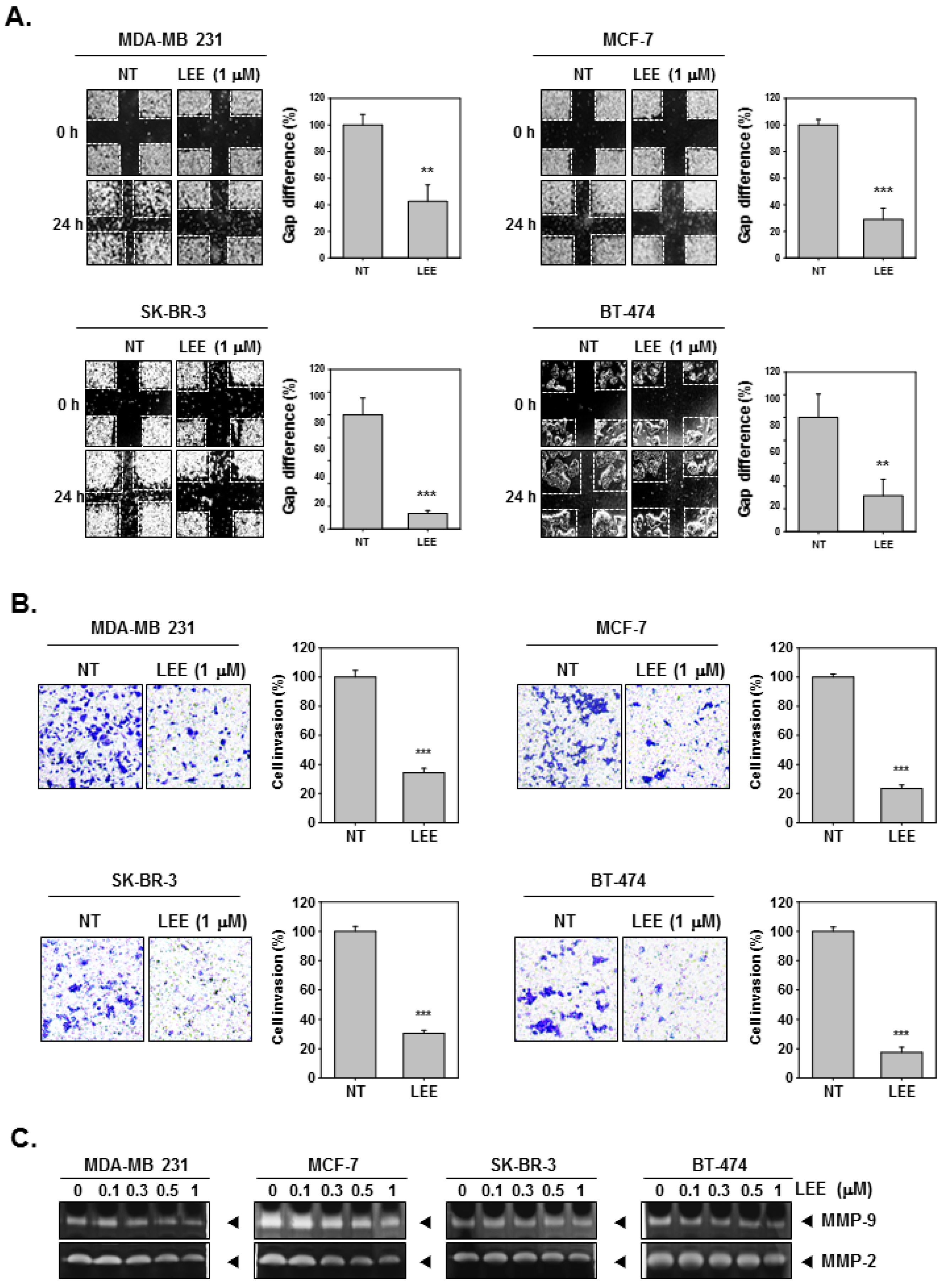
2.2. LEE Abrogated the Migration and Invasion in Human Breast Cancer Cells
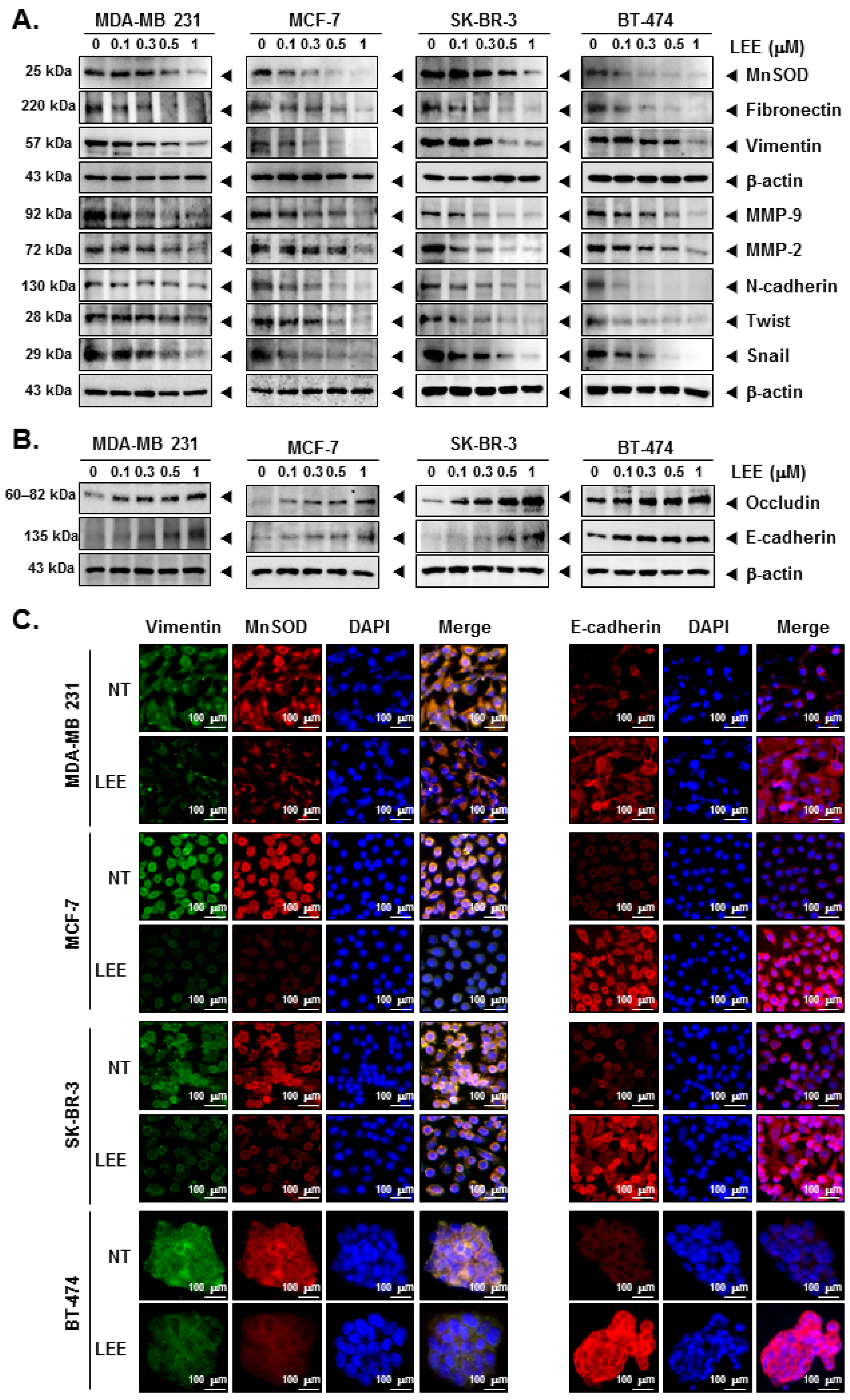
2.3. LEE Suppressed the EMT Process through Modulating EMT Markers
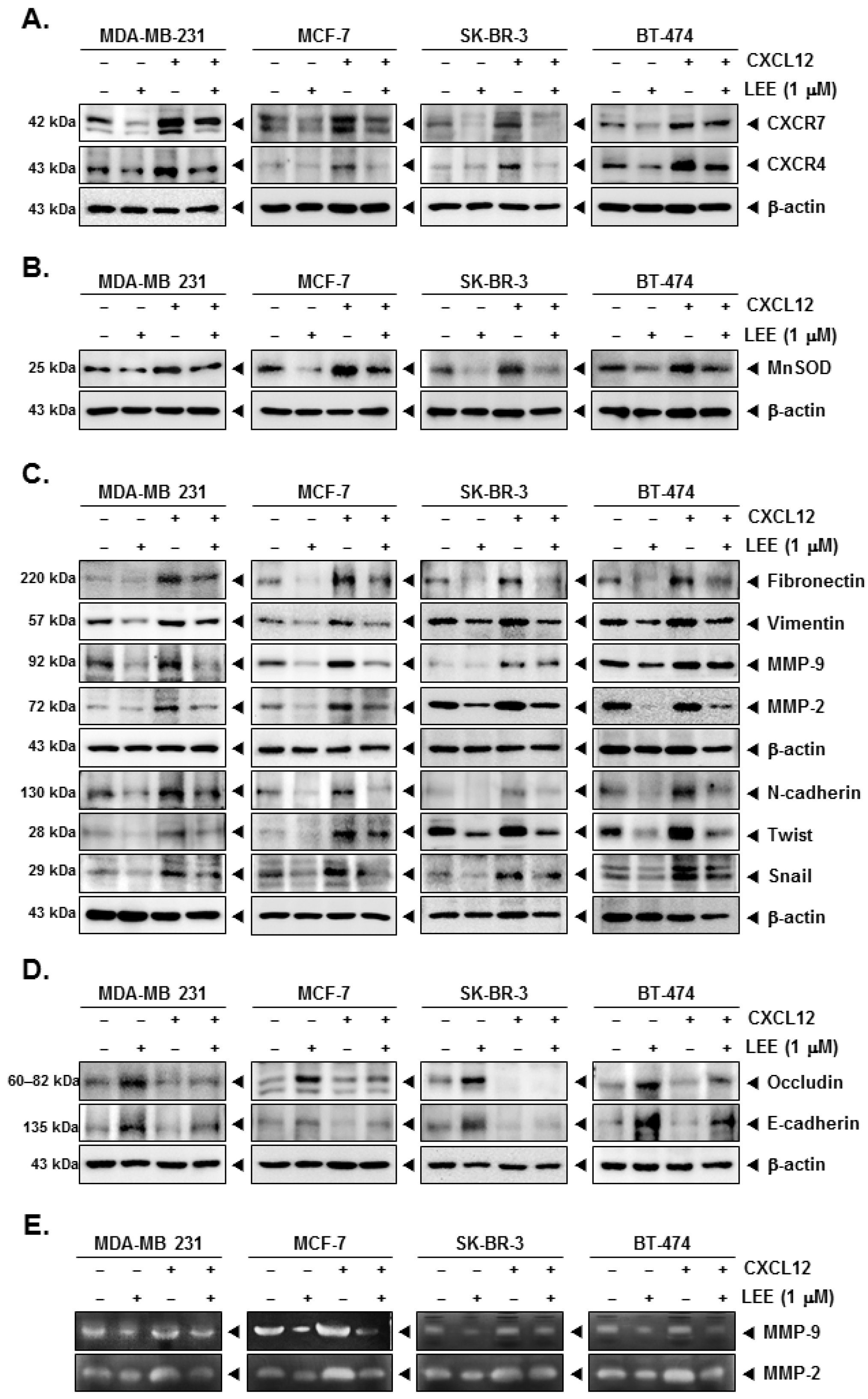
2.4. LEE Attenuated the EMT Process in CXCL12-Stimulated Breast Cancer Cells
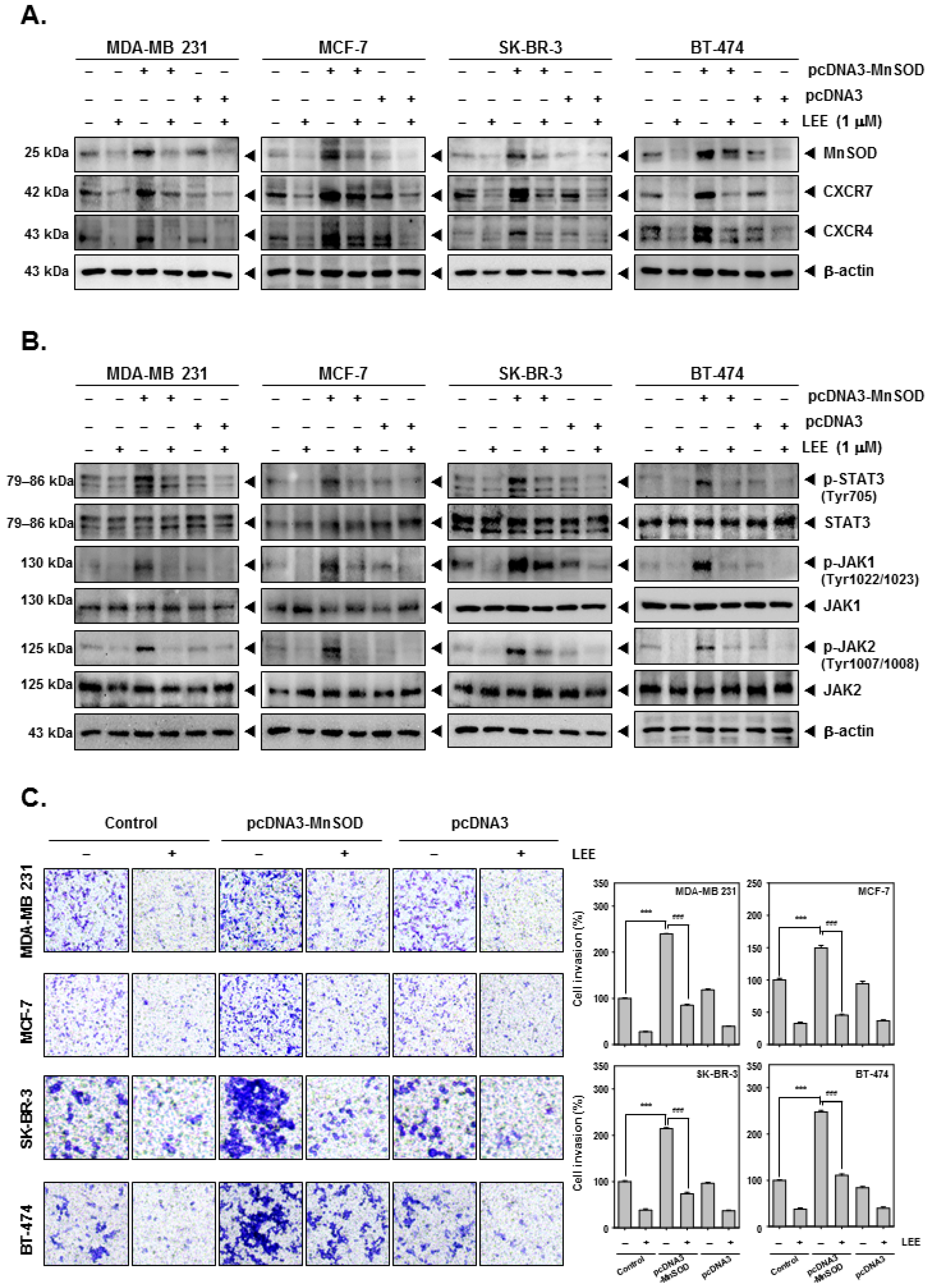
2.5. LEE Reduced the Various Tumorigenesis Proteins in MnSOD-Overexpressing Breast Cancer Cells
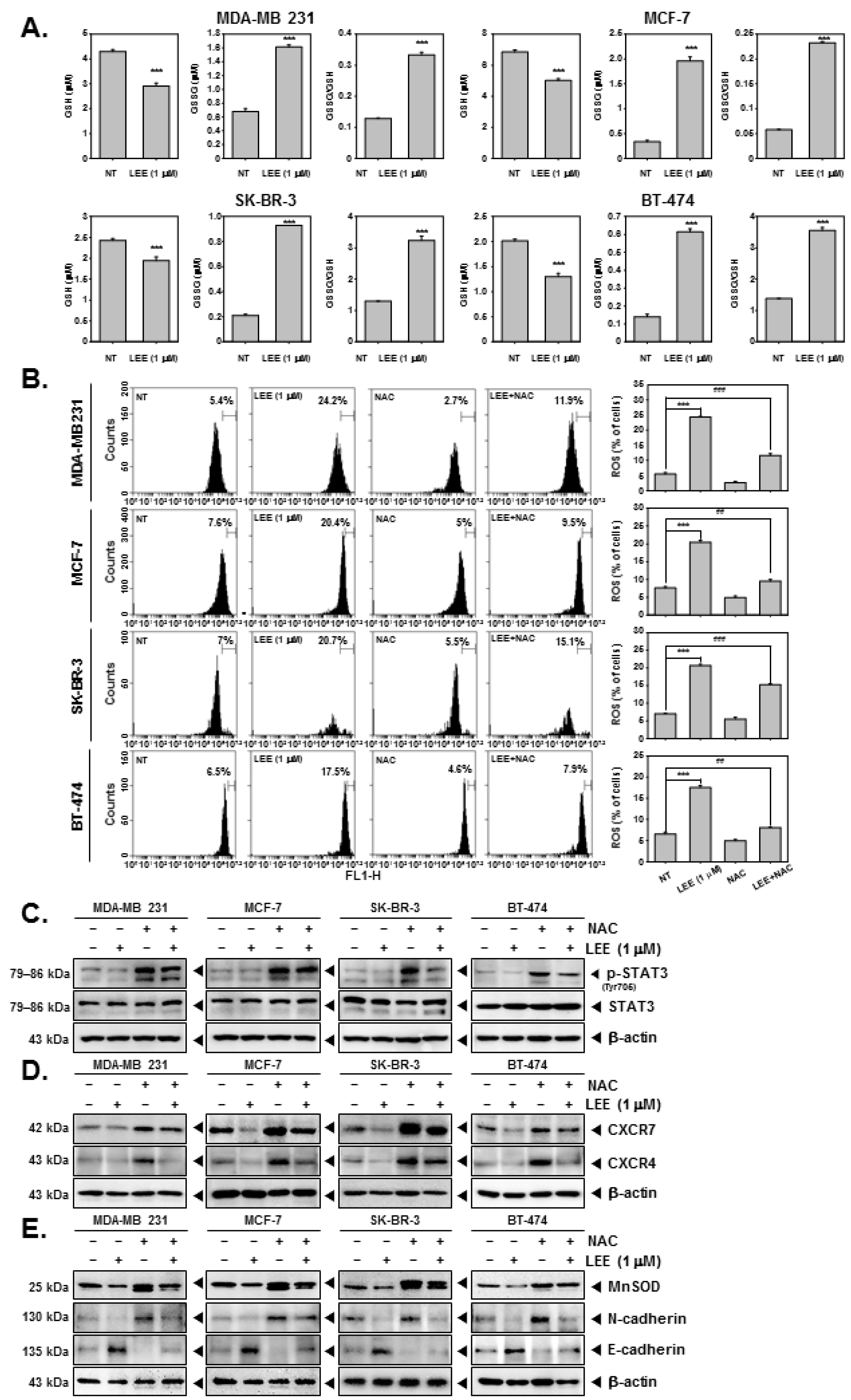
2.6. LEE Induced ROS Production through Promoting GSH/GSSG Imbalance
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture Conditions
4.3. MTT Assay
4.4. Western Blot Analysis
4.5. RT-PCR
4.6. Wound Healing Assay for Cell Migration Observation
4.7. Boyden Chamber Assay for Cell Invasion Observation
4.8. Gelatin Zymography
4.9. Immunocytochemistry
4.10. Transfection with MnSOD Overexpression
4.11. GSH/GSSG Assay
4.12. ROS Detection Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuzu, O.F.; Gowda, R.; Sharma, A.; Robertson, G.P. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Mol. Cancer Ther. 2014, 13, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Inamdar, G.S.; Kuzu, O.; Dinavahi, S.S.; Krzeminski, J.; Battu, M.B.; Voleti, S.R.; Amin, S.; Robertson, G.P. Identifying the structure-activity relationship of leelamine necessary for inhibiting intracellular cholesterol transport. Oncotarget 2017, 8, 28260–28277. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Um, J.Y.; Chinnathambi, A.; Govindasamy, C.; Sethi, G.; Ahn, K.S. Leelamine Modulates STAT5 Pathway Causing Both Autophagy and Apoptosis in Chronic Myelogenous Leukemia Cells. Biology 2022, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Um, J.Y.; Nasif, O.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Blockage of the JAK/STAT3 signaling pathway in multiple myeloma by leelamine. Phytomedicine 2021, 87, 153574. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, A.; Kim, S.H.; Hahm, E.R.; Arlotti, J.A.; Eiseman, J.; Shiva, S.S.; Rigatti, L.H.; Singh, S.V. Cancer-selective death of human breast cancer cells by leelamine is mediated by bax and bak activation. Mol. Carcinog. 2017, 56, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Ji, X.; Singh, S.V. Therapeutic Potential of Leelamine, a Novel Inhibitor of Androgen Receptor and Castration-Resistant Prostate Cancer. Mol. Cancer Ther. 2018, 17, 2079. [Google Scholar] [CrossRef]
- Singh, K.B.; Hahm, E.-R.; Pore, S.K.; Singh, S.V. Leelamine is a novel lipogenesis inhibitor in prostate cancer cells in vitro and in vivo. Mol. Cancer Ther. 2019, 18, 1800–1810. [Google Scholar] [CrossRef]
- Singh, K.B.; Hahm, E.R.; Singh, S.V. Leelamine suppresses cMyc expression in prostate cancer cells in vitro and inhibits prostate carcinogenesis in vivo. J. Cancer Metastasis Treat. 2021, 7, 16. [Google Scholar] [CrossRef]
- Chu, T.; Shields, L.B.E.; Zhang, Y.P.; Feng, S.Q.; Shields, C.B.; Cai, J. CXCL12/CXCR4/CXCR7 Chemokine Axis in the Central Nervous System: Therapeutic Targets for Remyelination in Demyelinating Diseases. Neuroscientist 2017, 23, 627–648. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Lee, J.H.; Kannaiyan, R.; Mustafa, N.; Manu, K.A.; Siveen, K.S.; Sethi, G.; Chng, W.J.; Kumar, A.P. Celastrol Attenuates the Invasion and Migration and Augments the Anticancer Effects of Bortezomib in a Xenograft Mouse Model of Multiple Myeloma. Front. Pharmacol. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Cui, Q.L.; Gottle, P.; Kuhlmann, T.; Hartung, H.P.; Antel, J.; Kury, P. CXCR7 Is Involved in Human Oligodendroglial Precursor Cell Maturation. PLoS ONE 2016, 11, e0146503. [Google Scholar] [CrossRef]
- Ceholski, D.K.; Turnbull, I.C.; Pothula, V.; Lecce, L.; Jarrah, A.A.; Kho, C.; Lee, A.; Hadri, L.; Costa, K.D.; Hajjar, R.J.; et al. CXCR4 and CXCR7 play distinct roles in cardiac lineage specification and pharmacologic beta-adrenergic response. Stem Cell Res. 2017, 23, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiu, L.; Zhang, Y.; Xu, D.; Zheng, J.C.; Jiang, L. CXCL12 enhances angiogenesis through CXCR7 activation in human umbilical vein endothelial cells. Sci. Rep. 2017, 7, 8289. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Du, Y. Clinicopathological significance and prognostic role of chemokine receptor CXCR4 expression in pancreatic ductal adenocarcinoma, a meta-analysis and literature review. Int. J. Surg. 2019, 65, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Jiang, Z.; Ge, C.; Zhao, F.; Jiang, J.; Tian, H.; Chen, T.; Xie, H.; Cui, Y.; et al. TCF12 promotes the tumorigenesis and metastasis of hepatocellular carcinoma via upregulation of CXCR4 expression. Theranostics 2019, 9, 5810–5827. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Mezzapelle, R. The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration. Front. Immunol. 2020, 11, 2109. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A. Endocytic trafficking of chemokine receptors. Curr. Opin. Cell Biol. 2014, 27, 72–77. [Google Scholar] [CrossRef]
- Odemis, V.; Boosmann, K.; Heinen, A.; Kury, P.; Engele, J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J. Cell Sci. 2010, 123, 1081–1088. [Google Scholar] [CrossRef]
- Hattermann, K.; Mentlein, R. An infernal trio: The chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Ann. Anat. Anat. Anz. 2013, 195, 103–110. [Google Scholar] [CrossRef]
- Singh, A.K.; Arya, R.K.; Trivedi, A.K.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.M.; Datta, D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.; Hay, H.S.; Rajendran, P.; Shanmugam, M.K.; Li, F.; Bist, P.; Koay, E.S.; Lim, L.H.; Kumar, A.P.; Sethi, G. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-kappaB activation in breast and pancreatic tumor cells. Biochem. Pharmacol. 2010, 80, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Um, J.Y.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Kumar, A.P.; Ahn, K.S. Identification of Matrine as a Novel Regulator of the CXCR4 Signaling Axis in Tumor Cells. Int. J. Mol. Sci. 2020, 21, 4731. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ong, T.H.; Subramaniam, A.; Siveen, K.S.; Perumal, E.; Samy, R.P.; Bist, P.; Lim, L.H.; Kumar, A.P.; et al. Emodin suppresses migration and invasion through the modulation of CXCR4 expression in an orthotopic model of human hepatocellular carcinoma. PLoS ONE 2013, 8, e57015. [Google Scholar] [CrossRef]
- Santagata, S.; Ierano, C.; Trotta, A.M.; Capiluongo, A.; Auletta, F.; Guardascione, G.; Scala, S. CXCR4 and CXCR7 Signaling Pathways: A Focus on the Cross-Talk between Cancer Cells and Tumor Microenvironment. Front. Oncol. 2021, 11, 591386. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Kumar, A.P.; Sethi, G.; Ahn, K.S. Fangchinoline targets epithelial-mesenchymal transition process by modulating activation of multiple cell-signaling pathways. J. Cell. Biochem. 2022, 123, 1222–1236. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Um, J.Y.; Kumar, A.P.; Sethi, G.; Ahn, K.S. Daidzin targets epithelial-to-mesenchymal transition process by attenuating manganese superoxide dismutase expression and PI3K/Akt/mTOR activation in tumor cells. Life Sci. 2022, 295, 120395. [Google Scholar] [CrossRef]
- Loo, S.Y.; Hirpara, J.L.; Pandey, V.; Tan, T.Z.; Yap, C.T.; Lobie, P.E.; Thiery, J.P.; Goh, B.C.; Pervaiz, S.; Clement, M.V.; et al. Manganese Superoxide Dismutase Expression Regulates the Switch between an Epithelial and a Mesenchymal-Like Phenotype in Breast Carcinoma. Antioxid Redox Signal. 2016, 25, 283–299. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Wu, J.; Pan, C.; Wang, H.; Ying, X.; Zhou, Y.; Yu, H.; Zuo, Y.; Pan, Z.; Liu, R.Y.; et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology 2013, 145, 426–436.e6. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cao, X.; Liu, L.; Cao, X.; Yuan, Q.; Li, X.; Cui, Y.; Xu, C.; Zou, C.; Ren, K.; et al. Modulation of MnSOD and FoxM1 Is Involved in Invasion and EMT Suppression by Isovitexin in Hepatocellular Carcinoma Cells. Cancer Manag. Res. 2020, 12, 5759–5771. [Google Scholar] [CrossRef] [PubMed]
- Shay, G.; Lynch, C.C.; Fingleton, B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015, 44–46, 200–206. [Google Scholar] [CrossRef]
- Sani, I.K.; Marashi, S.H.; Kalalinia, F. Solamargine inhibits migration and invasion of human hepatocellular carcinoma cells through down-regulation of matrix metalloproteinases 2 and 9 expression and activity. Toxicol. Vitr. 2015, 29, 893–900. [Google Scholar] [CrossRef]
- Ko, J.H.; Um, J.Y.; Lee, S.G.; Yang, W.M.; Sethi, G.; Ahn, K.S. Conditioned media from adipocytes promote proliferation, migration, and invasion in melanoma and colorectal cancer cells. J. Cell. Physiol. 2019, 234, 18249–18261. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.Y.; Sethi, G.; Ahn, K.S. Anti-myeloma Effects of Icariin Are Mediated Through the Attenuation of JAK/STAT3-Dependent Signaling Cascade. Front. Pharmacol. 2018, 9, 531. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Chang, Y.C.; Kumar, D.; et al. Ascochlorin Enhances the Sensitivity of Doxorubicin Leading to the Reversal of Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef] [Green Version]
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of tumor-derived exosomes in cancer metastasis. Biochim. Biophys. Acta Rev. Cancer 2019, 187, 12–19. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brustanol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020, 13, 83–94. [Google Scholar]
- Yang, M.H.; Lee, J.H.; Ko, J.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules 2019, 24, 1584. [Google Scholar] [CrossRef]
- Ko, J.H.; Nam, D.; Um, J.Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules 2018, 23, 1601. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, Y.; Qu, J.; Che, X.; Song, N.; Fan, Y.; Wen, T.; Xu, L.; Gong, J.; Wang, X.; et al. The Chemokine Receptor CXCR4 and c-MET Cooperatively Promote Epithelial-Mesenchymal Transition in Gastric Cancer Cells. Transl. Oncol. 2018, 11, 487–497. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Song, Y.; Si, M.; Sun, Y.; Liu, X.; Cui, S.; Qu, X.; Yu, X. Exosomal miR-146a-5p and miR-155-5p promote CXCL12/CXCR7-induced metastasis of colorectal cancer by crosstalk with cancer-associated fibroblasts. Cell Death Dis. 2022, 13, 380. [Google Scholar] [CrossRef]
- Shishodia, S.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007, 74, 118–130. [Google Scholar]
- Zhuo, H.; Jiang, K.; Dong, L.; Zhu, Y.; Lü, L.; Lü, Y.; Zhang, Y.; Zhang, H.; Ye, Y.; Wang, S. Overexpression of N-cadherin is correlated with metastasis and worse survival in colorectal cancer patients. Chin. Sci. Bull. 2013, 58, 3529–3534. [Google Scholar]
- Singhai, R.; Patil, V.W.; Jaiswal, S.R.; Patil, S.D.; Tayade, M.B.; Patil, A.V. E-Cadherin as a diagnostic biomarker in breast cancer. N. Am. J. Med. Sci. 2011, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Mansel, R.E.; Jiang, W.G. Loss of occludin leads to the progression of human breast cancer. Int. J. Mol. Med. 2010, 26, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Vila-Coro, A.J.; Rodriguez-Frade, J.M.; Martin De Ana, A.; Moreno-Ortiz, M.C.; Martinez, A.C.; Mellado, M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999, 13, 1699–1710. [Google Scholar] [PubMed]
- Luo, Y.; Yang, X.; Basourakos, S.P.; Zuo, X.; Wei, D.; Zhao, J.; Li, M.; Li, Q.; Feng, T.; Guo, P.; et al. Enzalutamide-Resistant Progression of Castration-Resistant Prostate Cancer Is Driven via the JAK2/STAT1-Dependent Pathway. Front. Mol. Biosci. 2021, 8, 652443. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Ha, I.J.; Um, J.Y.; Sethi, G.; Ahn, K.S. Fangchinoline diminishes STAT3 activation by stimulating oxidative stress and targeting SHP-1 protein in multiple myeloma model. J. Adv. Res. 2022, 35, 245–257. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Flohe, L. The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta 2013, 1830, 3139–3142. [Google Scholar] [CrossRef]
- Peng, B.; Xu, L.; Cao, F.; Wei, T.; Yang, C.; Uzan, G.; Zhang, D. HSP90 inhibitor, celastrol, arrests human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way. Mol. Cancer 2010, 9, 79. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Lee, S.G.; Sethi, G.; Ahn, K.S. Ophiopogonin D, a Steroidal Glycoside Abrogates STAT3 Signaling Cascade and Exhibits Anti-Cancer Activity by Causing GSH/GSSG Imbalance in Lung Carcinoma. Cancers 2018, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Zukowski, P.; Maciejczyk, M.; Matczuk, J.; Kurek, K.; Waszkiel, D.; Zendzian-Piotrowska, M.; Zalewska, A. Effect of N-Acetylcysteine on Antioxidant Defense, Oxidative Modification, and Salivary Gland Function in a Rat Model of Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6581970. [Google Scholar] [CrossRef]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Rajendran, P.; Ong, T.H.; Chen, L.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin. Cancer Res. 2011, 17, 1425–1439. [Google Scholar]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z.; et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–8338. [Google Scholar]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-kappaB activation cascade in estrogen receptor-negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sandur, S.K.; Lin, X.; Chaturvedi, M.M.; Aggarwal, B.B. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J. Biol. Chem. 2006, 281, 23425–23435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid Redox Signal. 2016, 24, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.Y.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Potential Application of Leelamine as a Novel Regulator of Chemokine-Induced Epithelial-to-Mesenchymal Transition in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9848. https://doi.org/10.3390/ijms23179848
Jung YY, Um J-Y, Sethi G, Ahn KS. Potential Application of Leelamine as a Novel Regulator of Chemokine-Induced Epithelial-to-Mesenchymal Transition in Breast Cancer Cells. International Journal of Molecular Sciences. 2022; 23(17):9848. https://doi.org/10.3390/ijms23179848
Chicago/Turabian StyleJung, Young Yun, Jae-Young Um, Gautam Sethi, and Kwang Seok Ahn. 2022. "Potential Application of Leelamine as a Novel Regulator of Chemokine-Induced Epithelial-to-Mesenchymal Transition in Breast Cancer Cells" International Journal of Molecular Sciences 23, no. 17: 9848. https://doi.org/10.3390/ijms23179848




