Abstract
BmSuc1, a novel animal-type β-fructofuranosidase (β-FFase, EC 3.2.1.26) encoding gene, was cloned and identified for the first time in the silkworm, Bombyx mori. BmSuc1 was specifically and highly expressed in the midgut and silk gland of Bombyx mori. Until now, the function of BmSuc1 in the silk gland was unclear. In this study, it was found that the expression changes of BmSuc1 in the fifth instar silk gland were consistent with the growth rate of the silk gland. Next, with the aid of the CRISPR/Cas9 system, the BmSuc1 locus was genetically mutated, and homozygous mutant silkworm strains with truncated β-FFase (BmSUC1) proteins were established. BmSuc1 mutant larvae exhibited stunted growth and decreased body weight. Interestingly, the molecular weight of part of Sericin1 (Ser1) in the silk gland of the mutant silkworms was reduced. The knockout of BmSuc1 reduced the sericin content in the silkworm cocoon shell, and the mechanical properties of the mutant line silk fibers were also negatively affected. These results reveal that BmSUC1 is involved in the synthesis of Ser1 protein in silk glands and helps to maintain the homeostasis of silk protein content in silk fibers and the mechanical properties of silk fibers, laying a foundation for the study of BmSUC1 regulation of silk protein synthesis in silk glands.
1. Introduction
Sucrose is one of the main products of photosynthesis and the most common transported sugar in plants, and it is also an easily assimilated macronutrient that provides a carbon or energy source for insects to fulfil the requirement of physiological metabolism [1,2]. Sucrose can be hydrolysed by α-glucosidase (EC 3.2.1.20) acts on α-glucosyl residue and by β-fructofuranosidase (β-FFase, EC 3.2.1.26) acts on β-fructosyl residue. α-glucosidase belongs to the hydrolase GH13 family and is widely found in animals, plants, and microorganisms [3]. β-FFase, which belongs to the glycoside hydrolase GH32 family, is abundant in microorganisms and plants and can release monosaccharide units by hydrolysis of sucrose [4]. Moreover, the β-FFase also has transfructosylation activity, which can convert sucrose into fructooligosaccharides (FOS) [5,6]. However, there are few reports on animal β-FFase.
Insects can utilize plant-derived sucrose as a source of food nutrition. However, insect sucrase activity is generally thought to depend mainly on α-glucosidase [1,7]. As the research continued, the researchers discovered the presence of β-FFase activity in insects and speculated that β-FFase is not synthesized by intestinal bacteria [8,9]. In 2008, a novel gene (named BmSuc1) of β-FFase was cloned and identified in the silkworm Bombyx mori. This provided the first direct evidence for the existence of β-FFase in an animal genome [10]. Subsequently, the β-FFase gene was successively discovered in the larvae of Helicoverpa armigera [11], Manduca sexta [12], Dendroctonus ponderosae [13], Sphenophorus levis [14], and Agrilus planipennis [15]. The insect β-FFase gene is thought to have been acquired via horizontal gene transfer from bacteria, including the origin of the silkworm gene BmSuc1 [10,12,13]. The BmSuc1 gene was highly expressed in the midgut and in the anterior and middle parts of the silk gland, and BmSuc1 encodes a functional β-fructofuranosidase, whose enzymatic activity was not inhibited by 1-deoxynojirimycin (DNJ) [2,10]. Our previous study reported that BmSUC1 acted as an essential sucrase by directly modulating the degree of sucrose hydrolysis in the silkworm larval midgut. Silencing BmSuc1 significantly reduced glucose, activated maltase and trehalase in the midgut, and decreased glycogen and trehalose in the fat body, which resulted in larval malnutrition and abnormal petite phenotypes [2]. These results strongly support the hypothesis that silkworms can evade the toxic effects of mulberry leaf alkaloids by high expression of β-FFase in the midgut, then hydrolyse sucrose to absorb sugar nutrition [10].
The silk gland is vitally important for Bombyx mori, as it biosynthesizes the silk protein and spins the silk fiber. The silkworm synthesizes fibroin and sericin proteins in the silk gland to cocoon and pupates inside that shelter at the mature stage [16]. The silk gland is located on the left and right sides of the digestive tract and consists of the anterior silk gland (ASG), middle silk gland (MSG), and posterior silk gland (PSG). Sericin is mainly synthesized in MSG cells and silk fibroin is mainly synthesized in PSG cells [17]. In addition to silk proteins, numerous functional proteins exist in silk glands. Through comparative proteomics, Li et al. found that some proteins highly expressed in the anterior part of the MSG are associated with silk gland development and silk protein protection [18]. BmSerpin-16 is specifically and highly expressed in ASG and MSG of Bombyx mori, which is important for maintaining the stability of the silk secretion environment [19]. BmOsiris9a is specifically expressed in silk glands and exists in cocoon silk, and the overexpression of BmOsiris9a can improve the mechanical properties of silk fibers [20,21]. For BmSuc1, which is also highly expressed in silk glands, previous studies have already been carried out. For example, through the proteomic analysis of the silk glands of silkworms, Dong et al. found that the expression of β-FFase gradually decreased from the 5th instar to the wandering stage and speculated that β-FFase may be involved in the glycosylation of flavonoids [22]. Subsequently, Guo et al. detected the presence of β-FFase in cocoon silk, indicating that β-FFase is also a component of cocoon silk protein [23]. However, it is not clear whether BmSUC1 is related to the development of silk glands and the synthesis of silk protein.
In the present study, we investigated the relationship between silk gland growth rates and BmSuc1 expression patterns. The BmSuc1 mutant silkworm was obtained using the Cas9/small guide RNA (sgRNA) system, and the changes of silk proteins in silk gland tissues before and after BmSuc1 deletion were analyzed. Finally, we also investigated the effect of BmSuc1 deletion on the mechanical properties of silk fibers. Our findings lay the foundation for the exploration of the functions of specifically expressed genes in silk glands.
2. Results
2.1. Expression Pattern of BmSuc1 Is Identical to the Growth Rate of Silk Gland
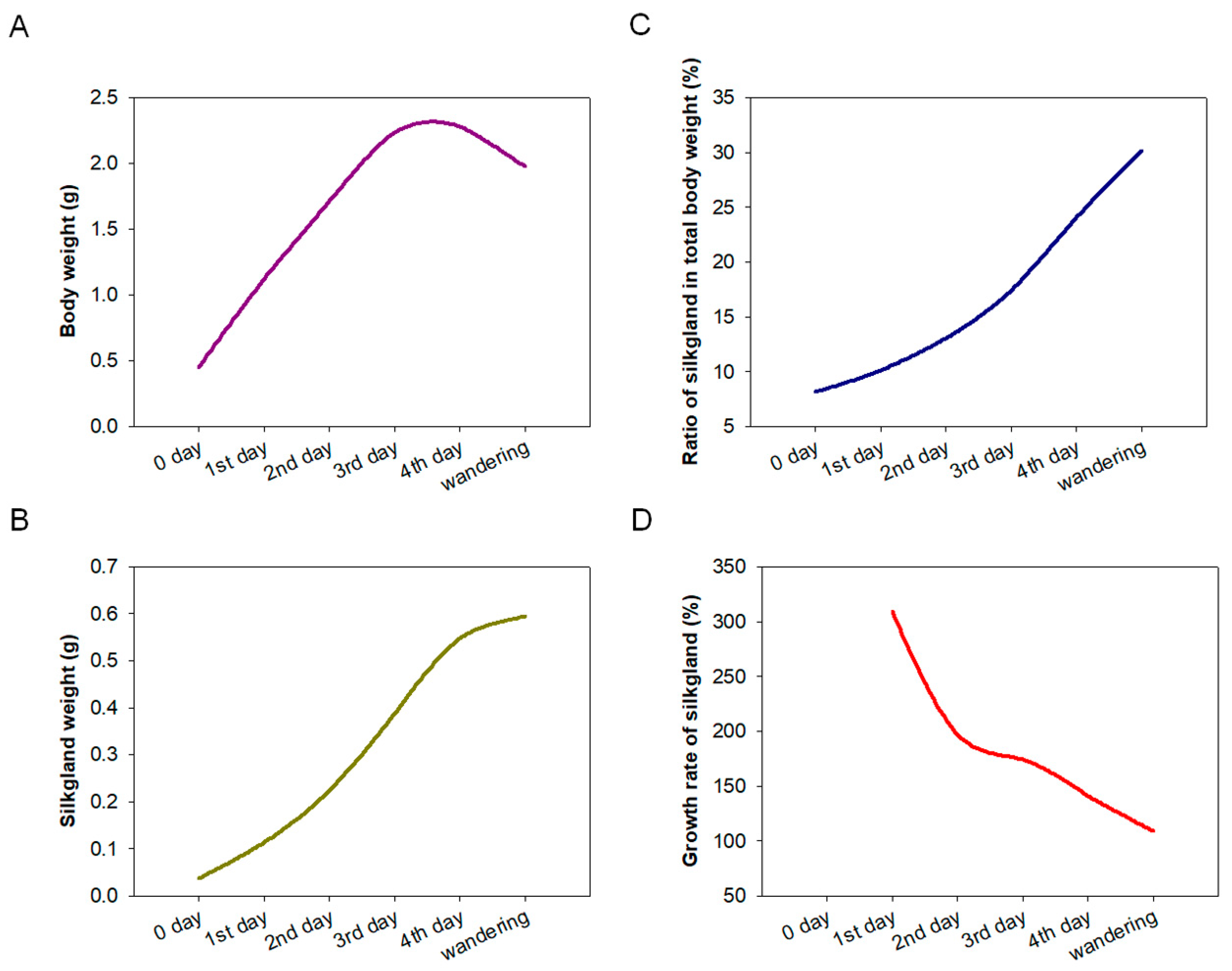
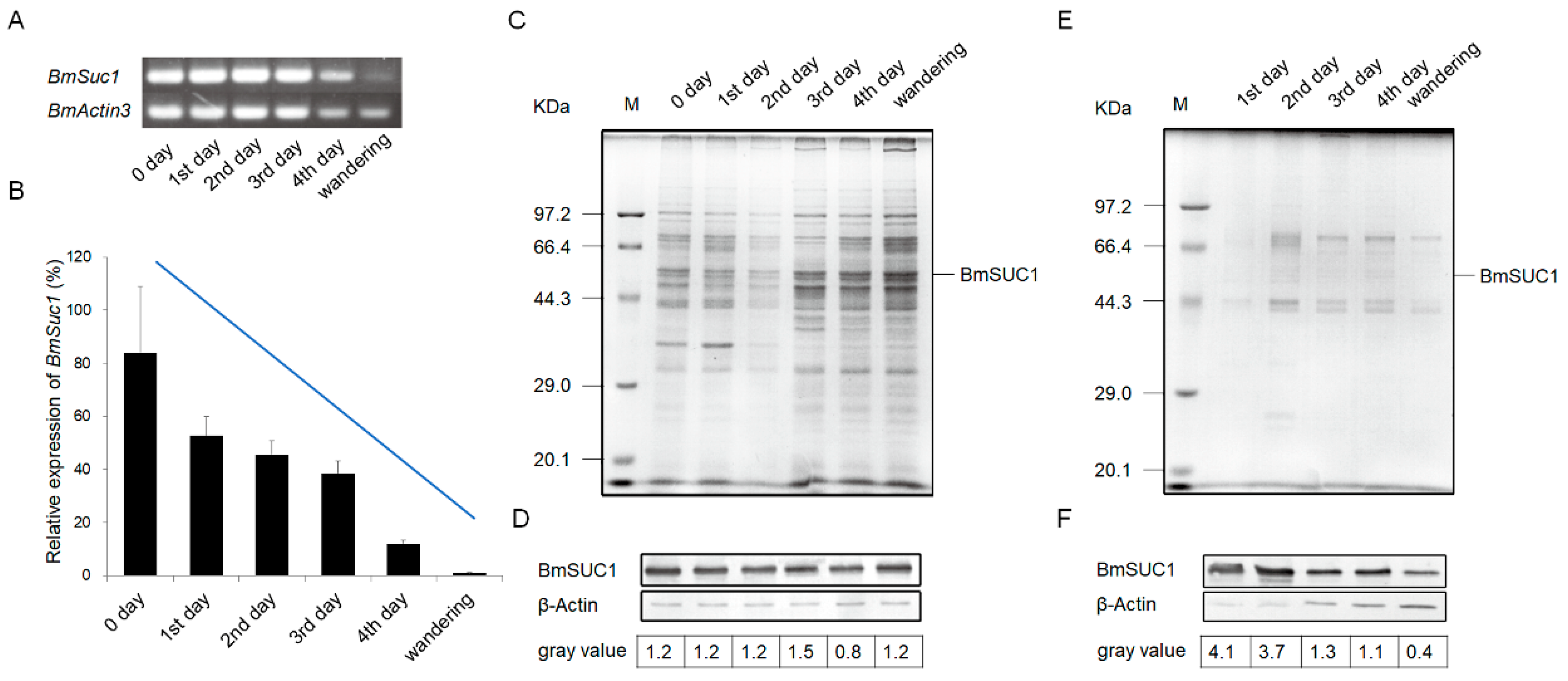
The development of silk glands and the synthesis of silk proteins in Bombyx mori are mainly completed at the fifth instar [24,25]. To determine the relationship between BmSuc1 expression and silk gland development, we first recorded the body weight of silkworm larvae from the fifth instar to the wandering stage and silk gland weights in these larvae to investigate the developmental pattern of silk glands throughout the fifth instar. It was found that the body weight of silkworms increased rapidly from the early fifth instar to the end of the fifth instar (Figure 1A). With the increase of silkworm body weight, the weight of the silk glands increased rapidly and reached the maximum value in the wandering stage (Figure 1B), and the proportion of silk glands in individuals gradually increased (Figure 1C). The growth rate of silk glands was the fastest in the early fifth instar, then gradually slowed down (Figure 1D). A previous study found that BmSuc1 was highly expressed in ASG and MSG of the day 3 fifth instar larvae of Bombyx mori [2]. We investigated the mRNA levels of BmSuc1 in the ASG and MSG tissues of the whole fifth instar silkworm by RT-PCR and qRT-PCR. It was found that BmSuc1 was highly expressed in the ASG and MSG of the fifth instar silkworm, with the highest expression in the early fifth instar, and gradually decreased over time (Figure 2A,B). At the same time, the expression pattern of BmSUC1 protein in fifth instar silkworms was analyzed by SDS-PAGE and western blot. The results showed that the protein abundance in the silk glandular of Bombyx mori was much higher than that in the silk gland luminal (Figure 2C,E). For the BmSUC1 protein, its expression changes were the same as the mRNA transcription level, and it was shown that the expression level in the luminal was higher than that in the glandular (Figure 2D,F). These results indicate that the expression pattern of BmSuc1 (Figure 2B) is consistent with the growth rate of silk glands (Figure 1D), suggesting that BmSUC1 in silk glands may be related to silk gland development.
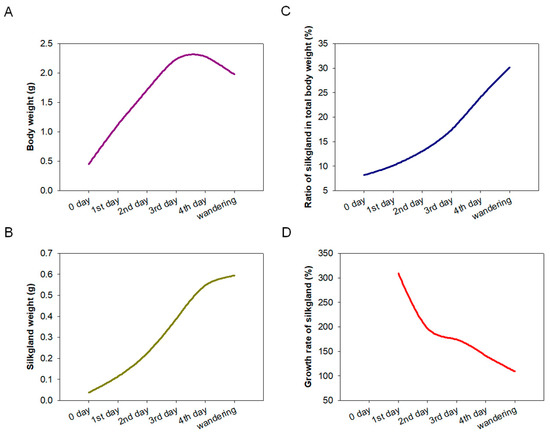
Figure 1.
Investigation of the development of the silk gland during the fifth instar stage. The silk gland of the silkworm was dissected from the 0 days of the fifth instar (5L0D) to the wandering stage, average body and silk gland weight of 3 individuals per group at each stage. (A) Body weight, (B) silk gland weight, (C) ratio of silk gland to total body weight, and (D) growth rate of silk gland.
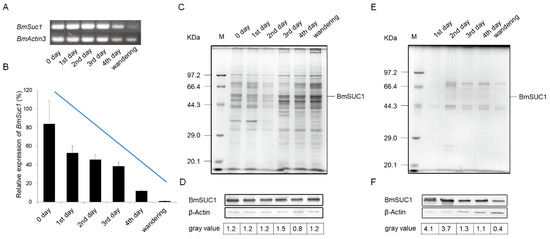
Figure 2.
Expression profiles of BmSuc1 in silkworm larvae anterior and middle parts of the silk gland during the fifth instar using (A) RT-PCR and (B) qRT-PCR. The total RNA was extracted from the 5L0D larvae to the wandering stage (expression level of BmSuc1 at 5L0D is 100%). BmActin3 was used as an internal control. Data in B represent mean ± SEM (n = 3). The SDS-PAGE and western blot analysis of (C,D) glandular and (E,F) lumina protein in anterior silk gland (ASG) and middle silk gland (MSG). (C,E) Total protein expression of ASG and MSG during the fifth instar by SDS-PAGE analysis. (D,F) Western blot analysis of ASG and MSG proteins obtained from fifth instar larvae using an anti-BmSUC1 antibody. β-Actin was used as the control. The amount of BmSUC1 is calculated by grayscale analysis.
2.2. Body Weight Decreased in BmSuc1 Knockout Mutant Larvae
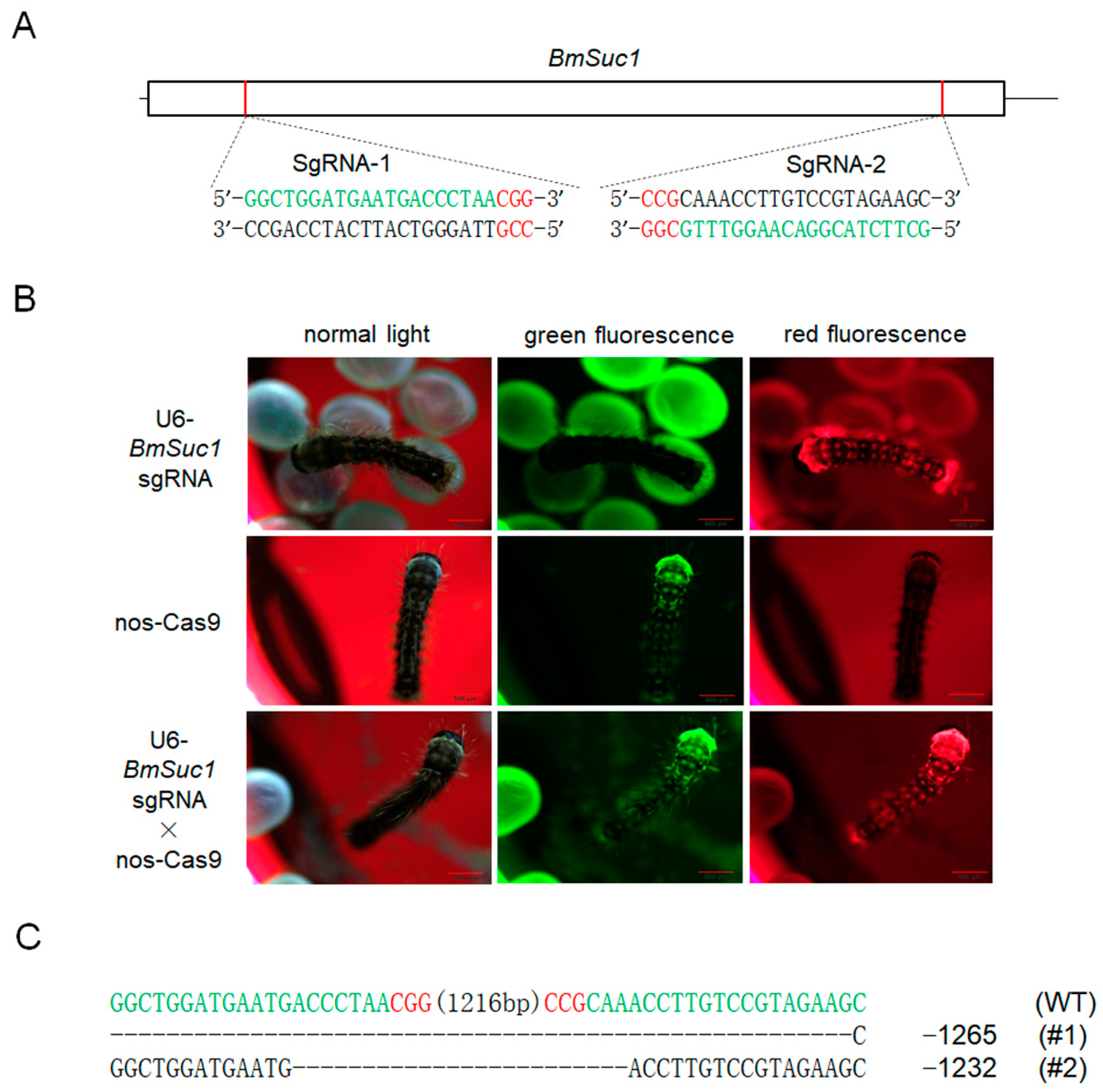
To investigate the function of BmSuc1 in the silk gland of silkworms, we genetically ablated BmSuc1 using a transposon-based, Cas9/sgRNA-mediated mutagenesis system [26,27]. Two independent transgenic lines were established by transposon-mediated germline transformation. One transgenic line expressed two sequence-specific sgRNAs against BmSuc1 (Figure 3A) under the control of the BmU6 promoter [28,29], while the other line expressed Cas9 under the control of the germ-cell-specific promoter Bmnos [27]. Each line also expressed an IE1 promoter-derived fluorescent marker (DsRed2 in the sgRNA-expressing lines and EGFP in the Cas9-expressing lines) (Figure S1A) to facilitate the screening of positive individuals from the embryonic stage [27]. In the F1 hybrid progeny of the sgRNA and Cas9 lines, positive individuals were screened for containing both red and green fluorescently tagged proteins (Figure 3B), and their somatic mutagenesis was identified by PCR-based analysis (Figure S2A) and subsequent sequencing (Figure S1B), indicating that successful mutagenesis was induced by the transgenic CRISPR/Cas9 system. Compared with the wild-type (WT) animals, the knockout of BmSuc1 did not interfere with silkworm fertility. To obtain heritable, non-transgenic, homologous mutants, two adults with complete deletion events were selected based on PCR identification and sequencing results of F2 generation adults with fluorescent deletion (Figure S2B), then backcrossed with WT moths, followed by a series of hybridization strategies and PCR-based screening experiments (Figure S1C) as previously described [26]. Ultimately, two independent BmSuc1-deficient homozygous lines were established (Figure 3C and Figure S2C).
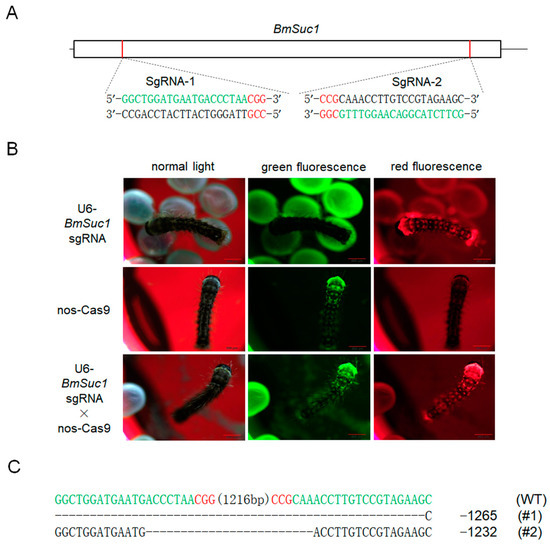
Figure 3.
SgRNA target selection of the BmSuc1 transgenic CRISPR/Cas9 system and fluorescence screening of transgenic silkworm and two selected mutation events. (A) Schematic diagram of the BmSuc1 target sites. The black line represents the genome locus, the sgRNA-targeting sequence is in green, and the protospacer adjacent motif (PAM) sequence is in red. (B) Fluorescence screening of F1 transgenic silkworm. (C) The sequence shows the two selected mutation events. The sgRNA-targeting sequence is in green, and the PAM sequence is shown in red.
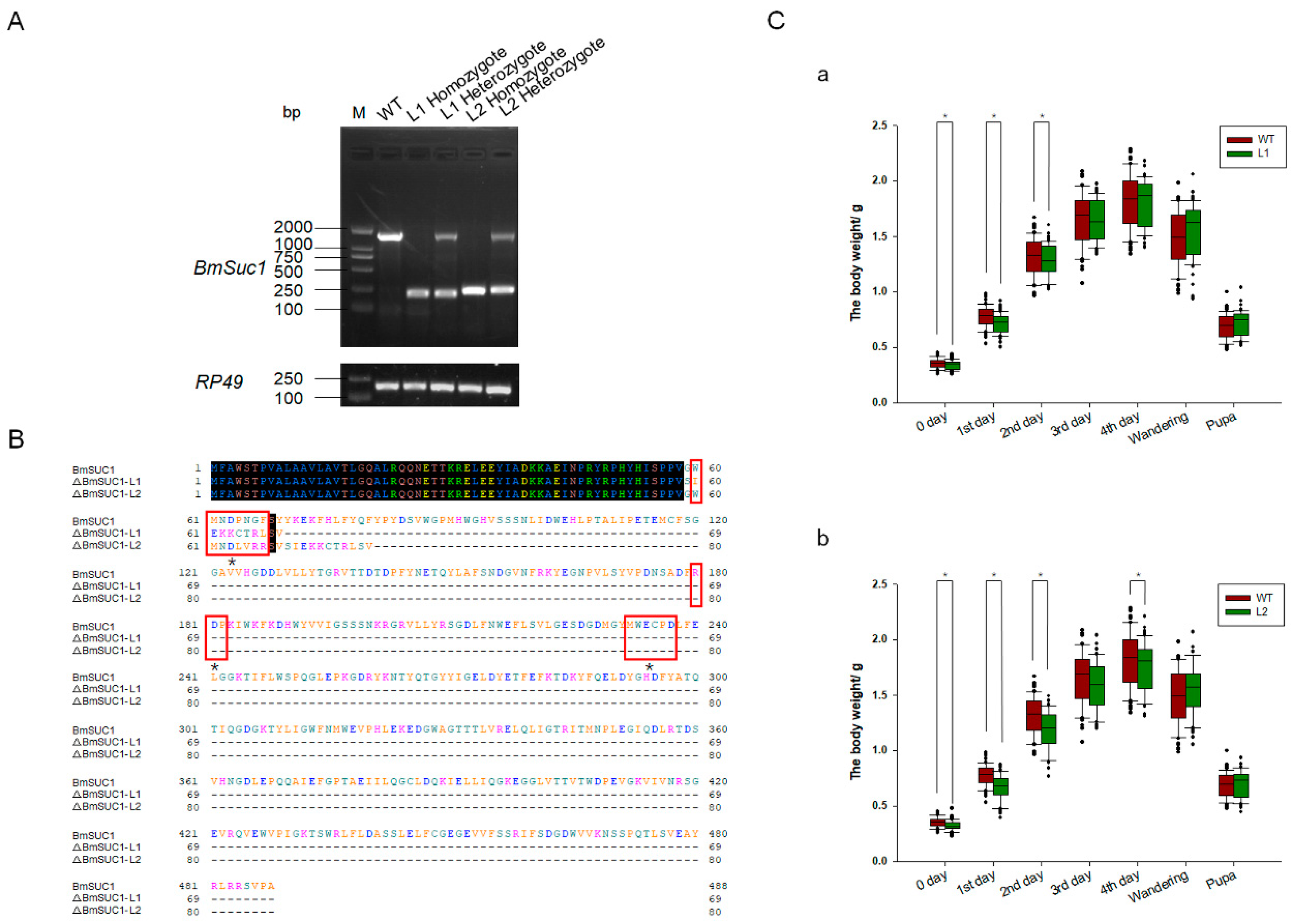
One mutant line (ΔBmSuc1-L1) had a 1265-bp genomic DNA deletion, resulting in a 1265-bp deletion in the ORF and yielded a truncated 69-aa protein that was 419-aa shorter than the WT BmSUC1 protein (Figure 4A,B). Another mutant line (ΔBmSuc1-L2) has a 1232-bp genomic DNA deletion, resulting in a 1232-bp deletion in the ORF and yielded a truncated 80-aa protein that was 408-aa shorter than the WT BmSUC1 protein (Figure 4A,B). Furthermore, compared with WT, BmSuc1 mutant larvae extended the duration of the final larval stage by approximately 12 h, and the individual weight of the mutant was lower than that of the WT at the early fifth instar (Figure 4C). It indicated that the knockout of BmSuc1 had a negative impact on the growth and development of silkworm larvae, which were consistent with previous reports [2].
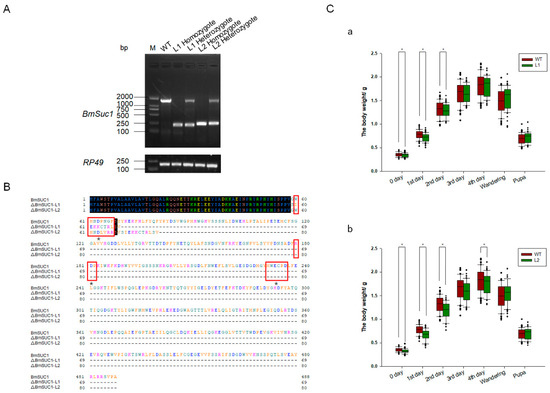
Figure 4.
Comparison of BmSuc1 among mutants and WT. (A) RT-PCR of BmSuc1 of wild-type (WT), line 1 (L1) homozygote, L1 heterozygote, line 2 (L2) homozygote, and L2 heterozygote. ΔBmSuc1 of mutant L1 had a 1265-bp deletion, and ΔBmSuc1 of mutant L2 had a 1232-bp deletion in the ORF. (B) Amino acid sequence alignment of the BmSUC1 protein in WT, L1 homozygote, and L2 homozygote. ΔBmSUC1-L1 is 69 aa, and ΔBmSUC1-L2 is 80 aa. Red boxes represent three conserved domains (60–66, 180–182, 232–237). * Signify active sites (D63, D181, E234). (C) Effect of BmSuc1 knockout on body weight development of silkworms. Comparison of body weight between (a) L1 mutant and WT and (b) L2 mutant and WT. The error bars represent SD; * p < 0.05.
2.3. Sucrose Hydrolase Activity Lost in Silk Gland of BmSuc1 Mutant
Subsequently, we investigated the sucrose hydrolase activity of ΔBmSUC1 in vitro and in vivo. We first synthesized and purified two truncated BmSUC1 and WT BmSUC1 proteins in vitro using a prokaryotic expression system (Figure S3). The purified recombinant protein was confirmed by western blot with different antibodies (Figure 5A). The level of sucrose hydrolase activity of the recombinant protein was detected by DNS (dinitrosalicylic acid) method, and it was found that both ΔBmSUC1 had lost the enzymatic activity of hydrolyzing sucrose (Figure 5B). Next, in order to explore the changes in the sucrose hydrolysis activity of BmSUC1 in the mutant strain silkworm, and the role of BmSUC1 in the sucrose hydrolysis of silk gland tissue of the silkworm larvae, we explored the catalytic ability of sucrase in ASG and MSG total proteins (distinguishing glandular and luminal) of the fifth instar silkworm by adding DNJ, an inhibitor of α-glucosidase, to the reaction mix (Figure 5C). When the DNJ was added to the reaction, the sucrose hydrolase activity in the silk gland of the WT strain did not change significantly. Compared with the WT line, the sucrose hydrolase activity in the silk glands of the mutant larval was almost lost, which was consistent with the loss of sucrose hydrolase activity in the purified recombinant protein ΔBmSUC1 in vitro (Figure 5B), indicating that ΔBmSUC1 in the mutant silkworm had been lost the typical β-FFase character.
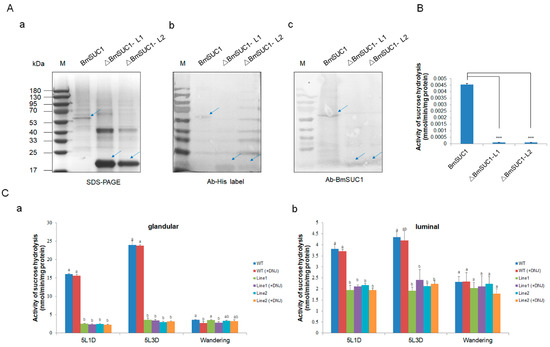
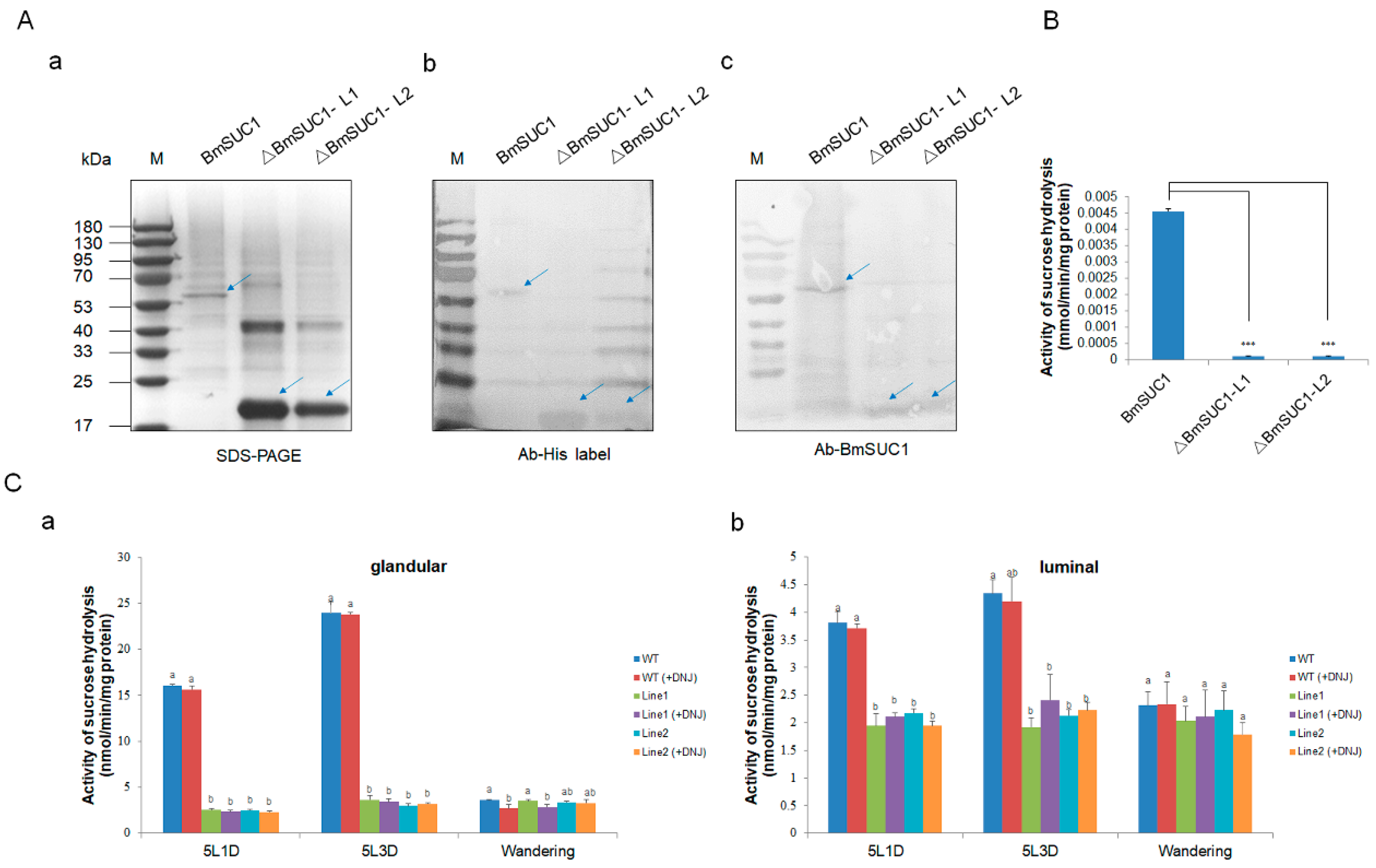
Figure 5.
Determination of sucrose hydrolase activity. (A) Identification of three purified recombinant proteins by (a) SDS-PAGE, (b) western blot using anti-His labelled antibody, and (c) western blot using an anti-BmSUC1 antibody. (B) Sucrose hydrolase activity of ΔBmSUC1, which is expressed in prokaryotes. Error bars, SD; *** p < 0.001. (C) Determination of sucrose hydrolase activity in ASG and MSG of mutant and WT larva. Sucrose hydrolase activity (-DNJ) and β-fructofuranosidase activity (+DNJ) of (a) glandular proteins and (b) luminal proteins at the 5L1D, 5L3D, and wandering stages. Error bars, SD; p-values ≤ 0.05 are considered statistically significant (significant differences are represented by different letters a, b, and c).
2.4. Molecular Weight of Ser1 Decreased in BmSuc1 Mutant
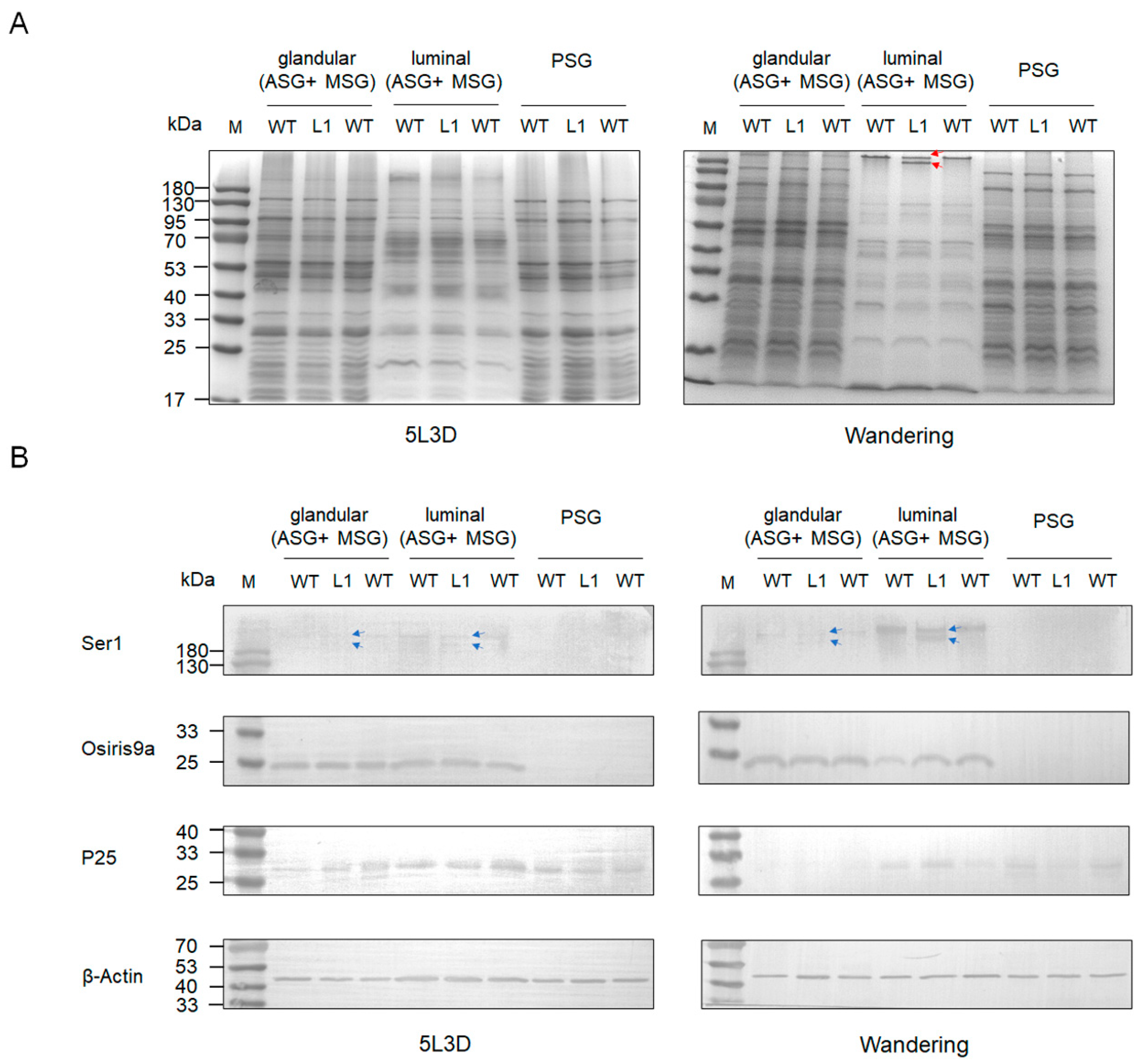
To explore the effect of knocking out BmSUC1 on silk protein synthesis in silkworm silk glands, we performed SDS-PAGE and western blot analysis on total proteins in silk gland tissues from different parts of the silkworm at day 3 fifth instar and wandering stage, respectively. The results showed that the total proteins of ASG and MSG glandular and PSG were not significantly different in both mutant and WT silkworms. Interestingly, in the ASG and MSG luminal of the wandering stage silkworm, compared to the WT, there is a protein band in mutant L1 that becomes lighter in color, and there is an additional protein band of smaller molecular weight below it (indicated by red arrows; Figure 6A). Subsequently, we examined the expression of tissue-specific silk proteins Ser1, BmOsiris9a, and fibrohexamerin/P25 fibroin in silk glands. The results showed that the expression levels of BmOsiris9a and P25 were not significantly different between mutant and WT silkworms. BmOsiris9a was only detected in ASG and MSG, but not in PSG; P25 was detected in both ASG and MSG and PSG (Figure 6B), which is consistent with previous results [17,21]. Interestingly, in ASG and MSG, the BmSer1 antibody detected only one product signal band in WT silkworm, whereas two product signal bands were detected in the mutant line, and the product signal was stronger in the wandering stage (indicated by a blue arrow; Figure 6B). Based on the above results, we speculate that the smaller molecular weight band in the mutant L1 in the SDS-PAGE results is likely to be Ser1.
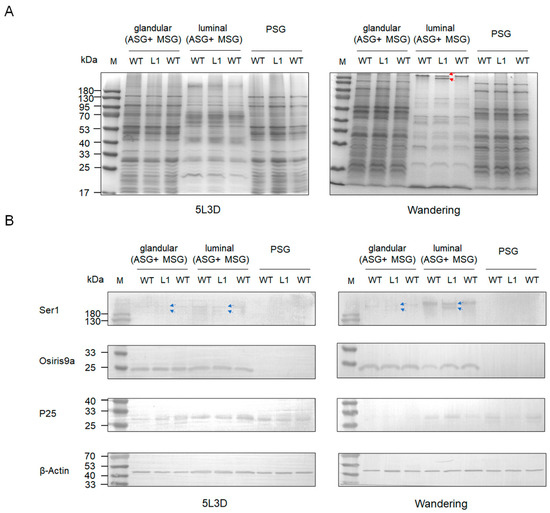
Figure 6.
Protein analysis in different parts (glandular of ASG and MSG, luminal of ASG and MSG, and PSG) of larva silk gland tissue in different stages among mutant and WT by (A) SDS-PAGE. One protein band became lighter in color and a smaller molecular weight protein band appeared below it in the wandering stage of ASG and MSG luminal of mutant L1. The red lines represent the positions of light-colored protein bands and smaller molecular weight protein bands. (B) Western blot of glandular and luminal protein using anti-Ser1, anti-Osiris9a, and anti-P25 antibodies. β-Actin was used as the control. The blue lines indicate the position of the two Ser1 protein molecular weight bands that appear in the mutant L1.
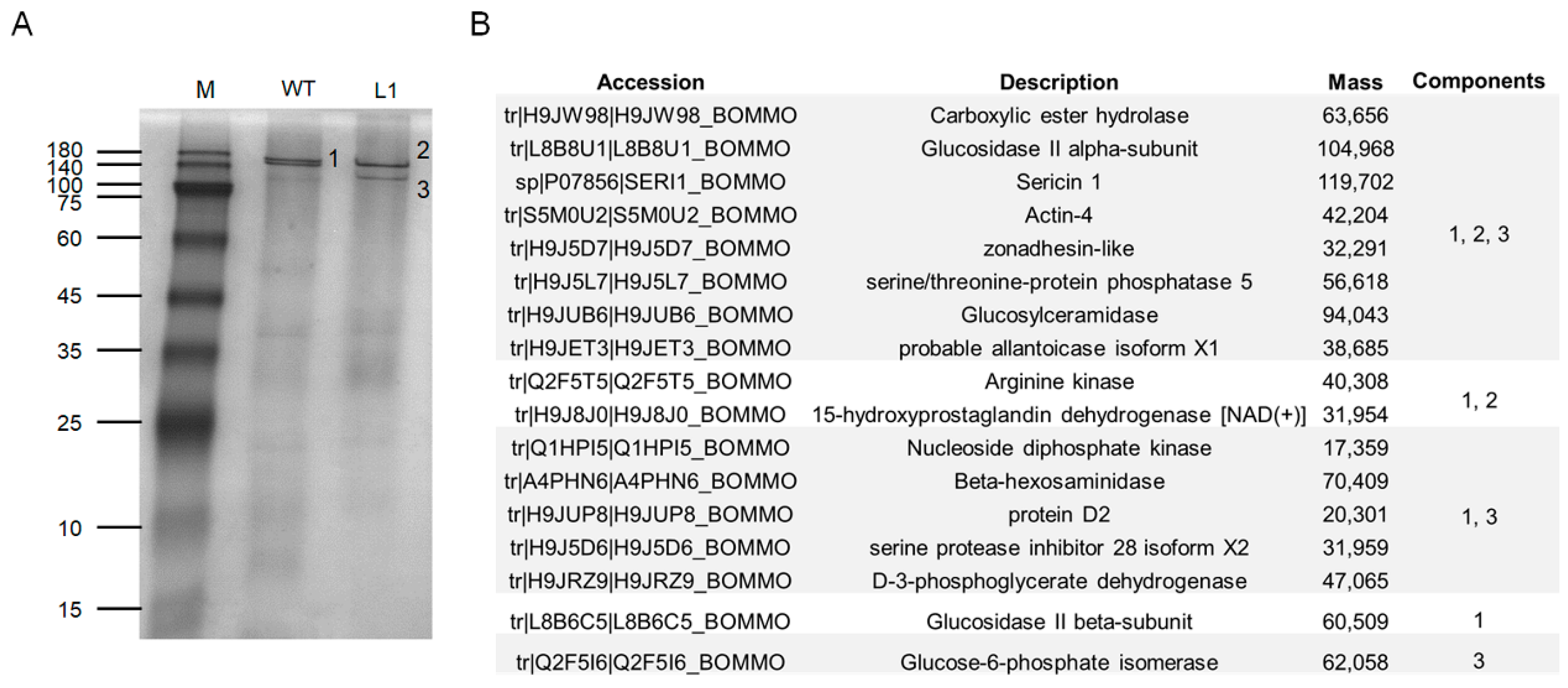
To further confirm our speculation, we performed native PAGE separation of total proteins in the luminal of ASG and MSG in the wandering stage of the mutant L1 and WT silkworms. In the WT silkworm, two closely spaced bands were marked as differential band 1, and two protein bands appeared at the corresponding positions in the mutant strain, marked as differential band 2 and differential band 3 (Figure 7A). The results of mass spectrometry analysis of the three differential bands found that Ser1 components and various other protein components were contained in all three samples (Figure 7B), indicating that the three differential protein bands were protein complexes. Combined with the Western blot results (Figure 6B), it was indicated that the knockout of BmSuc1 resulted in the decrease in molecular weight of some Ser1 proteins.
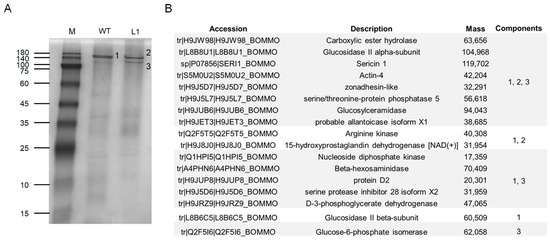
Figure 7.
Mass spectrometry identification of differential components in the silk gland luminal (ASG and MSG) of wandering stage mutant and WT larva. (A) Total protein expression of ASG and MSG luminal in wandering stage by native PAGE analysis. (B) Summary of LC-MSMS analysis identification results for the 3 different protein bands (1, 2, and 3) obtained in (A).
2.5. Knockout of BmSuc1 Reduces the Strength of Silk Fibres
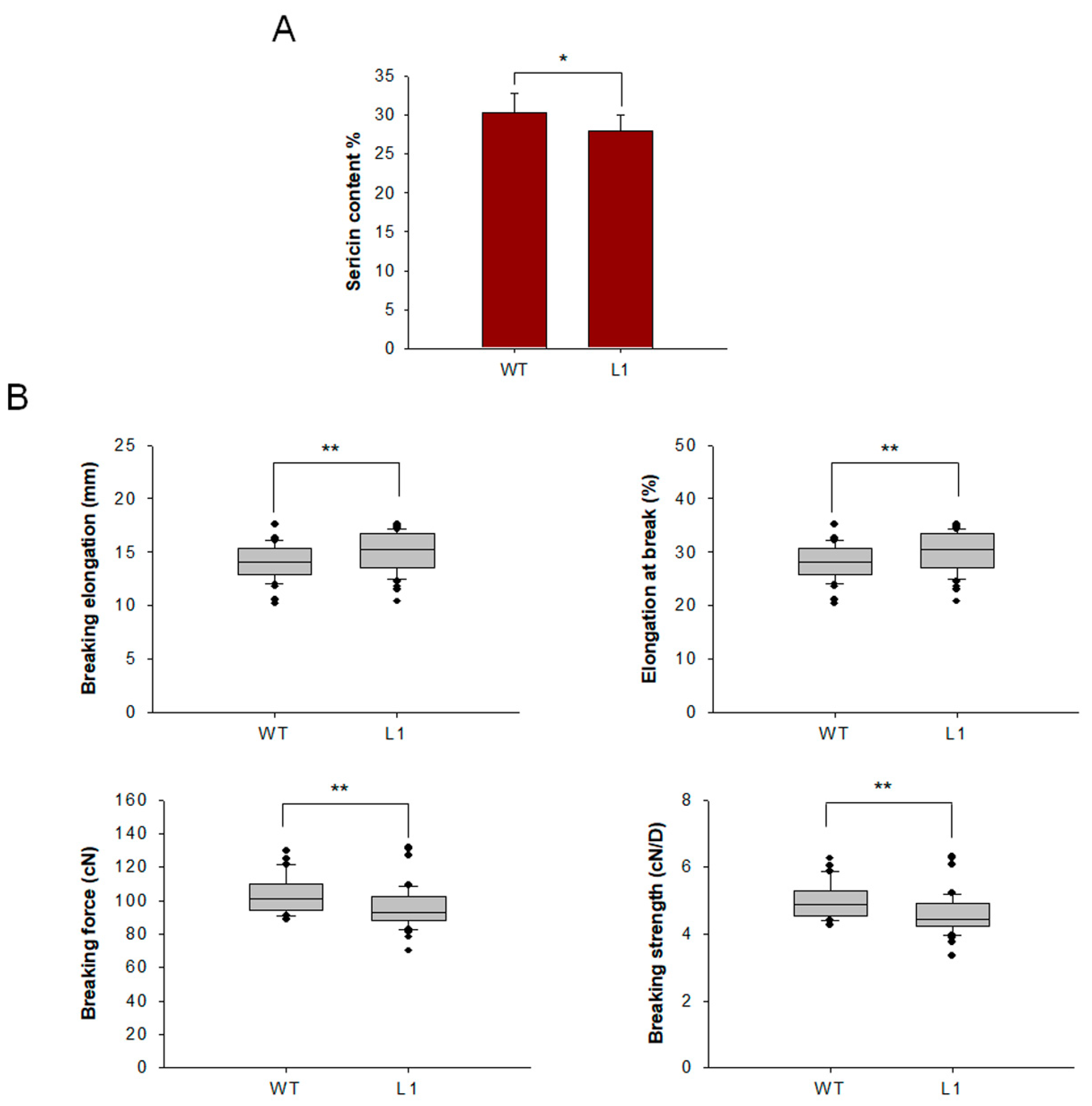
Cocoons of mutant and WT lines were harvested, and raw silk was coiled from the cocoons. To characterize the effect of knocking out BmSuc1 on sericin content, sericin in cocoon shells was extracted and weighed, and it was found that sericin content in mutant L1 was decreased (Figure 8A). To determine the effect of BmSuc1 knockout on silk fiber mechanical properties, mechanical tests were performed using raw silk fibers. The results showed that the breaking elongation and elongation at the break of the mutant silk were higher than those of the WT silk. The breaking force and breaking strength of WT filaments were greater than those of mutant tethered filaments (Figure 8B). It is speculated that the knockout of BmSuc1 reduces the molecular weight of part of Ser1, resulting in a decrease in the overall quality of sericin, which in turn negatively affects the mechanical properties of silk fibers.
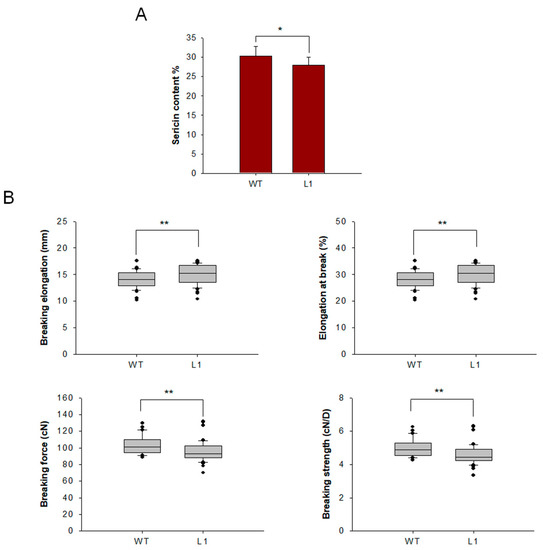
Figure 8.
Comparison of sericin content and mechanical properties test of cocoon silks from mutant L1 and WT. (A) Sericin content in cocoons. (B) Three groups of measurements were made for each strain, 20 times for each group, and a total of 60 measurements were made. The loading distance of raw silk was 50 mm, and the tensile speed was 150 mm/min. Breaking elongation, Elongation at break, Breaking force and Breaking strength. Error bars, SD; * p < 0.05, ** p < 0.01.
3. Discussion
As the first animal-type β-FFase gene to be cloned and identified, BmSuc1 is specifically and highly expressed in the midgut and silk gland tissues of silkworms [2,10]. BmSUC1 plays an important role in the metabolism of sugar nutrition in the midgut of silkworms and has helped silkworm larvae to break into a new and essential path to absorb sugar nutrients through alkaloid sugar mimics in the mulberry leaves [2]. However, the function of BmSuc1 in silkworm silk glands is poorly understood. To broaden our understanding of BmSuc1 function in silk gland tissue of Bombyx mori, we genetically abolished BmSuc1 by Cas9/sgRNA-mediated targeted mutagenesis, revealing that BmSUC1 is involved in the synthesis of sericin 1 protein in the silk gland and plays an important role in maintaining the excellent mechanical properties of silk fibers.
The silk gland of silkworms is a specialized tissue; morphologically and functionally, it can be divided into ASG, MSG for synthesizing and secreting sericin, and PSG for synthesizing and secreting fibroin [30]. The silk glands of the fifth instar larvae developed rapidly, accounting for 30.1% of the body weight of larvae (Figure 1C), which corresponds to the previous findings that the fifth instar is an important period for silk gland development and silk protein synthesis [24,25]. The research on silk glands is relatively clear about the expression and transcriptional regulation of silk protein genes, the development of silk glands, and the synthesis of silk proteins; for example, the transcription factor Bmsage participates in the regulation of silk fibroin heavy chain genes through the interaction with SGF1 [31] and the discovery and characterization of the silk protein constituent BmOsiris9a in the silk gland [21]. However, studies on functional proteins in silk glands are still very scarce. To understand the function of BmSuc1 in silk glands, we first investigated the expression of BmSuc1 in ASG and MSG from the transcriptional and protein levels. The time expression profiling results showed that BmSuc1 was highly expressed in the early fifth instar of Bombyx mori (Figure 2), which was consistent with the higher growth rate of silk glands in the early fifth instar (Figure 1D). We speculated that BmSuc1 might be involved in the development of silk glands.
The amino acid sequence alignment results (Figure 4A) showed that BmSUC1 in the CRISPR/Cas9-BmSuc1 mutant lines lacked most of the amino acids, including three conserved sequences and three restriction sites [32,33]. It is speculated that the β-FFase function has been lost in the mutant line, which was also confirmed by in vivo and in vitro sucrose hydrolase activity experiments (Figure 5 and Figure S4). It is well known that sucrose can be hydrolyzed by α-glucosidase and β-FFase. When the BmSUC1 activity was lost in the midgut of mutant larvae, α-glucosidase activity was increased (Figure S4). However, even with α-glucosidase as compensation, the mutant larvae still showed developmental retardation and lighter weight (Figure 4C). The reduction of mutant larvae weight is most likely due to the loss of BmSUC1 sucrose hydrolase function. This result further confirms the previous findings that the sugar metabolism of silkworms is a complex process, in which BmSUC1 plays a major role [2].
The silk gland of silkworms can be divided into two parts in space: silk glandular and silk gland luminal. Silk protein is mainly synthesized in the glandular and then secreted into the luminal for transportation and storage [30]. The expression of BmSUC1 in the luminal of the silk gland is higher than that in the glandular (Figure 2D,F), and Guo et al. also detected the presence of BmSUC1 protein in cocoon silk [23], indicating that silkworm BmSUC1 is synthesized in the glandular and secreted into the luminal to directly interact with silk protein to play its function. The findings of Dai et al. suggest that BmSUC1 has a role in maintaining silk gland metabolic homeostasis [34]. Loss of BmSUC1 resulted in a disturbance of the silk secretion environment, resulting in a decrease in the secretion of sericin protein and a decrease in the content of sericin in the cocoons (Figure 8). However, by using an anti-Ser1 antibody, we found no significant changes in Ser1 content in the BmSuc1-L1 mutant (Figure 6). It is well known that sericin protein is a collective term for several proteins (17), and the constant content of one sericin does not contradict the decrease of the total sericin content (Figure 8). Interestingly, the molecular weight of part of Ser1 protein was reduced in the ASG and MSG of mutants in our study (Figure 6 and Figure 7). It has been reported that Ser1 has multiple splicing isoforms and is different among strains [35]. In this study, one Ser1 band was mainly detected in the WT strain, so the two bands detected in the mutant strain may represent different isomers or dimers of Ser1 protein. β-FFase, also known as invertase, can transfer fructosyl to sucrose molecules to form FOS, such as kestose [36,37]. Studies have confirmed that BmSUC1 in silkworms has both sucrose hydrolase and glycosyltransferase activities [2,32]. The activity of sucrose hydrolase in WT silk glands did not change with the addition of DNJ, indicating that only β-FFase, but not α-glucosidase, was present in silk gland tissue (Figure 5C). Compared with the sucrose hydrolase function of BmSUC1 in the midgut, BmSUC1 may mainly function as a glycosyltransferase in the silk gland. Glycosyltransferases play an important role in the process of protein glycosylation. Therefore, we speculate that the post-translational modification of the mutant silkworm Ser1 has changed. In particular, glycosylation modifications are more common in silk fibroin. For example, both silk fibroin P25 and Fib-L proteins have glycosylation modifications and lead to protein bands of different molecular weights [38]. However, the specific process of BmSUC1 involved in Ser1 protein synthesis and possibly involved in Ser1 post-translational modification processing in silkworms remains unclear and awaits further study.
Cocoon silk is mainly composed of fibroin and sericin proteins, and the composition of the protein determines the performance of the silk [30]. For example, by overexpressing BmOsiris9a in the silk gland, Cheng et al. found that the crystallinity of cocoon silk was reduced and the mechanical properties of silk fibers were better [39]. In the present study, deletion of BmSuc1 caused a decrease in sericin content in the cocoon shell and a decrease in the breaking strength of raw silk (Figure 8). It is generally believed that fibroin plays a dominant role in the mechanical properties of silk, while the contribution of sericin to the mechanical properties is generally negligible [40]. However, sericin in raw silk has a certain contribution to the mechanical properties of raw silk, and it was found that the breaking strength decreased by 20% when sericin was completely removed [41]. Studies have confirmed that sericin has low crystallinity, high fluidity, and high hydrophilicity. After the sericin is completely removed, the mechanical properties of the raw silk decrease. When a certain amount of sericin is retained in the raw silk, the sericin acts as a matrix in it, which can be regarded as a kind of fiber with multiple silk fibroin fibers as reinforcement materials [42]. The results of this study indicated that the deletion of BmSuc1 resulted in the reduction of sericin content in silk fibers, which in turn negatively affected the mechanical properties of silk fibers.
In conclusion, BmSUC1 contributes to the normal synthesis of Ser1 protein in silk glands and plays an important role in maintaining the homeostasis of silk protein content in silk fibers and the mechanical properties of silk fibers. However, it is still unclear whether the loss of BmSUC1, as we have speculated, hinders the glycosylation modification of the Ser1 protein, resulting in the reduction of the molecular weight of part of Ser1, which requires further experiments to determine. Therefore, in the following research, we will have a deeper understanding of the relationship between BmSUC1 and Ser1, hoping to further reveal the molecular regulation mechanism of BmSUC1 acting on Ser1.
4. Materials and Methods
4.1. Silkworms and Vectors
The multivoltine and monophagous B. mori strain, Nistari, was used as the basic transgenic strain. Silkworm larvae were fed fresh mulberry leaves and maintained at 25 °C under standard conditions [43]. The pMD19T plasmid was purchased from TaKaRa (Dalian, China). The PUC57 plasmid was purchased from TSINGKE Biological Technology (Nanjing, China). The pET32a plasmid, pET24b-BmSuc1 recombinant plasmid, and anti-BmSUC1 polyclonal antibody were maintained in our laboratory. The plasmid PXL-IE1-DsRed2-U6-U6 (Sal I, Nhe I), containing the B. mori U6 promoter, was used to construct the sgRNA expression vector.
4.2. RNA Isolation, cDNA Synthesis, and PCR
Total RNA was extracted from silk gland tissues on the fifth instar larval using TRIzol reagent (Sangon, Shanghai, China) according to the manufacturer’s instructions. Subsequently, the RNA was treated with DNase I (TaKaRa, Dalian, China) to remove genomic DNA. For reverse transcription, the ReverAid First Strand cDNA Synthesis Kit (Sangon, Shanghai, China) was used with 1 μg of total RNA according to the manufacturer’s instructions. Reverse transcription PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR) methods were performed to analyse the transcription level of BmSuc1 in silk gland tissues of silkworm larvae. The cycling conditions of RT-PCR were 95 °C for 5 min, followed by 29 cycles of 95 °C for 10 s, 51 °C for 20 s, and 72 °C for 20 s. PCR products were separated by 1.0% agarose gel electrophoresis. qRT-PCR was conducted using the CFX96 system (Bio-Rad, Hercules, CA, USA) and SYBR green PCR master mix (Bio-Rad, Hercules, CA, USA). Each qRT-PCR was performed under the following conditions: denaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 10 s, 51 °C for 15 s, and 72 °C for 20 s. The 2−∆∆Ct method [44] was used to calculate the relative expression level of the target gene. B. mori BmActin3 was used as an internal control. The primers used in RT-PCR and qRT-PCR are listed in Table S1 (Invitrogen, Shanghai, China). All experiments were performed in triplicate and repeated three times independently.
4.3. Western Blot
Total protein was extracted from different tissues of larvae using the Tissue or Cell Total Protein Extraction Kit (Sangon, Shanghai, China) [2]. Extracted total protein or purified recombinant protein were separated, transferred, and analyzed by immunoblotting as described in previous reports [2,45]. The anti-β-Actin or the anti-BmSUC1 rabbit polyclonal antibodies prepared in our previous study [32] was diluted to 1:2500 or 1:500 and in 5% (v/v) skimmed milk in PBST, and the secondary antibody of goat anti-rabbit IgG conjugated with HRP (Sangon, Shanghai, China) were diluted 1:5000 in the same blocking solution. The final detection was performed by using HRP-DAB Horseradish Peroxidase Color Development Kit (Sangon, Shanghai, China). Other primary antibodies used in this study included anti-BmSer1 (1:10,000), anti-BmOsiri9a (1:10,000), and anti-BmP25 (1:5000) [21,39,46].
4.4. Investigation of the Developmental Pattern of the Silk Gland in Wild-Type Silkworm Larvae
To evaluate the developmental pattern of the silk gland in silkworm larvae, 3 individuals of the Nistari strain were randomly selected every day (24 h) from the newly molted fifth instar to the wandering stage. The total larval bodies were first weighed, then the silk glands isolated from 3 larvae were weighed. The larvae were fed fresh mulberry leaves in large quantities every day during this period. Daily total larval body or silk gland weights were averaged and compared.
4.5. Plasmid Construction
According to the target design principle of the CRISPR/Cas9 system GN19 + NGG (protospacer adjacent motif (PAM) recognition site, not involved in constituting sgRNA sequence) [47], two sgRNAs (siRNAs) were designed based on the open reading frame (ORF) of BmSuc1 (GenBank: AB366559.1) on an online prediction website (www.crispr.dbcls.jp accessed on 28 July 2019). The sequences are shown in Table S2. The sgRNA was synthesized by TSINGKE Biological Technology (Nanjing, China), and the sgRNA product was cloned into PUC57 according to the instructions. Next, the sgRNA1 fragment was digested with Sal I restriction endonuclease and ligated into PXL-IE1-DsRed2-U6-U6 (Sal I digested) vector by T4 ligase at 16 °C for 2 h, and the recombinant plasmid was named PXL-IE1-DsRed2-U6-sgRNA1-U6. The sgRNA2 fragment was then digested with Nhe I restriction endonuclease and ligated into the PXL-IE1-DsRed2-U6-sgRNA1-U6 (Nhe I digested) vector by T4 ligase at 16 °C for 2 h, and the recombinant plasmid was named PXL-IE1-DsRed2-U6-sgRNA1-U6-sgRNA2. The primers used are listed in Table S1.
4.6. Silkworm Germline Transformation and Mutagenesis Analysis
For silkworm germline transformation, G0 preblastodermal Nistari embryos were injected with a mixture of transformation plasmids and helper plasmids, followed by incubation in a humidified chamber at 25 °C and 75% relative humidity for 10–12 days until larvae hatching [28,48]. Hatched larvae are raised to adults and sib-mated or backcrossed with WT adults. Next, G1 progeny was scored for the presence of the marker gene at the embryonic or newly-hatched silkworm stage under a fluorescence microscope (OLYMPUS DP72, Tokyo, Japan). For the transgenic CRISPR/Cas9 system, the Nos-Cas9 line (donated by Shanghai Institute of Plant Physiology and Biochemistry) was crossed with the U6-BmSuc1-sgRNA line as previously described [49]. Subsequently, genomic PCR and sequencing were performed using BmSuc1 ORF primers to identify BmSuc1 mutant alleles. The primers used are listed in Table S1.
4.7. Screening Strategy and Establishment of Homozygous Mutant Strains and Phenotype Screening
To establish stable homozygous mutant lines, Nos-Cas9:U6-BmSuc-sgRNA (F1) somatic mutants were backcrossed to WT moths, and PCR was used to identify heterozygous F2 mutant animals (lacking fluorescence). The extraction method of animal genomic DNA refers to the previous method [26]. The selected F2 mutants were again backcrossed with WT moths, and the offspring of the cross would be approximately 50% heterozygotes and 50% WT animals. The F3 heterozygous animals were then sib-mated, and the offspring of the cross will yield approximately 25% WT, 50% heterozygous mutants, and 25% homozygous mutant animals. Finally, F4 homozygous mutants were then sib-mated to obtain 100% homozygous mutant animals for subsequent experiments. Details about the hybridization process are shown in Figure S1C.
To investigate the growth and development of silkworm larvae, 20 individuals from the WT or CRISPR/Cas9-mediated mutants were randomly selected from a group (3 groups each) and fed abundantly with fresh mulberry leaves. At the fifth instar stage, each larva was weighed every 24 h intervals until the wandering stage. The individual weight of daily larvae was compared among strains.
4.8. Cloning, Expression, and Purification of Mutant and WT BmSUC1
DNAMAN software was used to predict the amino acids of the protein encoded by the mutant BmSuc1 (ΔBmSuc1) gene. Genetyx_version 7 software (Version 7.03, Genetyx Corporation, Tokyo, Japan) was used to overlap amino acid sequences. To obtain the ΔBmSUC1 protein for biochemical analysis, the ORF of ΔBmSuc1 was amplified by PCR, using specific primers designed according to the cDNA sequence of ΔBmSuc1 and the mutant silk gland cDNA as the template. The PCR product was inserted into a pET32a-derived vector and transformed into E. coli BL21 (DE3) strain. Recombinant ΔBmSUC1 was expressed and purified in E. coli as described by Guo et al. [23]. The WT protein BmSUC1 was expressed using the pET24b-BmSuc1 recombinant plasmid maintained in our laboratory [2,32] and was expressed and purified in the same manner as the mutant protein. The purity of the eluted protein was assessed by SDS-PAGE and detected by western blot using the anti-histidine antibody (1:2500), and the protein samples were stored at 80 °C until use. The primers used are shown in Supplementary Table S1.
4.9. Sucrase Activity Assays
The hydrolytic activity of sucrase on total protein or purified recombinant protein was determined according to the method of Daimon et al. [10] with some modifications, and the detailed reaction procedure was as previously described [2,32]. Reactions without enzyme addition served as controls. In addition, 0.25% DNJ (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to the sucrose substrate to inhibit the activity of α-glucosidase in total protein. All experiments were performed independently in triplicate. One unit of enzyme is defined as the amount of enzyme per μg protein that catalyzes the production of 1 mmol glucose per minute (mmol/min/μg).
4.10. Native PAGE and Mass Spectrometry Analysis
Total ASG and MSG luminal proteins extracted from wandering stage silkworm larvae of mutant line 1 (L1) and WT lines were separated by 15% native PAGE. Then three differential protein bands (WT: 1; mutant L1: 2, 3) were excised from the native PAGE for mass spectrometry analysis in Beijing Huada Protein Research and Development Center Co., Ltd. (Huada, Beijing, China). Samples were digested by trypsin overnight at 37 °C and analyzed on the mass spectrometer (Q Exactive, Thermo-Fisher, Waltham, MA, USA). Raw data from mass spectrometry was used to identify peptides by searching against the silkworm proteins downloaded from the GenBank database using the software MasCot (version 2.2; Matrix Science, London, UK).
4.11. Extraction of Sericin from Silkworm Cocoon Shell
The cocoons used in this study were harvested from the rearing of mutant and WT silkworms and were kept at room temperature in a dry fume hood before use. To extract sericin, cocoon shells from WT or mutants were first washed with warm water to remove the spoils, dried at 60 °C (6–8 h), and cut into 1 cm2 pieces. Two grams of cocoon shell fragments and 200 mL of distilled water were added to a 125 °C-sterilization pot for 2.5 h to separate sericin. After filtering with gauze, the raw silk was washed with 100 mL of warm water 3 times, the washing solution was mixed with degumming liquid, and the solution was filtered with filter paper. The sericin solution was then rotary evaporated and concentrated in vacuo at 125 rpm, 60 °C, and a pressure of 0.1 MPa. The sample is concentrated to a volume of about 100 mL, pre-cooled (−80 °C, 2 h), and dried with a vacuum freeze dryer (sample temperature −33 °C, pressure 1 Pa) until it freezes into powder. The sericin content was calculated after weighing the powder. Three replicates were used for each silkworm line.
4.12. Mechanical Properties of Silk
The mutant silkworm and WT silkworm cocoons were reeled in the same way. The size of cocoon filament (WT: 1.381 deniers; mutant L1: 1.606 deniers) was determined by the fixed-length weighing method [50,51], and the number of cocoon filament silk fiber strands (WT: 15 strands; mutant L1: 13 strands) constituting the raw silk were determined according to the target cocoon filament size (about 21 D). The tensile sample tests were performed under ambient conditions using a constant rate elongation tester (CRE) with a gauge length of 50 mm and a constant speed of 150 mm/min of moving gripper movement. The strength reading accuracy was ≤0.01 kg (0.1 N), and the elongation reading accuracy was ≤0.1%. For each group, statistical analysis was performed using 20 ± 5 measurements with 3 replicates. Elongation at break, breaking force, and breaking strength were calculated.
4.13. Statistical Analysis
Data are presented as mean standard deviation (SD). Differences between groups were examined by a two-tailed Student t-test. Differences were considered statistically significant when the p-value was less than 0.05. Statistically significant differences were indicated with ‘*’ or ‘a, b, c’.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23179891/s1.
Author Contributions
Conceptualization, L.Y., Y.Z. and Y.M.; methodology, L.Y., Y.Z. and S.J.; software, L.Y., R.S. and R.X.; validation, Y.Z. and R.X.; formal analysis, L.Y.; investigation, L.Y., D.L. and Y.M.; resources, Y.M.; data curation, L.Y., Q.G. and Y.M.; writing original draft preparation, L.Y. and Q.G.; writing review and editing, L.Y. and Y.M.; visualization, L.Y., Y.Z. and R.S.; supervision, S.J. and Y.M.; project administration, Y.M.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 31872423), the International Science & Technology Cooperation Plan of Anhui Province (Grant No. 2022h11020020) and the Innovative Research Groups of Anhui Agricultural University (Grant No. ANRC2019032) to Y.M.
Acknowledgments
We want to thank Anjiang Tan (Jiangsu University of Science and Technology, China) for his warm assistance in silkworm egg microinjection, and thank Chun Liu (Southwest University, China) for her warm assistance in providing silk protein antibodies of anti-BmSer1, anti-BmOsiris9a, and anti-BmP25, and thank Jiangchao Song (Jiangsu University of Science and Technology) for his warm assistance in measuring the mechanical properties of silk fibers.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| β-FFase | β-fructofuranosidase |
| DNJ | 1-deoxynojirimycin |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| Cas9 | CRISPR-associated protein-9 nuclease |
| Ser1 | Sericin1 |
| FOS | fructooligosaccharides |
| D-AB1 | 1,4-dideoxy-1,4-imino-D-arabinitol |
| ASG | anterior silk gland |
| MSG | middle silk gland |
| PSG | posterior silk gland |
| sgRNA | small guide RNA |
| PAM | protospacer adjacent motif |
| Nos-Cas9 | pBac[IE1-EGFP-Nos-Cas9] |
| U6-sgRNA | pBac[IE1-DsRed2-U6-BmSuc1-sgRNA] |
| RT-PCR | Reverse transcription PCR |
| qRT-PCR | quantitative real-time PCR |
| anti-BmSUC1 | the antibody against BmSUC1 |
| anti-β-actin | the antibody against β-actin |
| anti-BmSer1 | the antibody against BmSer1 |
| anti-BmOsiri9a | the antibody against BmOsiri9a |
| anti-BmP25 | the antibody against BmP25 |
| ΔBmSuc1 | the mutant BmSuc1 gene |
| WT | wild-type |
| L1 | BmSuc1 homozygous mutant strains Line1 |
| L2 | BmSuc1 homozygous mutant strains Line2 |
| CRE | constant rate elongation tester |
| 5L0D | 0 day of 5th instar |
| 5L1D | 1st day of 5th instar |
| 5L3D | 3rd day of 5th instar |
References
- Li, X.T.; Shi, L.G.; Zhou, Y.Y.; Xie, H.Q.; Dai, X.P.; Li, R.Q.; Chen, Y.Y.; Wang, H.B. Molecular evolutionary mechanisms driving functional diversification of α-glucosidase in Lepidoptera. Sci. Rep. 2017, 7, 45787. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Zhang, X.W.; Zhang, D.B.; Shi, L.; Zhou, Y.; Sun, T.T.; Jiang, S.; Gao, J.S.; Meng, Y. BmSUC1 is essential for glycometabolism modulation in the silkworm, Bombyx mori. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Park, E.Y. Structure–function analysis of silkworm sucrose hydrolase uncovers the mechanism of substrate specificity in GH13 subfamily 17 exo -α-glucosidases. J. Biol. Chem. 2020, 295, 8784–8797. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.; Bignon, C.; Sulzenbacher, G.; Henrissat, B.; Czjzek, M. The Three-dimensional Structure of Invertase (β-Fructosidase) from Thermotoga maritima Reveals a Bimodular Arrangement and an Evolutionary Relationship between Retaining and Inverting Glycosidases. J. Biol. Chem. 2004, 279, 18903–18910. [Google Scholar] [CrossRef]
- Mercedes, R.E.; María, G.P.; Beatriz, G.; Dolores, L.; Zoran, M.; María, F.L.; Julia, S.A. Structural Analysis of β-Fructofuranosidase from Xanthophyllomyces dendrorhous Reveals Unique Features and the Crucial Role of N-Glycosylation in Oligomerization and Activity. J. Biol. Chem. 2016, 291, 6843–6857. [Google Scholar]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Production of fructo-oligosaccharides by fructosyl transferase from Aspergillus oryzae CFR 202 and Aureobasidium pullulans CFR 77. Process Biochem. 2004, 39, 755–760. [Google Scholar] [CrossRef]
- Krasikov, V.V.; Karelov, D.V.; Firsov, L.M. alpha-Glucosidases. Biochemistry 2001, 66, 267–281. [Google Scholar]
- Carneiro, C.N.B.; Isejima, E.M.; Samuels, R.I.; Silva, C.P. Sucrose hydrolases from the midgut of the sugarcane stalk borer Diatraea saccharalis. J. Insect Physiol. 2004, 50, 1093–1101. [Google Scholar] [CrossRef]
- Santos, C.D.; Terra, W.R. Midgut α-glucosidase and β-fructosidase from Erinnyis ello larvae and imagoes: Physical and kinetic properties. Insect Biochem. 1986, 16, 819–824. [Google Scholar] [CrossRef]
- Daimon, T.; Taguchi, T.; Meng, Y.; Katsuma, S.; Mita, K.; Shimada, T. Beta-fructofuranosidase genes of the silkworm, Bombyx mori: Insights into enzymatic adaptation of B. mori to toxic alkaloids in mulberry latex. J. Biol. Chem. 2008, 283, 15271–15279. [Google Scholar] [CrossRef]
- Pauchet, Y.; Muck, A.; Svatos, A.; Heckel, D.G.; Preiss, S. Mapping the larval midgut lumen proteome of Helicoverpa armigera, a generalist herbivorous insect. J. Proteome Res. 2008, 7, 1629–1639. [Google Scholar] [CrossRef]
- Pauchet, Y.; Wilkinson, P.; Vogel, H.; Nelson, D.R.; Reynolds, S.E.; Heckel, D.G.; Ffrench-Constant, R.H. Pyrosequencing the Manduca sexta larval midgut transcriptome: Messages for digestion, detoxification and defence. Insect Mol. Biol. 2010, 19, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Yuen, M.M.S.; Liao, N.Y.; Docking, T.R.; Chan, S.K.; Taylor, G.A.; Palmquist, D.L.; Jackman, S.D.; Nguyen, A.; Li, M.; et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 2013, 14, R27. [Google Scholar] [CrossRef] [PubMed]
- Pedezzi, R.; Fonseca, F.P.P.; Júnior, C.D.S.; Kishi, L.T.; Terra, W.R.; Henrique-Silva, F. A novel β-fructofuranosidase in Coleoptera: Characterization of a β-fructofuranosidase from the sugarcane weevil, Sphenophorus levis. Insect Biochem. Mol. Biol. 2014, 55, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Doucet, D.; Mittapalli, O. Characterization of horizontally transferred β-fructofuranosidase (ScrB) genes in Agrilus planipennis. Insect Mol. Biol. 2015, 23, 821–832. [Google Scholar] [CrossRef]
- Xin, H.H.; Zhang, D.P.; Chen, R.T.; Cai, Z.Z.; Lu, Y.; Liang, S.; Miao, Y.G. TRANSCRIPTION FACTOR Bmsage PLAYS A CRUCIAL ROLE IN SILK GLAND GENERATION IN SILKWORM, Bombyx mori. Arch. Insect Biochem. Physiol. 2015, 90, 59–69. [Google Scholar] [CrossRef]
- Gamo, T.; Inokuchi, T.; Laufer, H. Polypeptides of fibroin and sericin secreted from the different sections of the silk gland in Bombyx mori. Insect Biochem. 1977, 7, 285–295. [Google Scholar] [CrossRef]
- Li, J.Y.; Ye, L.P.; Che, J.Q.; Song, J.; You, Z.Y.; Yun, K.C.; Wang, S.H.; Zhong, B.X. Comparative proteomic analysis of the silkworm middle silk gland reveals the importance of ribosome biogenesis in silk protein production. J. Proteom. 2015, 126, 109–120. [Google Scholar] [CrossRef]
- Guo, P.C.; Dong, Z.M.; Xiao, L.; Li, T.; Zhang, Y.; He, H.W.; Xia, Q.Y.; Zhao, P. Silk gland-specific proteinase inhibitor serpin16 from the Bombyx mori shows cysteine proteinase inhibitory activity. Biochem. Biophys. Res. Commun. 2015, 457, 31–36. [Google Scholar] [CrossRef]
- Smith, C.R.; Morandin, C.; Noureddine, M.; Pant, S. Conserved roles of Osiris genes in insect development, polymorphism and protection. J. Evol. Biol. 2018, 31, 516–529. [Google Scholar] [CrossRef]
- Liu, C.; Hu, W.B.; Cheng, T.C.; Peng, Z.C.; Mita, K.; Xia, Q.Y. Osiris9a is a major component of silk fiber in lepidopteran insects. Insect Biochem. Mol. Biol. 2017, 89, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Zhao, P.; Zhang, Y.; Song, Q.R.; Zhang, X.L.; Guo, P.C.; Wang, D.D.; Xia, Q.Y. Analysis of proteome dynamics inside the silk gland lumen of Bombyx mori. Sci. Rep. 2016, 6, 21158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, P.C.; Wang, Q.; Wang, Z.; Dong, Z.M.; He, H.W.; Zhao, P. Biochemical characterization and functional analysis of invertase Bmsuc1 from silkworm, Bombyx mori. Int. J. Biol. Macromol. 2018, 107, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Morimoto, T.; Matsuura, S.; Nagata, S. Studies on the posterior silk gland of the silkworm, Bombyx mori. I. Growth of posterior silk gland cells and biosynthesis of fibroin during the fifth larval instar. J. Cell Biol. 1968, 38, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.R.; Kafatos, F.C. Developmentally Regulated Genes in Silkmoths. Annu. Rev. Genet. 1984, 18, 443–487. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, S.S.; Niu, B.L.; Ji, D.F.; Liu, X.J.; Li, M.W.; Bai, H.; Palli, S.; Wang, C.Z.; Tan, A.J. A determining factor for insect feeding preference in the silkworm, Bombyx mori. PLoS Biol 2019, 17, e3000162. [Google Scholar] [CrossRef]
- Xu, J.; Chen, S.Q.; Zeng, B.S.; James, A.; Huang, Y.P. Bombyx mori P-element Somatic Inhibitor (BmPSI) Is a Key Auxiliary Factor for Silkworm Male Sex Determination. PLoS Genet. 2017, 13, e1006576. [Google Scholar] [CrossRef]
- Tan, A.J.; Fu, G.L.; Jin, L.; Guo, Q.H.; Li, Z.Q.; Niu, B.L.; Meng, Z.Q.; Morrison, N.I.; Alphey, L.; Huang, Y.P. Transgene-based, female-specific lethality system for genetic sexing of the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2013, 110, 6766–6770. [Google Scholar] [CrossRef]
- Li, Z.Q.; You, L.; Zeng, B.S.; Ling, L.; Xu, J.; Chen, X.; Zhang, Z.J.; Palli, S.R.; Huang, Y.P.; Tan, A.J. Ectopic expression of ecdysone oxidase impairs tissue degeneration in Bombyx mori. Proc. Biol. Sci. 2015, 282, 20150513. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Li, S.; Feng, Q.L. Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu. Rev. Entomol. 2014, 59, 513–536. [Google Scholar] [CrossRef]
- Zhao, X.M.; Liu, C.; Li, Q.Y.; Hu, W.B.; Zhou, M.T.; Nie, H.Y.; Zhang, Y.X.; Peng, Z.C.; Zhao, P.; Xia, Q.Y. Basic helix-loop-helix transcription factor Bmsage is involved in regulation of fibroin H-chain gene via interaction with SGF1 in Bombyx mori. PLoS ONE 2014, 9, e94091. [Google Scholar]
- Gan, Q.; Li, X.; Zhang, X.W.; Wu, L.L.; Ye, C.J.; Wang, Y.; Gao, J.S.; Meng, Y. D181A Site-Mutagenesis Enhances Both the Hydrolyzing and Transfructosylating Activities of BmSUC1, a Novel β-Fructofuranosidase in the Silkworm Bombyx mori. Int. J. Mol. Sci. 2018, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.; Hernández, L.; Batista, F.R.; Chinea, G. Prediction of a common beta-propeller catalytic domain for fructosyltransferases of different origin and substrate specificity. Protein Sci. 2010, 9, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.P.; Kiuchi, T.; Zhou, Y.Y.; Jia, S.Z.; Xu, Y.S.; Katsuma, S.; Shimada, T.; Wang, H.B. Horizontal Gene Transfer and Gene Duplication of β-Fructofuranosidase Confer Lepidopteran Insects Metabolic Benefits. Mol. Biol. Evol. 2021, 38, 2897–2914. [Google Scholar] [CrossRef]
- Yukuhiro, K.; Sezutsu, H.; Tsubota, T.; Takasu, Y.; Yonemura, N. Insect Silks and Cocoons: Structural and Molecular Aspects. In Extracellular Composite Matrices in Arthropods; Springer: Cham, Switzerland, 2016; pp. 515–555. [Google Scholar]
- Hernalsteens, S.; Maugeri, F. Purification and characterisation of a fructosyltransferase from Rhodotorula sp. Appl. Microbiol. Biotechnol. 2008, 79, 589–596. [Google Scholar] [CrossRef]
- Miguel, L.B.; Sainz-Polo, M.A.; David, G.P.; Beatriz, G.; Plou, F.J.; María, F.L.; Julia, S.A. Structural and Kinetic Insights Reveal That the Amino Acid Pair Gln-228/Asn-254 Modulates the Transfructosylating Specificity of Schwanniomyces occidentalis β-Fructofuranosidase, an Enzyme That Produces Prebiotics. J. Biol. Chem. 2012, 287, 19674–19686. [Google Scholar]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-chain, L-chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Cheng, T.C.; Zhang, X.; Peng, Z.C.; Fan, Y.F.; Zhang, L.; Liu, C. Effects of Osiris9a on Silk Properties in Bombyx mori Determined by Transgenic Overexpression. Int. J. Mol. Sci. 2020, 21, 1888. [Google Scholar] [CrossRef]
- Pérez-Rigueiro, J.; Viney, C.; Llorca, J.; Elices, M. Mechanical properties of single-brin silkworm silk. J. Appl. Polym. Sci. 2000, 75, 1270–1277. [Google Scholar] [CrossRef]
- Jauzein, V.; Bunsell, A. Bio-composite aspects of silk: The sericin sheath acting as a matrix. J. Mater. Sci. 2012, 47, 3082–3088. [Google Scholar] [CrossRef]
- Xie, Q.F.; Hu, B.H.; Yang, M.Y.; Zhu, L.J. A Review on Mechanical Properties of Silkworm Cocoon, Cocoon Filament and RawSilk. Sci. Sericul. 2015, 6, 1120–1126. [Google Scholar]
- Daimon, T.; Katsuma, S.; Iwanaga, M.; Kang, W.K.; Shimada, T. The BmChi-h gene, a bacterial-type chitinase gene of Bombyx mori, encodes a functional exochitinase that plays a role in the chitin degradation during the molting process. Insect Biochem. Mol. Biol. 2005, 35, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2013, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Gong, M.X.; Shu, R.; Li, X.; Gao, J.S.; Meng, Y. Molecular and enzymatic characterization of two enzymes BmPCD and BmDHPR involving in the regeneration pathway of tetrahydrobiopterin from the silkworm Bombyx mori. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 186, 20–27. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zheng, H.S.; Fan, Y.F.; Liu, C. Identification and location of sericin in silkworm with anti-sericin antibodies. Int. J. Biol. Macromol. 2021, 184, 522–529. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Tan, A.J.; Tanaka, H.; Tamura, T.; Shiotsuki, T. Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc. Natl. Acad. Sci. USA 2005, 102, 11751–11756. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Liu, X.; Shiotsuki, T.; Wang, Z.S.; Xu, X.; Huang, Y.P.; Li, M.W.; Li, K.; Tan, A.J. Depletion of juvenile hormone esterase extends larval growth in Bombyx mori. Insect Biochem. Mol. Biol. 2017, 81, 72–79. [Google Scholar] [CrossRef]
- Nakamae, K.; Nishino, T.; Ohkubo, H. Elastic modulus of the crystalline regions of silk fibroin. Polymer 1989, 30, 1243–1246. [Google Scholar] [CrossRef]
- Peng, Z.C.; Liu, C.; Zhang, L.; Li, W.; Hu, W.B.; Ma, S.Y.; Xia, Q.Y. A Simple Method for the Cross-Section Area Determination of Single Profiled Fibers and Its Application. Microsc. Microanal. 2018, 24, 17–28. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).