Critical Players and Therapeutic Targets in Chronic Itch
Abstract
:1. Introduction
2. Neuropeptide Targets in Itch Transmission
2.1. CGRP
2.2. SP
2.3. BNP
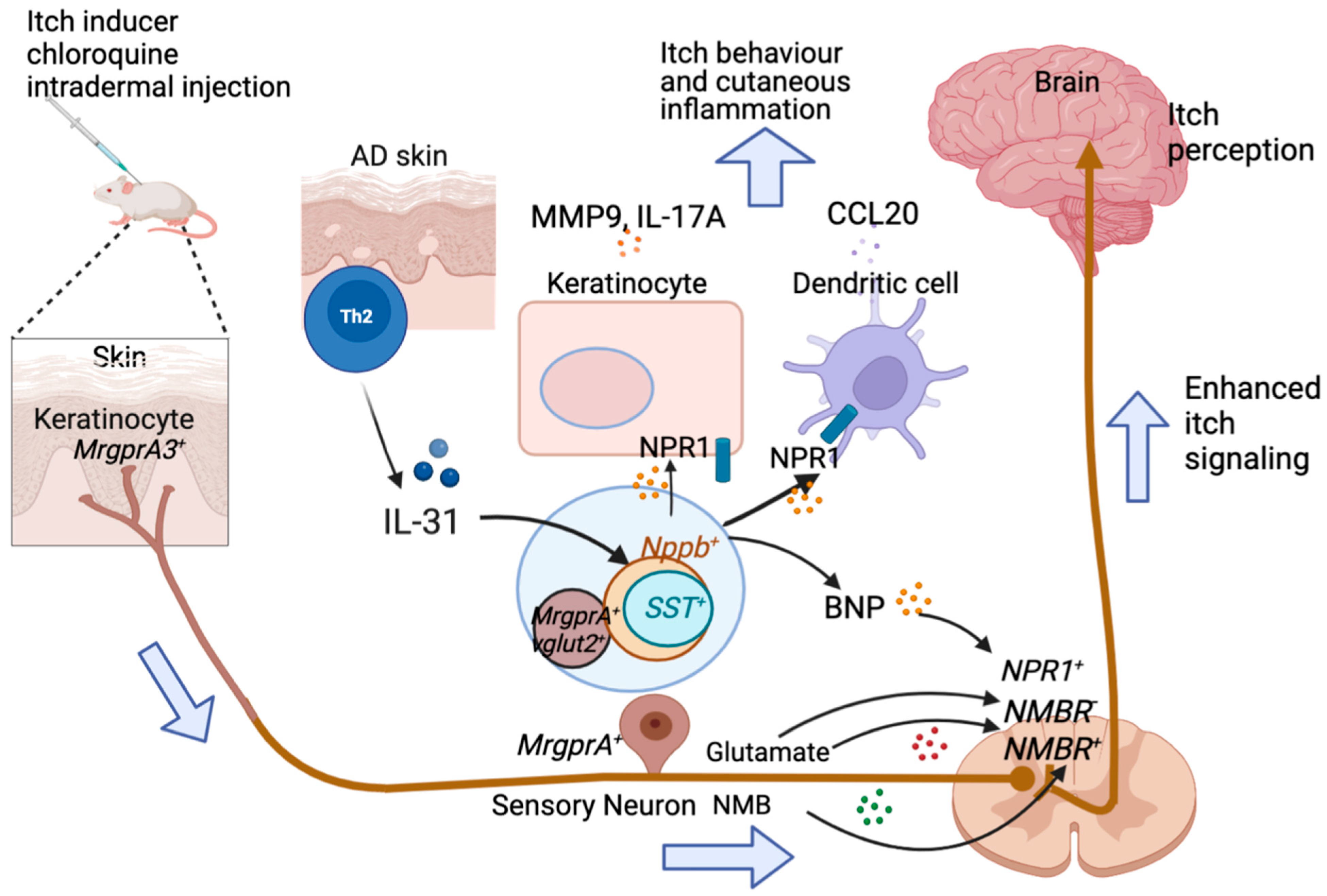
2.4. GRP
2.5. NMB
2.6. SST
2.7. Endothelin-1 (ET-1)
3. Itch Targets in G-Protein-Coupled Receptor Pathways
3.1. Histamine
3.2. Proteases, Tryptase, and Kallikreins (KLK) 5 and 14
3.3. Mas-Related G-Protein Coupled Receptors (Mrgprs)
3.4. 5-Hydroxytryptamine (5-HT, Serotonin)
3.5. Leukotrienes
4. Cytokine and Chemotactic Factor Induced Itch
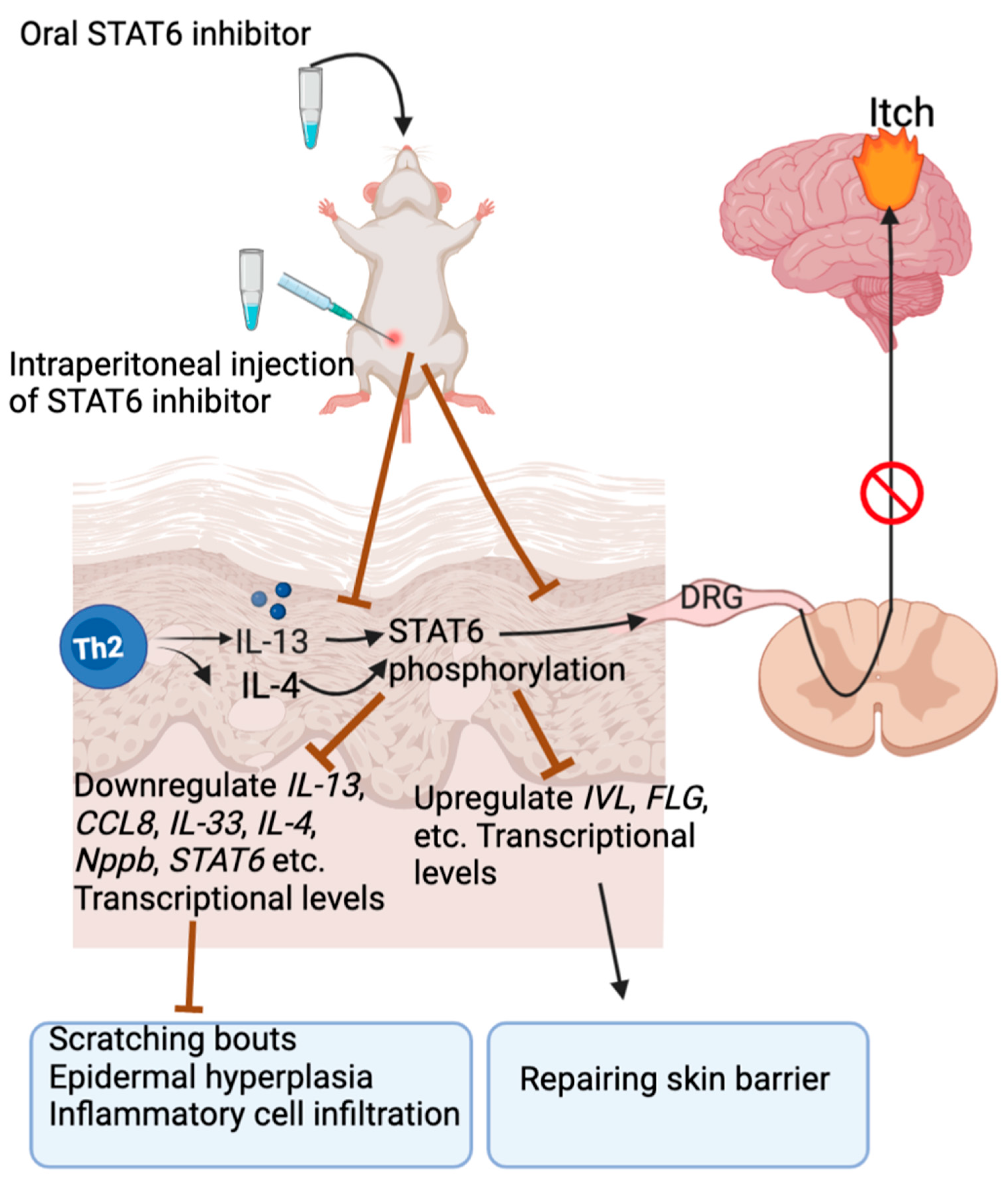
4.1. IL-13
4.2. IL-31
4.3. IL-33
4.4. IL-6
4.5. IL-2
4.6. TSLP
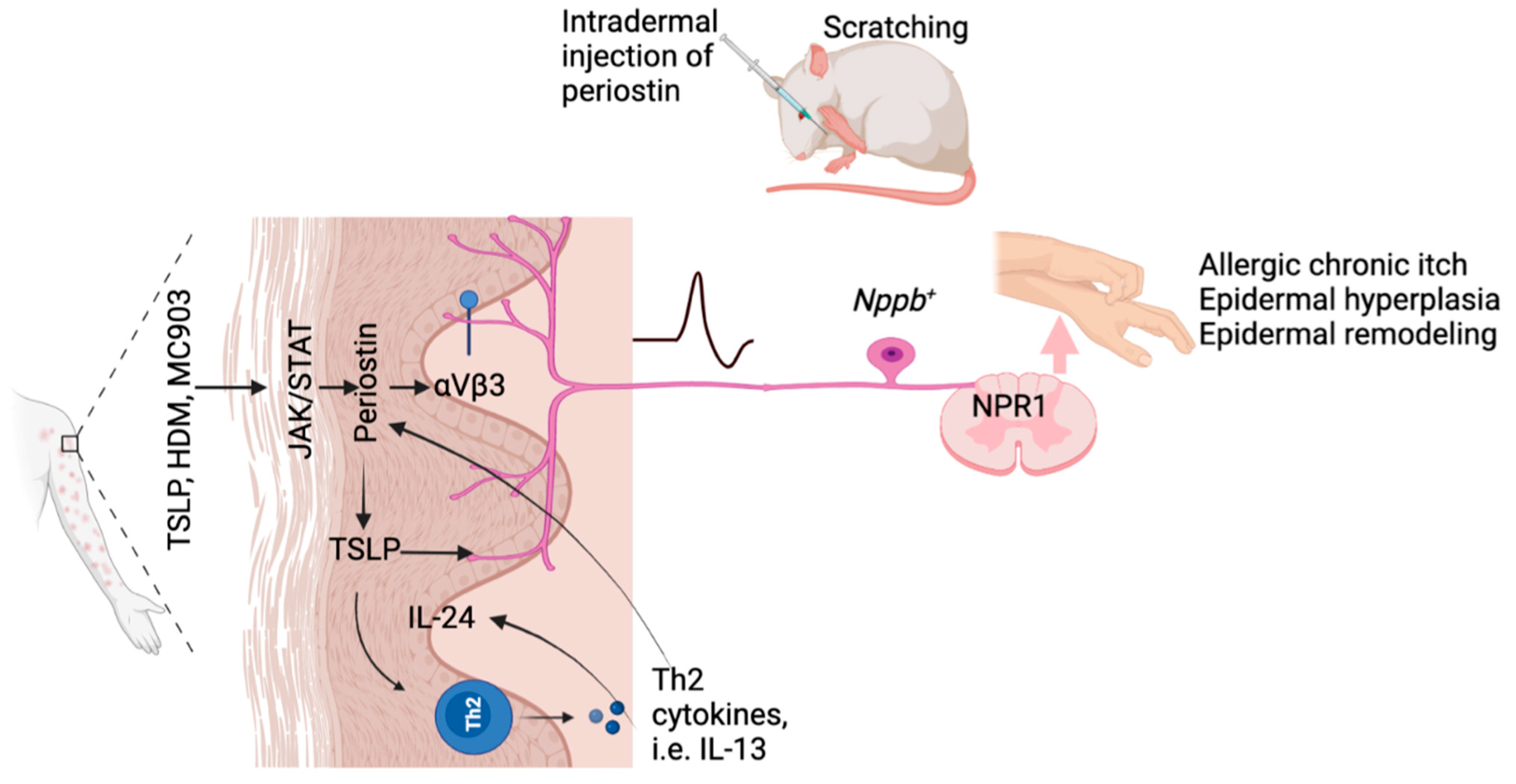
4.7. Periostin
4.8. Lipocalin-2 (LCN2)
4.9. Serpin E1
4.10. Oncostatin M (OSM)
4.11. C-X-C Motif Chemokine Ligand 10 (CXCL10)
4.12. Chemokine C-C Motif Chemokine 2 (CCL2)
5. Research Progress on Itch Therapeutical Development
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Mu, D.; Deng, J.; Liu, K.F.; Wu, Z.Y.; Shi, Y.F.; Guo, W.M.; Mao, Q.Q.; Liu, X.J.; Li, H.; Sun, Y.G. A central neural circuit for itch sensation. Science 2017, 357, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chen, W.; Wang, J. Interventions in the B-type natriuretic peptide signalling pathway as a means of controlling chronic itch. Br. J. Pharmacol. 2020, 177, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Eedy, D.J.; Johnston, C.F.; Shaw, C.; Buchanan, K.D. Neuropeptides in psoriasis: An immunocytochemical and radioimmunoassay study. J. Investig. Dermatol. 1991, 96, 434–438. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Wen, Y.; Zeng, L.; Li, Y.; Tao, T.; Zhao, Z.; Tao, A. Spinal GRPR and NPRA Contribute to Chronic Itch in a Murine Model of Allergic Contact Dermatitis. J. Investig. Dermatol. 2020, 140, 1856–1866.e7. [Google Scholar] [CrossRef]
- Andoh, T.; Kuwazono, T.; Lee, J.B.; Kuraishi, Y. Gastrin-releasing peptide induces itch-related responses through mast cell degranulation in mice. Peptides 2011, 32, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Tominaga, M.; Takamori, K.; Carstens, M.I.; Carstens, E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 2014, 155, 80–92. [Google Scholar] [CrossRef]
- Tseng, P.Y.; Hoon, M.A. Interactions of the NeuroImmuneStromal Triad in Itch. J. Investig. Dermatol. 2022, 142, 42–46. [Google Scholar] [CrossRef]
- Bell, J.K.; McQueen, D.S.; Rees, J.L. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br. J. Pharmacol. 2004, 142, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Inami, Y.; Andoh, T.; Sasaki, A.; Kuraishi, Y. Topical surfactant-induced pruritus: Involvement of histamine released from epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2013, 344, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Datsi, A.; Steinhoff, M.; Ahmad, F.; Alam, M.; Buddenkotte, J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy 2021, 76, 2982–2997. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef]
- Gomes, L.O.; Hara, D.B.; Rae, G.A. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012, 91, 628–633. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef]
- Meng, J.; Moriyama, M.; Feld, M.; Buddenkotte, J.; Buhl, T.; Szöllösi, A.; Zhang, J.; Miller, P.; Ghetti, A.; Fischer, M.; et al. New mechanism underlying IL-31-induced atopic dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1677–1689.e8. [Google Scholar] [CrossRef]
- Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Tsuji, G.; Furue, K.; Hashimoto-Hachiya, A.; Furue, M. Scratching Counteracts IL-13 Signaling by Upregulating the Decoy Receptor IL-13Rα2 in Keratinocytes. Int. J. Mol. Sci. 2019, 20, 3324. [Google Scholar] [CrossRef]
- Qu, L.; Fu, K.; Yang, J.; Shimada, S.G.; LaMotte, R.H. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain 2015, 156, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Cui, H.; Wang, T.; Shimada, S.G.; Sun, R.; Tan, Z.; Ma, C.; LaMotte, R.H. CCL2/CCR2 signaling elicits itch- and pain-like behavior in a murine model of allergic contact dermatitis. Brain Behav. Immun. 2019, 80, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Merrill, A.W.; Zanotto, K.; Carstens, M.I.; Carstens, E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J. Pharmacol. Exp. Ther. 2009, 329, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.B.; Shimada, S.G.; Sikand, P.; Lamotte, R.H.; Lerner, E.A. Cathepsin S elicits itch and signals via protease-activated receptors. J. Investig. Dermatol. 2010, 130, 1468–1470. [Google Scholar] [CrossRef]
- Reddy, V.B.; Iuga, A.O.; Shimada, S.G.; LaMotte, R.H.; Lerner, E.A. Cowhage-evoked itch is mediated by a novel cysteine protease: A ligand of protease-activated receptors. J. Neurosci. 2008, 28, 4331–4335. [Google Scholar] [CrossRef]
- Ui, H.; Andoh, T.; Lee, J.B.; Nojima, H.; Kuraishi, Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur. J. Pharmacol. 2006, 530, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.E.; van Dijk, R.; Leckie, P.; Schaap, F.G.; Kuiper, E.M.; Mettang, T.; Reiners, K.S.; Raap, U.; van Buuren, H.R.; van Erpecum, K.J.; et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology 2012, 56, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Haza, S.; Saito, A.; Kuraishi, Y. Involvement of leukotriene B4 in spontaneous itch-related behaviour in NC mice with atopic dermatitis-like skin lesions. Exp. Dermatol. 2011, 20, 894–898. [Google Scholar] [CrossRef]
- Andoh, T.; Nishikawa, Y.; Yamaguchi-Miyamoto, T.; Nojima, H.; Narumiya, S.; Kuraishi, Y. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J. Investig. Dermatol. 2007, 127, 2042–2047. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Petersen, M.B.; Benfeldt, E.; Jensen, S.B.; Serup, J. Scratch induction in the rat by intradermal serotonin: A model for pruritus. Acta Derm.-Venereol. 2001, 81, 250–254. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, C.; Yao, H.; Chen, O.; Rahman, S.; Gu, Y.; Zhao, J.; Huh, Y.; Ji, R.R. Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition. Brain J. Neurol. 2021, 144, 665–681. [Google Scholar] [CrossRef]
- Ostrowski, S.M.; Belkadi, A.; Loyd, C.M.; Diaconu, D.; Ward, N.L. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J. Investig. Dermatol. 2011, 131, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Albisetti, G.W.; Pagani, M.; Platonova, E.; Hosli, L.; Johannssen, H.C.; Fritschy, J.M.; Wildner, H.; Zeilhofer, H.U. Dorsal Horn Gastrin-Releasing Peptide Expressing Neurons Transmit Spinal Itch But Not Pain Signals. J. Neurosci. 2019, 39, 2238–2250. [Google Scholar] [CrossRef]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Wildner, H.; Yévenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Benke, D.; Yevenes, G.E. Chronic pain states: Pharmacological strategies to restore diminished inhibitory spinal pain control. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 111–133. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Schaer, D.J.; Zulewski, H.; Keller, U.; Müller, B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit. Care Med. 2004, 32, 1715–1721. [Google Scholar] [CrossRef]
- He, Y.; Ding, G.; Wang, X.; Zhu, T.; Fan, S. Calcitonin gene-related peptide in Langerhans cells in psoriatic plaque lesions. Chin. Med. J. 2000, 113, 747–751. [Google Scholar]
- Hou, Q.; Barr, T.; Gee, L.; Vickers, J.; Wymer, J.; Borsani, E.; Rodella, L.; Getsios, S.; Burdo, T.; Eisenberg, E.; et al. Keratinocyte expression of calcitonin gene-related peptide β: Implications for neuropathic and inflammatory pain mechanisms. Pain 2011, 152, 2036–2051. [Google Scholar] [CrossRef]
- Mantyh, P.W.; Gates, T.; Mantyh, C.R.; Maggio, J.E. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. J. Neurosci. 1989, 9, 258–279. [Google Scholar] [CrossRef]
- Granstein, R.D.; Wagner, J.A.; Stohl, L.L.; Ding, W. Calcitonin gene-related peptide: Key regulator of cutaneous immunity. Acta Physiol. 2015, 213, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Antúnez, C.; Torres, M.J.; López, S.; Rodriguez-Pena, R.; Blanca, M.; Mayorga, C.; Santamaría-Babi, L.F. Calcitonin gene-related peptide modulates interleukin-13 in circulating cutaneous lymphocyte-associated antigen-positive T cells in patients with atopic dermatitis. Br. J. Dermatol. 2009, 161, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.; Baran, E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 223–228. [Google Scholar] [CrossRef]
- McCoy, E.S.; Taylor-Blake, B.; Street, S.E.; Pribisko, A.L.; Zheng, J.; Zylka, M.J. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 2013, 78, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.; Klede, M.; Rukwied, R.; Lischetzki, G.; Neisius, U.; Skov, P.S.; Petersen, L.J.; Schmelz, M. Acute effects of substance P and calcitonin gene-related peptide in human skin—A microdialysis study. J. Investig. Dermatol. 2000, 115, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.M. Calcitonin gene-related peptide receptor antagonists: New therapeutic agents for migraine. J. Med. Chem. 2014, 57, 7838–7858. [Google Scholar] [CrossRef]
- Payan, D.G.; Brewster, D.R.; Goetzl, E.J. Specific stimulation of human T lymphocytes by substance P. J. Immunol. 1983, 131, 1613–1615. [Google Scholar]
- Raap, M.; Rüdrich, U.; Ständer, S.; Gehring, M.; Kapp, A.; Raap, U. Substance P activates human eosinophils. Exp. Dermatol. 2015, 24, 557–559. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Amponnawarat, A.; Ali, H. Substance P Serves as a Balanced Agonist for MRGPRX2 and a Single Tyrosine Residue Is Required for beta-Arrestin Recruitment and Receptor Internalization. Int. J. Mol. Sci. 2021, 22, 5318. [Google Scholar] [CrossRef]
- Paus, R.; Schmelz, M.; Biro, T.; Steinhoff, M. Frontiers in pruritus research: Scratching the brain for more effective itch therapy. J. Clin. Investig. 2006, 116, 1174–1186. [Google Scholar] [CrossRef]
- Luger, T.A. Neuromediators—A crucial component of the skin immune system. J. Dermatol. Sci. 2002, 30, 87–93. [Google Scholar] [CrossRef]
- Furutani, K.; Koro, O.; Hide, M.; Yamamoto, S. Substance P- and antigen-induced release of leukotriene B4, prostaglandin D2 and histamine from guinea pig skin by different mechanisms in vitro. Arch. Dermatol. Res. 1999, 291, 466–473. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Marincsák, R.; Dobrosi, N.; Géczy, T.; Paus, R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta 2007, 1772, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Andoh, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J. Pharmacol. Exp. Ther. 1998, 286, 1140–1145. [Google Scholar] [PubMed]
- Stander, S.; Yosipovitch, G. Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br. J. Dermatol. 2019, 181, 932–938. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Buddenkotte, J.; Buhl, T.; Steinhoff, M. Role of SNAREs in Atopic Dermatitis-Related Cytokine Secretion and Skin-Nerve Communication. J. Investig. Dermatol. 2019, 139, 2324–2333. [Google Scholar] [CrossRef]
- Huang, J.; Polgár, E.; Solinski, H.J.; Mishra, S.K.; Tseng, P.Y.; Iwagaki, N.; Boyle, K.A.; Dickie, A.C.; Kriegbaum, M.C.; Wildner, H.; et al. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 2018, 21, 707–716. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hoon, M.A. The cells and circuitry for itch responses in mice. Science 2013, 340, 968–971. [Google Scholar] [CrossRef]
- Pitake, S.; Ralph, P.C.; DeBrecht, J.; Mishra, S.K. Atopic Dermatitis Linked Cytokine Interleukin-31 Induced Itch Mediated via a Neuropeptide Natriuretic Polypeptide B. Acta Derm.-Venereol. 2018, 98, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.T.; Liu, X.Y.; Liu, X.T.; Liu, J.; Munanairi, A.; Barry, D.M.; Liu, B.; Jin, H.; Sun, Y.; Yang, Q.; et al. BNP facilitates NMB-encoded histaminergic itch via NPRC-NMBR crosstalk. eLife 2021, 10, e71689. [Google Scholar] [CrossRef] [PubMed]
- Larkin, C.; Chen, W.; Szabó, I.L.; Shan, C.; Dajnoki, Z.; Szegedi, A.; Buhl, T.; Fan, Y.; O’Neill, S.; Walls, D.; et al. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1110–1114.e5. [Google Scholar] [CrossRef]
- Solinski, H.J.; Dranchak, P.; Oliphant, E.; Gu, X.; Earnest, T.W.; Braisted, J.; Inglese, J.; Hoon, M.A. Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci. Transl. Med. 2019, 11, eaav5464. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.S.; Ramos, D.; Han, S.B.; Zhao, J.; Son, Y.J.; Luo, W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol. Pain 2012, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Uta, D.; Ding, H.; Uchida, H.; Saika, F.; Matsuzaki, S.; Fukazawa, Y.; Abe, M.; Sakimura, K.; Ko, M.C.; et al. GRP receptor and AMPA receptor cooperatively regulate itch-responsive neurons in the spinal dorsal horn. Neuropharmacology 2020, 170, 108025. [Google Scholar] [CrossRef]
- Pagani, M.; Albisetti, G.W.; Sivakumar, N.; Wildner, H.; Santello, M.; Johannssen, H.C.; Zeilhofer, H.U. How Gastrin-Releasing Peptide Opens the Spinal Gate for Itch. Neuron 2019, 103, 102–117.e5. [Google Scholar] [CrossRef]
- Sun, S.; Xu, Q.; Guo, C.; Guan, Y.; Liu, Q.; Dong, X. Leaky Gate Model: Intensity-Dependent Coding of Pain and Itch in the Spinal Cord. Neuron 2017, 93, 840–853.e5. [Google Scholar] [CrossRef]
- Sun, Y.G.; Chen, Z.F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007, 448, 700–703. [Google Scholar] [CrossRef]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef]
- Jensen, R.T.; Battey, J.F.; Spindel, E.R.; Benya, R.V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.M.; Liu, X.-Y.; Chen, Z.-F. GRP and NMB: Distinct functions in itch transmission. Itch 2021, 6, e44. [Google Scholar] [CrossRef]
- Wan, L.; Jin, H.; Liu, X.Y.; Jeffry, J.; Barry, D.M.; Shen, K.F.; Peng, J.H.; Liu, X.T.; Jin, J.H.; Sun, Y.; et al. Distinct roles of NMB and GRP in itch transmission. Sci. Rep. 2017, 7, 15466. [Google Scholar] [CrossRef] [PubMed]
- Ehling, S.; Fukuyama, T.; Ko, M.C.; Olivry, T.; Bäumer, W. Neuromedin B Induces Acute Itch in Mice via the Activation of Peripheral Sensory Neurons. Acta Derm.-Venereol. 2019, 99, 587–893. [Google Scholar] [CrossRef]
- Barry, D.M.; Liu, X.T.; Liu, B.; Liu, X.Y.; Gao, F.; Zeng, X.; Liu, J.; Yang, Q.; Wilhelm, S.; Yin, J.; et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat. Commun. 2020, 11, 1397. [Google Scholar] [CrossRef]
- Cui, L.; Guo, J.; Cranfill, S.L.; Gautam, M.; Bhattarai, J.; Olson, W.; Beattie, K.; Challis, R.C.; Wu, Q.; Song, X.; et al. Glutamate in primary afferents is required for itch transmission. Neuron 2022, 110, 809–823.e5. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z. Intrathecal somatostatin modulates spinal sensory and reflex mechanisms: Behavioral and electrophysiological studies in the rat. Neurosci. Lett. 1985, 62, 69–74. [Google Scholar] [CrossRef]
- Solinski, H.J.; Kriegbaum, M.C.; Tseng, P.Y.; Earnest, T.W.; Gu, X.; Barik, A.; Chesler, A.T.; Hoon, M.A. Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell Rep. 2019, 26, 3561–3573.e4. [Google Scholar] [CrossRef]
- Steele, H.R.; Xing, Y.; Zhu, Y.; Hilley, H.B.; Lawson, K.; Nho, Y.; Niehoff, T.; Han, L. MrgprC11+ sensory neurons mediate glabrous skin itch. Proc. Natl. Acad. Sci. USA 2021, 118, e2022874118. [Google Scholar] [CrossRef]
- Fatima, M.; Ren, X.; Pan, H.; Slade, H.F.E.; Asmar, A.J.; Xiong, C.M.; Shi, A.; Xiong, A.E.; Wang, L.; Duan, B. Spinal somatostatin-positive interneurons transmit chemical itch. Pain 2019, 160, 1166–1174. [Google Scholar] [CrossRef]
- Giaid, A.; Gibson, S.J.; Ibrahim, B.N.; Legon, S.; Bloom, S.R.; Yanagisawa, M.; Masaki, T.; Varndell, I.M.; Polak, J.M. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc. Natl. Acad. Sci. USA 1989, 86, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Kido-Nakahara, M.; Buddenkotte, J.; Kempkes, C.; Ikoma, A.; Cevikbas, F.; Akiyama, T.; Nunes, F.; Seeliger, S.; Hasdemir, B.; Mess, C.; et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J. Clin. Investig. 2014, 124, 2683–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Trentin, P.G.; Fernandes, M.B.; D’Orléans-Juste, P.; Rae, G.A. Endothelin-1 causes pruritus in mice. Exp. Biol. Med. 2006, 231, 1146–1151. [Google Scholar]
- Dahlöf, B.; Gustafsson, D.; Hedner, T.; Jern, S.; Hansson, L. Regional haemodynamic effects of endothelin-1 in rat and man: Unexpected adverse reaction. J. Hypertens. 1990, 8, 811–817. [Google Scholar] [CrossRef]
- Magnusdottir, E.I.; Grujic, M.; Bergman, J.; Pejler, G.; Lagerstrom, M.C. Mouse connective tissue mast cell proteases tryptase and carboxypeptidase A3 play protective roles in itch induced by endothelin-1. J. Neuroinflamm. 2020, 17, 123. [Google Scholar] [CrossRef]
- Santer, M.; Rumsby, K.; Ridd, M.J.; Francis, N.A.; Stuart, B.; Chorozoglou, M.; Roberts, A.; Liddiard, L.; Nollett, C.; Hooper, J.; et al. Adding emollient bath additives to standard eczema management for children with eczema: The BATHE RCT. Health Technol. Assess. 2018, 22, 1–116. [Google Scholar] [CrossRef]
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef]
- Szollosi, A.G.; McDonald, I.; Szabo, I.L.; Meng, J.; van den Bogaard, E.; Steinhoff, M. TLR3 in Chronic Human Itch: A Keratinocyte-Associated Mechanism of Peripheral Itch Sensitization. J. Investig. Dermatol. 2019, 139, 2393–2396.e6. [Google Scholar] [CrossRef]
- Kido-Nakahara, M.; Wang, B.; Ohno, F.; Tsuji, G.; Ulzii, D.; Takemura, M.; Furue, M.; Nakahara, T. Inhibition of mite-induced dermatitis, pruritus, and nerve sprouting in mice by the endothelin receptor antagonist bosentan. Allergy 2021, 76, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Ulzii, D.; Miake, S.; Fujishima, K.; Sakai, S.; Chiba, T.; Tsuji, G.; Furue, M. Topical application of endothelin receptor a antagonist attenuates imiquimod-induced psoriasiform skin inflammation. Sci. Rep. 2020, 10, 9510. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, H.J.; Kim, H.; Koh, J.Y.; Kim, K.M.; Noh, M.S.; Kim, J.J.; Lee, C.H. Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur. J. Pharmacol. 2008, 579, 390–394. [Google Scholar] [CrossRef]
- Rossbach, K.; Nassenstein, C.; Gschwandtner, M.; Schnell, D.; Sander, K.; Seifert, R.; Stark, H.; Kietzmann, M.; Baumer, W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 2011, 190, 89–102. [Google Scholar] [CrossRef]
- Han, S.K.; Mancino, V.; Simon, M.I. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 2006, 52, 691–703. [Google Scholar] [CrossRef]
- Green, D.; Dong, X. The cell biology of acute itch. J. Cell Biol. 2016, 213, 155–161. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol. 2016, 51, 263–292. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.S. Itch: A Paradigm of Neuroimmune Crosstalk. Immunity 2020, 52, 753–766. [Google Scholar] [CrossRef]
- Giustizieri, M.L.; Albanesi, C.; Fluhr, J.; Gisondi, P.; Norgauer, J.; Girolomoni, G. H1 histamine receptor mediates inflammatory responses in human keratinocytes. J. Allergy Clin. Immunol. 2004, 114, 1176–1182. [Google Scholar] [CrossRef]
- Glatzer, F.; Gschwandtner, M.; Ehling, S.; Rossbach, K.; Janik, K.; Klos, A.; Bäumer, W.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Histamine induces proliferation in keratinocytes from patients with atopic dermatitis through the histamine 4 receptor. J. Allergy Clin. Immunol. 2013, 132, 1358–1367. [Google Scholar] [CrossRef]
- Girard, B.M.; Merrill, L.; Malley, S.; Vizzard, M.A. Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J. Mol. Neurosci. 2013, 51, 602–614. [Google Scholar] [CrossRef]
- Schaper-Gerhardt, K.; Rossbach, K.; Nikolouli, E.; Werfel, T.; Gutzmer, R.; Mommert, S. The role of the histamine H4 receptor in atopic dermatitis and psoriasis. Br. J. Pharmacol. 2020, 177, 490–502. [Google Scholar] [CrossRef]
- Voisin, T.; Chiu, I.M. Molecular link between itch and atopic dermatitis. Proc. Natl. Acad. Sci. USA 2018, 115, 12851–12853. [Google Scholar] [CrossRef]
- Werfel, T.; Layton, G.; Yeadon, M.; Whitlock, L.; Osterloh, I.; Jimenez, P.; Liu, W.; Lynch, V.; Asher, A.; Tsianakas, A.; et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1830–1837.e4. [Google Scholar] [CrossRef]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef]
- Klein, A.; Carstens, M.I.; Carstens, E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011, 106, 1078–1088. [Google Scholar] [CrossRef]
- Zhao, J.; Munanairi, A.; Liu, X.Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S.; et al. PAR2 Mediates Itch via TRPV3 Signaling in Keratinocytes. J. Investig. Dermatol. 2020, 140, 1524–1532. [Google Scholar] [CrossRef]
- Barr, T.P.; Garzia, C.; Guha, S.; Fletcher, E.K.; Nguyen, N.; Wieschhaus, A.J.; Ferrer, L.; Covic, L.; Kuliopulos, A. PAR2 Pepducin-Based Suppression of Inflammation and Itch in Atopic Dermatitis Models. J. Investig. Dermatol. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Han, L.; Ma, C.; Liu, Q.; Weng, H.J.; Cui, Y.; Tang, Z.; Kim, Y.; Nie, H.; Qu, L.; Patel, K.N.; et al. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013, 16, 174–182. [Google Scholar] [CrossRef]
- Ray, P.; Torck, A.; Quigley, L.; Wangzhou, A.; Neiman, M.; Rao, C.; Lam, T.; Kim, J.Y.; Kim, T.H.; Zhang, M.Q.; et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018, 159, 1325–1345. [Google Scholar] [CrossRef]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef]
- Roy, S.; Chompunud Na Ayudhya, C.; Thapaliya, M.; Deepak, V.; Ali, H. Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 2021, 148, 293–308. [Google Scholar] [CrossRef]
- Song, M.H.; Shim, W.S. Lithocholic Acid Activates Mas-Related G Protein-Coupled Receptors, Contributing to Itch in Mice. Biomol. Ther. 2022, 30, 38–47. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, H.; Han, Y.; Yin, S.; Shen, B.; Wu, Y.; Li, W.; Cao, Z. Tick peptides evoke itch by activating MrgprC11/MRGPRX1 to sensitize TRPV1 in pruriceptors. J. Allergy Clin. Immunol. 2021, 147, 2236–2248.e16. [Google Scholar] [CrossRef]
- Zhang, S.; Edwards, T.N.; Chaudhri, V.K.; Wu, J.; Cohen, J.A.; Hirai, T.; Rittenhouse, N.; Schmitz, E.G.; Zhou, P.Y.; McNeil, B.D.; et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 2021, 184, 2151–2166.e16. [Google Scholar] [CrossRef]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019, 50, 1163–1171.e5. [Google Scholar] [CrossRef]
- Liu, Q.; Sikand, P.; Ma, C.; Tang, Z.; Han, L.; Li, Z.; Sun, S.; LaMotte, R.H.; Dong, X. Mechanisms of itch evoked by beta-alanine. J. Neurosci. 2012, 32, 14532–14537. [Google Scholar] [CrossRef]
- Meixiong, J.; Vasavda, C.; Snyder, S.H.; Dong, X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc. Natl. Acad. Sci. USA 2019, 116, 10525–10530. [Google Scholar] [CrossRef]
- Cao, C.; Kang, H.J.; Singh, I.; Chen, H.; Zhang, C.; Ye, W.; Hayes, B.W.; Liu, J.; Gumpper, R.H.; Bender, B.J.; et al. Structure, function and pharmacology of human itch GPCRs. Nature 2021, 600, 170–175. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Brown, J.M.; Wu, Y.; Kirshenbaum, A.; Metcalfe, D.D. Human mast cells are capable of serotonin synthesis and release. J. Allergy Clin. Immunol. 2007, 119, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res. 1999, 35, 77–83. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Carstens, E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J. Neurophysiol. 2010, 104, 2442–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelz, M.; Schmidt, R.; Weidner, C.; Hilliges, M.; Torebjork, H.E.; Handwerker, H.O. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003, 89, 2441–2448. [Google Scholar] [CrossRef]
- Weisshaar, E.; Dunker, N.; Rohl, F.W.; Gollnick, H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp. Dermatol. 2004, 13, 298–304. [Google Scholar] [CrossRef]
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.; Wong, J.F.; Stucky, C.L.; et al. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron 2015, 87, 124–138. [Google Scholar] [CrossRef]
- Akiyama, T.; Ivanov, M.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Kempkes, C.; Buddenkotte, J.; Steinhoff, M.; et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J. Investig. Dermatol. 2016, 136, 154–160. [Google Scholar] [CrossRef]
- Sanjel, B.; Kim, B.H.; Song, M.H.; Carstens, E.; Shim, W.S. Glucosylsphingosine evokes pruritus via activation of 5-HT2A receptor and TRPV4 in sensory neurons. Br. J. Pharmacol. 2022, 179, 2193–2207. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Liu, X.Y.; Jeffry, J.; Karunarathne, W.K.; Li, J.L.; Munanairi, A.; Zhou, X.Y.; Li, H.; Sun, Y.G.; Wan, L.; et al. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron 2014, 84, 821–834. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.L.; Huang, Y.; Chen, L.H.; Cheng, R.X.; Zhou, F.M.; Guo, R.; Li, J.C.; Liu, T. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci. Rep. 2016, 6, 36286. [Google Scholar] [CrossRef]
- Shen, M.; Cao, D.; Xiao, Y.; Kuang, Y.; Jing, D.; Li, Y.; Liu, P.; Chen, X.; Zhu, W. Serum 5-Hydroxytryptamine is Related to Psoriasis Severity in Patients with Comorbid Anxiety or Depression. Acta Derm.-Venereol. 2021, 101, adv00514. [Google Scholar]
- Voisin, T.; Perner, C.; Messou, M.A.; Shiers, S.; Ualiyeva, S.; Kanaoka, Y.; Price, T.J.; Sokol, C.L.; Bankova, L.G.; Austen, K.F.; et al. The CysLT(2)R receptor mediates leukotriene C(4)-driven acute and chronic itch. Proc. Natl. Acad. Sci. USA 2021, 118, e2022087118. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, H.J.; Sung, K.S.; Kim, H.; Cho, S.A.; Kim, K.M.; Lee, C.H.; Kim, J.J. 12(S)-HPETE induces itch-associated scratchings in mice. Eur. J. Pharmacol. 2007, 554, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Kuraishi, Y. Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur. J. Pharmacol. 1998, 353, 93–96. [Google Scholar] [CrossRef]
- Andoh, T.; Kuraishi, Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Brain Res. Mol. Brain Res. 2005, 137, 263–266. [Google Scholar] [CrossRef]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440.e17. [Google Scholar] [CrossRef]
- Hilger, R.A.; Neuber, K.; Konig, W. Conversion of leukotriene A4 by neutrophils and platelets from patients with atopic dermatitis. Immunology 1991, 74, 689–695. [Google Scholar]
- Zinn, S.; Sisignano, M.; Kern, K.; Pierre, S.; Tunaru, S.; Jordan, H.; Suo, J.; Treutlein, E.M.; Angioni, C.; Ferreiros, N.; et al. The leukotriene B4 receptors BLT1 and BLT2 form an antagonistic sensitizing system in peripheral sensory neurons. J. Biol. Chem. 2017, 292, 6123–6134. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; He, R.; Li, Y.; Mondal, S.; Yoon, J.; Afshar, R.; Chen, M.; Lee, D.M.; Luo, H.R.; Luster, A.D.; et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 2012, 37, 747–758. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Steinhoff, M.; Dolly, J.O. TNFalpha induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci. Rep. 2016, 6, 21226. [Google Scholar] [CrossRef]
- Meng, J.; Steinhoff, M. Molecular mechanisms of pruritus. Curr. Res. Transl. Med. 2016, 64, 203–206. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Trier, A.M.; Mack, M.R.; Fredman, A.; Tamari, M.; Ver Heul, A.M.; Zhao, Y.; Guo, C.J.; Avraham, O.; Ford, Z.K.; Oetjen, L.K.; et al. IL-33 signaling in sensory neurons promotes dry skin itch. J. Allergy Clin. Immunol. 2022, 149, 1473–1480.e6. [Google Scholar] [CrossRef] [PubMed]
- Miron, Y.; Miller, P.E.; Hughes, C.; Indersmitten, T.; Lerner, E.A.; Cevikbas, F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Grabinski, N.S.; Liu, Q. Peripheral Mechanisms of Itch. J. Investig. Dermatol. 2022, 142, 31–41. [Google Scholar] [CrossRef]
- Cevikbas, F.; Lerner, E.A. Physiology and Pathophysiology of Itch. Physiol. Rev. 2020, 100, 945–982. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef]
- Van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Z.; Steinhoff, M.; Li, Y.; Buhl, T.; Fischer, M.; Chen, W.; Cheng, W.; Zhu, R.; Yan, X.; et al. Innate immune regulates cutaneous sensory IL-13 receptor alpha 2 to promote atopic dermatitis. Brain Behav. Immun. 2021, 98, 28–39. [Google Scholar] [CrossRef]
- Brightling, C.E.; Saha, S.; Hollins, F. Interleukin-13: Prospects for new treatments. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 42–49. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, R.; Chen, W.; Yang, H.; Wang, J.; Meng, J. Inhibiting Keratinocyte-Derived Signal Transducer and Activator of Transcription 6 Improved Atopic Dermatitis in Mice. J. Investig. Dermatol. 2022; in press. [Google Scholar] [CrossRef]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Takamori, A.; Nambu, A.; Sato, K.; Yamaguchi, S.; Matsuda, K.; Numata, T.; Sugawara, T.; Yoshizaki, T.; Arae, K.; Morita, H.; et al. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci. Rep. 2018, 8, 6639. [Google Scholar] [CrossRef] [PubMed]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef] [Green Version]
- Dubin, C.; Del Duca, E.; Guttman-Yassky, E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev. Clin. Immunol. 2021, 17, 835–852. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Cevikbas, F.; Steinhoff, M. IL-33: A novel danger signal system in atopic dermatitis. J. Investig. Dermatol. 2012, 132, 1326–1329. [Google Scholar] [CrossRef]
- Huang, J.; Gandini, M.A.; Chen, L.; M’Dahoma, S.; Stemkowski, P.L.; Chung, H.; Muruve, D.A.; Zamponi, G.W. Hyperactivity of Innate Immunity Triggers Pain via TLR2-IL-33-Mediated Neuroimmune Crosstalk. Cell Rep. 2020, 33, 108233. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef]
- Wallrapp, A.; Riesenfeld, S.J.; Burkett, P.R.; Kuchroo, V.K. Type 2 innate lymphoid cells in the induction and resolution of tissue inflammation. Immunol. Rev. 2018, 286, 53–73. [Google Scholar] [CrossRef]
- Rincón, M.; Anguita, J.; Nakamura, T.; Fikrig, E.; Flavell, R.A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 1997, 185, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Yamaguchi, C.; Eguchi, K.; Shiraishi, Y.; Kohno, K.; Mikoshiba, K.; Inoue, K.; Nishida, M.; Tsuda, M. Astrocytic STAT3 activation and chronic itch require IP3R1/TRPC-dependent Ca2+ signals in mice. J. Allergy Clin. Immunol 2021, 147, 1341–1353. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Koga, K.; Tozaki-Saitoh, H.; Kohro, Y.; Toyonaga, H.; Yamaguchi, C.; Hasegawa, A.; Nakahara, T.; Hachisuka, J.; Akira, S.; et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015, 21, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Yamagata, R.; Kohno, K.; Yamane, T.; Shiratori-Hayashi, M.; Kohro, Y.; Tozaki-Saitoh, H.; Tsuda, M. Sensitization of spinal itch transmission neurons in a mouse model of chronic itch requires an astrocytic factor. J. Allergy Clin. Immunol. 2020, 145, 183–191.e10. [Google Scholar] [CrossRef]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. IL-6/p-BTK/p-ERK signaling mediates calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef]
- Navarini, A.A.; French, L.E.; Hofbauer, G.F. Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J. Allergy Clin. Immunol. 2011, 128, 1128–1130. [Google Scholar] [CrossRef]
- Spencer, S.; Kostel Bal, S.; Egner, W.; Lango Allen, H.; Raza, S.I.; Ma, C.A.; Gurel, M.; Zhang, Y.; Sun, G.; Sabroe, R.A.; et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J. Exp. Med. 2019, 216, 1986–1998. [Google Scholar] [CrossRef]
- Wahlgren, C.F.; Tengvall Linder, M.; Hägermark, O.; Scheynius, A. Itch and inflammation induced by intradermally injected interleukin-2 in atopic dermatitis patients and healthy subjects. Arch. Dermatol. Res. 1995, 287, 572–580. [Google Scholar] [CrossRef]
- Hsieh, K.H.; Chou, C.C.; Huang, S.F. Interleukin 2 therapy in severe atopic dermatitis. J. Clin. Immunol. 1991, 11, 22–28. [Google Scholar] [CrossRef]
- Lee, S.H.; Baig, M.; Rusciano, V.; Dutcher, J.P. Novel management of pruritus in patients treated with IL-2 for metastatic renal cell carcinoma and malignant melanoma. J. Immunother. 2010, 33, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Wheeler, J.J.; Pitake, S.; Ding, H.; Jiang, C.; Fukuyama, T.; Paps, J.S.; Ralph, P.; Coyne, J.; Parkington, M.; et al. Periostin Activation of Integrin Receptors on Sensory Neurons Induces Allergic Itch. Cell Rep. 2020, 31, 107472. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 2013, 66, 129–155. [Google Scholar] [PubMed]
- Nakajima, S.; Kabata, H.; Kabashima, K.; Asano, K. Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses. Allergol. Int. 2020, 69, 197–203. [Google Scholar] [CrossRef]
- Jessup, H.K.; Brewer, A.W.; Omori, M.; Rickel, E.A.; Budelsky, A.L.; Yoon, B.R.; Ziegler, S.F.; Comeau, M.R. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J. Immunol. 2008, 181, 4311–4319. [Google Scholar] [CrossRef]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bruin-Weller, M.; Flohr, C.; Ardern-Jones, M.R.; Barbarot, S.; Deleuran, M.; Bieber, T.; Vestergaard, C.; Brown, S.J.; Cork, M.J.; et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J. Am. Acad. Dermatol. 2017, 77, 623–633. [Google Scholar] [CrossRef]
- Shannon, J.L.; Corcoran, D.L.; Murray, J.C.; Ziegler, S.F.; MacLeod, A.S.; Zhang, J.Y. Thymic stromal lymphopoietin controls hair growth. Stem Cell Rep. 2022, 17, 649–663. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nattkemper, L.A.; Kim, H.S.; Kursewicz, C.D.; Fowler, E.; Shah, S.M.; Nanda, S.; Fayne, R.A.; Romanelli, P.; Yosipovitch, G. Dermal Periostin: A New Player in Itch of Prurigo Nodularis. Acta Derm.-Venereol. 2021, 101, adv00375. [Google Scholar] [CrossRef]
- Kou, K.; Okawa, T.; Yamaguchi, Y.; Ono, J.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; Ohta, S.; Izuhara, K.; et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br. J. Dermatol. 2014, 171, 283–291. [Google Scholar] [CrossRef]
- Arima, K.; Ohta, S.; Takagi, A.; Shiraishi, H.; Masuoka, M.; Ontsuka, K.; Suto, H.; Suzuki, S.; Yamamoto, K.; Ogawa, M.; et al. Periostin contributes to epidermal hyperplasia in psoriasis common to atopic dermatitis. Allergol. Int. 2015, 64, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Cao, T.; Jin, L.; Li, B.; Fang, H.; Zhang, J.; Zhang, Y.; Hu, J.; Wang, G. Increased Lipocalin-2 Contributes to the Pathogenesis of Psoriasis by Modulating Neutrophil Chemotaxis and Cytokine Secretion. J. Investig. Dermatol. 2016, 136, 1418–1428. [Google Scholar] [CrossRef]
- Adebiyi, A.; Narayanan, D.; Jaggar, J.H. Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J. Biol. Chem. 2011, 286, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Salido, G.M.; Sage, S.O.; Rosado, J.A. TRPC channels and store-operated Ca2+ entry. Biochim. Biophys. Acta 2009, 1793, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, N.; Ishiuji, Y.; Tominaga, M.; Sakata, S.; Takahashi, N.; Yanaba, K.; Umezawa, Y.; Asahina, A.; Kimura, U.; Suga, Y.; et al. Relationship between the Degrees of Itch and Serum Lipocalin-2 Levels in Patients with Psoriasis. J. Immunol. Res. 2019, 2019, 8171373. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.Y.; Hoon, M.A. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci. Transl. Med. 2021, 13, eabe3037. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Steinhoff, M.; Zhang, W.; Buddenkotte, J.; Buhl, T.; Zhu, R.; Yan, X.; Lu, Z.; Xiao, S.; et al. The PLAUR signaling promotes chronic pruritus. FASEB J. 2022, 36, e22368. [Google Scholar] [CrossRef]
- Cully, M. Anti-oncostatin M antibody puts brakes on asthma exacerbations. Nat. Rev. Drug Discov. 2022, 21, 178. [Google Scholar] [CrossRef]
- Walsh, C.M.; Hill, R.Z.; Schwendinger-Schreck, J.; Deguine, J.; Brock, E.C.; Kucirek, N.; Rifi, Z.; Wei, J.; Gronert, K.; Brem, R.B.; et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife 2019, 8, e48448. [Google Scholar] [CrossRef]
- Mizumoto, N.; Iwabichi, K.; Nakamura, H.; Ato, M.; Shibaki, A.; Kawashima, T.; Kobayashi, H.; Iwabuchi, C.; Ohkawara, A.; Onoé, K. Enhanced contact hypersensitivity in human monocyte chemoattractant protein-1 transgenic mouse. Immunobiology 2001, 204, 477–493. [Google Scholar] [CrossRef]
- Mennini, M.; Dahdah, L.; Fiocchi, A. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 1090. [Google Scholar] [PubMed]
- Napolitano, M.; Fabbrocini, G.; Scalvenzi, M.; Nisticò, S.P.; Dastoli, S.; Patruno, C. Effectiveness of Dupilumab for the Treatment of Generalized Prurigo Nodularis Phenotype of Adult Atopic Dermatitis. Dermatitis 2020, 31, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Kanazawa, N.; Muraoka, K.; Yariyama, A.; Kawaguchi, A.; Kunimoto, K.; Kaminaka, C.; Yamamoto, Y.; Tsujioka, K.; Yoshida, A.; et al. Dupilumab Improves Pruritus in Netherton Syndrome: A Case Study. Children 2022, 9, 310. [Google Scholar] [CrossRef]
- Ariëns, L.F.M.; van der Schaft, J.; Bakker, D.S.; Balak, D.; Romeijn, M.L.E.; Kouwenhoven, T.; Kamsteeg, M.; Giovannone, B.; Drylewicz, J.; van Amerongen, C.C.A.; et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: First clinical and biomarker results from the BioDay registry. Allergy 2020, 75, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Chan, V.P.Y.; Leung, A.K.C. Experimental Drugs with the Potential to Treat Atopic Eczema. J. Exp. Pharmacol. 2021, 13, 487–498. [Google Scholar] [CrossRef]
- Agboola, F.; Atlas, S.J.; Brouwer, E.; Carlson, J.J.; Hansen, R.N.; Herron-Smith, S.; Nhan, E.; Rind, D.M.; Pearson, S.D. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: Effectiveness and value. J. Manag. Care Spec. Pharm. 2022, 28, 108–114. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef]
- Goncalves, F.; Freitas, E.; Torres, T. Selective IL-13 inhibitors for the treatment of atopic dermatitis. Drugs Context 2021, 10, 2021-1-7. [Google Scholar] [CrossRef] [PubMed]
- Noonan, M.; Korenblat, P.; Mosesova, S.; Scheerens, H.; Arron, J.R.; Zheng, Y.; Putnam, W.S.; Parsey, M.V.; Bohen, S.P.; Matthews, J.G. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. J. Allergy Clin. Immunol. 2013, 132, 567–574.e12. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Mihara, R. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 2093. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; Nemolizumab, J.P. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: Results from two phase III, long-term studies. Br. J. Dermatol. 2022, 186, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Labib, A.; Ju, T.; Vander Does, A.; Yosipovitch, G. Immunotargets and Therapy for Prurigo Nodularis. Immunotargets Ther. 2022, 11, 11–21. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef]
- Lebwohl, M.; Strober, B.; Menter, A.; Gordon, K.; Weglowska, J.; Puig, L.; Papp, K.; Spelman, L.; Toth, D.; Kerdel, F.; et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N. Engl. J. Med. 2015, 373, 1318–1328. [Google Scholar] [CrossRef]
- Papp, K.A.; Reich, K.; Paul, C.; Blauvelt, A.; Baran, W.; Bolduc, C.; Toth, D.; Langley, R.G.; Cather, J.; Gottlieb, A.B.; et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 2016, 175, 273–286. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Gordon, K.; Hsu, S.; Elewski, B.; Eichenfield, L.F.; Kircik, L.; Rastogi, S.; Pillai, R.; Israel, R. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1305–1313. [Google Scholar] [CrossRef]
- Paul, C.; Lacour, J.P.; Tedremets, L.; Kreutzer, K.; Jazayeri, S.; Adams, S.; Guindon, C.; You, R.; Papavassilis, C. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1082–1090. [Google Scholar] [CrossRef]
- Ungar, B.; Pavel, A.B.; Li, R.; Kimmel, G.; Nia, J.; Hashim, P.; Kim, H.J.; Chima, M.; Vekaria, A.S.; Estrada, Y.; et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.H.; Balogh, E.A.; Ghamrawi, R.I.; Feldman, S.R. A review of secukinumab in psoriasis treatment. Immunotherapy 2021, 13, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Li, G.G.; Liu, Q.; Niu, X.; Li, R.; Ma, H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Immunol Res. 2019, 2019, 2546161. [Google Scholar] [PubMed]
- Griffiths, C.E.; Reich, K.; Lebwohl, M.; van de Kerkhof, P.; Paul, C.; Menter, A.; Cameron, G.S.; Erickson, J.; Zhang, L.; Secrest, R.J.; et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet 2015, 386, 541–551. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Blauvelt, A.; Gooderham, M.; Iversen, L.; Ball, S.; Zhang, L.; Agada, N.O.; Reich, K. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3). J. Am. Acad. Dermatol. 2017, 77, 855–862. [Google Scholar] [CrossRef]
- Abe, F.; Mitsuyama, S.; Nagao, E.; Kimura, M.; Higuchi, T. Atopic Dermatitis-like Eruption Induced by Two Different Biologics in a Patient with Psoriatic Arthritis. Acta Derm.-Venereol. 2019, 99, 1291–1292. [Google Scholar] [CrossRef]
- Reddy, M.; Torres, G.; McCormick, T.; Marano, C.; Cooper, K.; Yeilding, N.; Wang, Y.; Pendley, C.; Prabhakar, U.; Wong, J.; et al. Positive treatment effects of ustekinumab in psoriasis: Analysis of lesional and systemic parameters. J. Dermatol. 2010, 37, 413–425. [Google Scholar] [CrossRef]
- Shroff, A.; Guttman-Yassky, E. Successful use of ustekinumab therapy in refractory severe atopic dermatitis. JAAD Case Rep. 2015, 1, 25–26. [Google Scholar] [CrossRef]
- Khattri, S.; Brunner, P.M.; Garcet, S.; Finney, R.; Cohen, S.R.; Oliva, M.; Dutt, R.; Fuentes-Duculan, J.; Zheng, X.; Li, X.; et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp. Dermatol. 2017, 26, 28–35. [Google Scholar] [CrossRef]
- Reich, K.; Griffiths, C.E.M.; Gordon, K.B.; Papp, K.A.; Song, M.; Randazzo, B.; Li, S.; Shen, Y.K.; Han, C.; Kimball, A.B.; et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: Results from the VOYAGE 1 and VOYAGE 2 trials. J. Am. Acad. Dermatol. 2020, 82, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Tsai, T.F.; Flavin, S.; Song, M.; Randazzo, B.; Wasfi, Y.; Jiang, J.; Li, S.; Puig, L. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: Results of the randomized, double-blind, phase III NAVIGATE trial. Br. J. Dermatol. 2018, 178, 114–123. [Google Scholar] [CrossRef]
- Iznardo, H.; Vilarrasa, E.; López-Ferrer, A.; Puig, L. Real-world drug survival of guselkumab, ixekizumab and secukinumab for psoriasis. Br. J. Dermatol. 2021, 185, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Armstrong, A.W.; Wasfi, Y.; Li, S.; Shen, Y.K.; Randazzo, B.; Song, M.; Kimball, A.B. Safety of guselkumab in patients with moderate-to-severe psoriasis treated through 100 weeks: A pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br. J. Dermatol. 2019, 180, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Al-Marri, F.; Al Chalabi, R.; Gieler, U.; Buddenkotte, J. Recalcitrant erythrodermic ichthyosis with atopic dermatitis successfully treated with Dupilumab in combination with Guselkumab. Skin Health Dis. 2022, 2, e87. [Google Scholar] [CrossRef]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): Results from two randomised controlled, phase 3 trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Arm, J.P.; Bottoli, I.; Skerjanec, A.; Floch, D.; Groenewegen, A.; Maahs, S.; Owen, C.E.; Jones, I.; Lowe, P.J. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014, 44, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Gimenez-Arnau, A.M.; Sussman, G.; Metz, M.; Baker, D.R.; Bauer, A.; Bernstein, J.A.; Brehler, R.; Chu, C.Y.; Chung, W.H.; et al. Ligelizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2019, 381, 1321–1332. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Cork, M.J.; Eckert, L.; Simpson, E.L.; Armstrong, A.; Barbarot, S.; Puig, L.; Girolomoni, G.; de Bruin-Weller, M.; Wollenberg, A.; Kataoka, Y.; et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: Analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J. Dermatol. Treat. 2020, 31, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e6. [Google Scholar] [CrossRef]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2169–2181. [Google Scholar] [CrossRef]
- Bohlius, J.; Schmidlin, K.; Brillant, C.; Schwarzer, G.; Trelle, S.; Seidenfeld, J.; Zwahlen, M.; Clarke, M.J.; Weingart, O.; Kluge, S.; et al. Erythropoietin or Darbepoetin for patients with cancer—Meta-analysis based on individual patient data. Cochrane Database Syst. Rev. 2009, 2009, CD007303. [Google Scholar] [CrossRef]
- Schabitz, A.; Eyerich, K.; Garzorz-Stark, N. So close, and yet so far away: The dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J. Intern. Med. 2021, 290, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Steinke, S.; Zeidler, C.; Riepe, C.; Bruland, P.; Soto-Rey, I.; Storck, M.; Augustin, M.; Bobko, S.; Garcovich, S.; Legat, F.J.; et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: Results of the European Academy of Dermatology and Venereology Network on Assessment of Severity and Burden of Pruritus (PruNet) cross-sectional trial. J. Am. Acad. Dermatol. 2018, 79, 457–463.e5. [Google Scholar] [CrossRef]

| Drug Category | Drug Names | Advantages | Disadvantages |
|---|---|---|---|
| Targeted monoclonal antibodies (mAb) | Dupilumab | Is a fully human mAb against IL-4Rα that inhibits both IL-4 and IL-13 signaling; is the first approved mAb for AD treatment; demonstrated efficacy and acceptable safety on patients with AD and some other chronic pruritic diseases [192,193]; currently, is ongoing second phase 3 trials on Prurigo Nodularis; is effective in Netherton syndrome, an itchy disease, in a case study [194]. | High cost and side effects that cause eye discomfort (especially conjunctivitis) [80,195,196]; administered subcutaneously twice weekly which is painful for children [197]; cannot treat sub-population of patients. |
| Tralokinumab | Is a fully human mAb that potently and specifically neutralizes IL-13; in phase 3 for moderate-to-severe adult AD; subcutaneous tralokinumab has an acceptable safety and tolerability profile and appears to provide early improvements in disease symptoms including itch, in participants with moderate-to-severe AD [2]; less costly than dupilumab. | Less effective than Dupilumab [198]. | |
| Lebrikizumab | Is a novel, high-affinity, monoclonal antibody targeting IL-13 that selectively inhibits IL-13 signaling [144,199]; in phase 2 for moderate-to-severe AD; significantly improves clinical manifestations of AD, pruritus, and quality of life in a rapid, dose-dependent manner; generally well tolerated [200,201]; might simultaneously target both inflammation and itch via blocking signals on both immune cells and neurons; less frequency in subcutaneous injection comparing to dupilumab [202]. | Might induce conjunctivitis in a few patients with AD [199,203]. | |
| Nemolizumab (CIM331) | Is a humanized antibody against IL-31RA, in the treatment of AD [204]; significantly improves pruritus in patients with moderate-to-severe AD; in two phase 3 trials, nemolizumab plus topical agents improved atopic AD and moderate-to-severe pruritus for up to 68 weeks, without safety issue [205]. | Subcutaneous injection might be associated with higher incidence of injection-site reaction than placebo [204]. | |
| Vixarelimab (KPL-716) | Is an OSMRβ antagonist and a fully-human antibody, inhibits the IL-31 signaling and OSM pathway by antagonizing the OSM beta receptor [206]; in phase 2a, subcutaneous injection improves Prurigo Nodularis signs and symptoms, with an average pruritus reduction of 70% by week 8 of treatment as well as significantly improved nodules as early as week 4; safe; currently, it just completed phase 2b in Prurigo Nodularis (ClinicalTrials.gov Identifier: NCT03816891). | No severe adverse effects [206]. | |
| Tezepelumab (AMG-157/ MEDI9929) | Is a human anti-TSLP antibody that prevents TSLP-TSLPR interactions; has high curative effect, good safety, and high tolerance level [207]; in phase 2a AD treatment (ClinicalTrials.gov Identifier: NCT02525094), tezepelumab achieved improvement on week 12 and 16 (post hoc), albeit not statistically significant over placebo and the itch relief is limited [177]. | The treatment cycle is longer and expensive, and a few (5.4%) patients developed injection-site erythema, which was not seen in placebo group [177]. | |
| Brodalumab (AMG 827) | Is a human anti–IL-17 receptor A IgG2 mAb; in phase 3, it significantly and rapidly improves moderate-to-severe psoriasis, including itch, in patients [208,209,210]; approved by FDA to treat adult moderate-to-severe plaque psoriasis. | Subcutaneous injection might be associated with higher incidence of injection-site reaction than placebo [204]. | |
| Secukinumab | Is a fully human anti-interleukin-17A IgG1 monoclonal antibody [211]; is well-tolerated, safe, and effective in psoriasis and associated itch and pain; approved by the US FDA and European Medicines Agency for moderate-to-severe plaque psoriasis and psoriatic arthritis [212,213]; in phase 2 for AD treatment including intrinsic, Asian, and pediatric AD, secukinumab is not effective in reduction of epidermal thickness, epidermal hyperplasia, and immune cell infiltrates, or inflammatory markers in relation to TH17/IL-23 at week 16 [212]. | Observed adverse events, all in secukinumab-treated patients: orbital cellulitis, upper respiratory infection, and streptococcal pharyngitis [212]; treatment of AD is not effective, however, may be helpful in conjunction with TH-2 biological agents [212]. | |
| Ixekizumab | Is an IgG4 monoclonal antibody that targets IL-17A; achieved outstanding performance in the itch and moderate-to-severe psoriasis treatment effect at 12 weeks [214]; can demonstrate persistent efficacy through 108 weeks (80 mg ixekizumab every 2 weeks up to week 12 and every 4 weeks thereafter); FDA approved for treatment of adult moderate to severe plaque psoriasis, active psoriatic arthritis [215,216]. | Mild or moderate adverse events included nasopharyngitis, upper respiratory tract infections, injection-site reactions, arthralgia, bronchitis, and headache [217]; some patients may be associated with eczematous eruptions in the face [218]. | |
| Ustekinumab | Is an IL-12/IL-23p40 IgG1κ monoclonal antibody that suppresses Th1, Th17, and Th22 activation; approved for psoriasis patients [219]; beneficial clinical effects in moderate-to-severe AD patients [220]; has unique mechanistic effects in AD as early as 4 weeks of treatment; strongest anti-inflammatory effects already occur within 4–8 weeks following an ustekinumab dose, with waning efficacy thereafter [221]. | Individual patients were excluded from analyses after week 28 due to newly developed contact dermatitis and due to worsening skin infection (eczema herpeticum) [221]. | |
| Risankizumab | Is a novel IL-23 mAb, with relatively high efficacy and low risk; had been approved by the FDA in April 2019 to treat AD [214], and in June 2022 to treat moderate-to-severe active Crohn’s disease in adults, an itchy disease. | The most relevant adverse events were nasopharyngitis, upper respiratory tract inflammation, and injection site reaction [214]. | |
| Guselkumab | Is an IL-23 mAb; approved for moderate-to-severe plaque psoriasis. It has demonstrated safety and efficacy in phase III clinical trials [222,223,224,225]; combined treatment by dupilumab and guselkumab rapidly and sustainably improved itch, erythroderma, and eczema in severe AD associated with congenital ichthyosiform erythroderma (CIE), whereas treatment with dupilumab or guselkumab was less or not effective [226]. | There are scarce data regarding its drug survival in clinical practice [224]; serious adverse events included serious infections, nonmelanoma skin cancer, malignancies other than nonmelanoma skin cancer, and major adverse cardiovascular events [222]. | |
| Tildrakizumab | Is a high-affinity, humanized, IgG1 κ antibody targeting p19 subunit of IL-23; demonstrated superior efficacy, safety, and long-term control of moderate-to-severe chronic plaque psoriasis; FDA-approved in 2018 for moderate-to-severe plaque psoriasis [214,227]. | Caused some minor adverse events, including body aches or pain, chills, cough, difficulty in breathing, ear congestion, fever, etc. [227]. | |
| IgE antibody | Omizumab | Is a humanized IgG1 mAb. It binds to the Ce3 domain of IgE with higher affinity; is highly selective for human and non-human primate IgE, with higher efficacy, good safety and high tolerance level in vivo; effective in AD, bullous pemphigoid, and urticaria [228]; received FDA breakthrough therapy designation for patients with chronic spontaneous urticaria in patients that cannot be treated effectively by H1 antihistamine. | Reported serious adverse events, viral upper respiratory tract infection (20%), injection-site reaction [229]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Chen, W.; Zhu, R.; Wang, J.; Meng, J. Critical Players and Therapeutic Targets in Chronic Itch. Int. J. Mol. Sci. 2022, 23, 9935. https://doi.org/10.3390/ijms23179935
Yang H, Chen W, Zhu R, Wang J, Meng J. Critical Players and Therapeutic Targets in Chronic Itch. International Journal of Molecular Sciences. 2022; 23(17):9935. https://doi.org/10.3390/ijms23179935
Chicago/Turabian StyleYang, Hua, Weiwei Chen, Renkai Zhu, Jiafu Wang, and Jianghui Meng. 2022. "Critical Players and Therapeutic Targets in Chronic Itch" International Journal of Molecular Sciences 23, no. 17: 9935. https://doi.org/10.3390/ijms23179935
APA StyleYang, H., Chen, W., Zhu, R., Wang, J., & Meng, J. (2022). Critical Players and Therapeutic Targets in Chronic Itch. International Journal of Molecular Sciences, 23(17), 9935. https://doi.org/10.3390/ijms23179935





