In Vivo Two-Photon Imaging Analysis of Dynamic Degradation of Hepatic Lipid Droplets in MS-275-Treated Mouse Liver
Abstract
:1. Introduction
2. Results
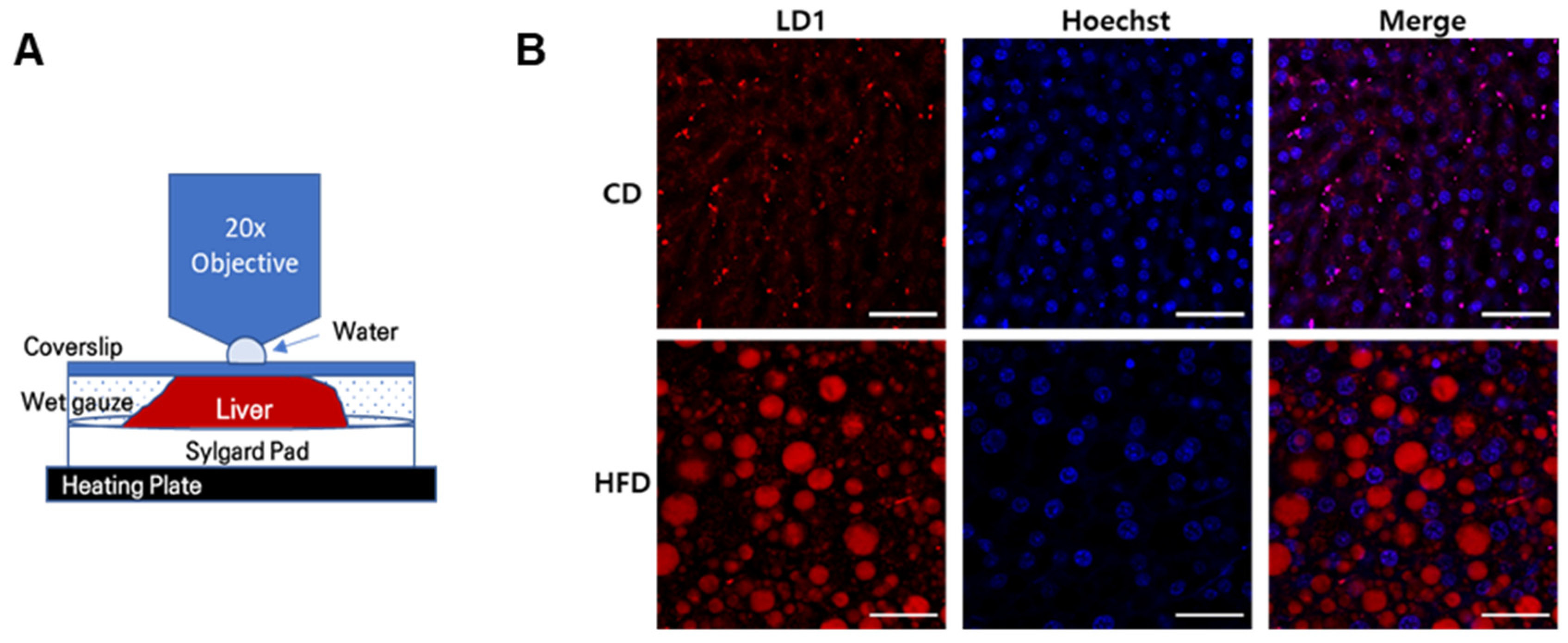
2.1. Formation of Hepatic LDs in HFD-Induced Fatty Liver
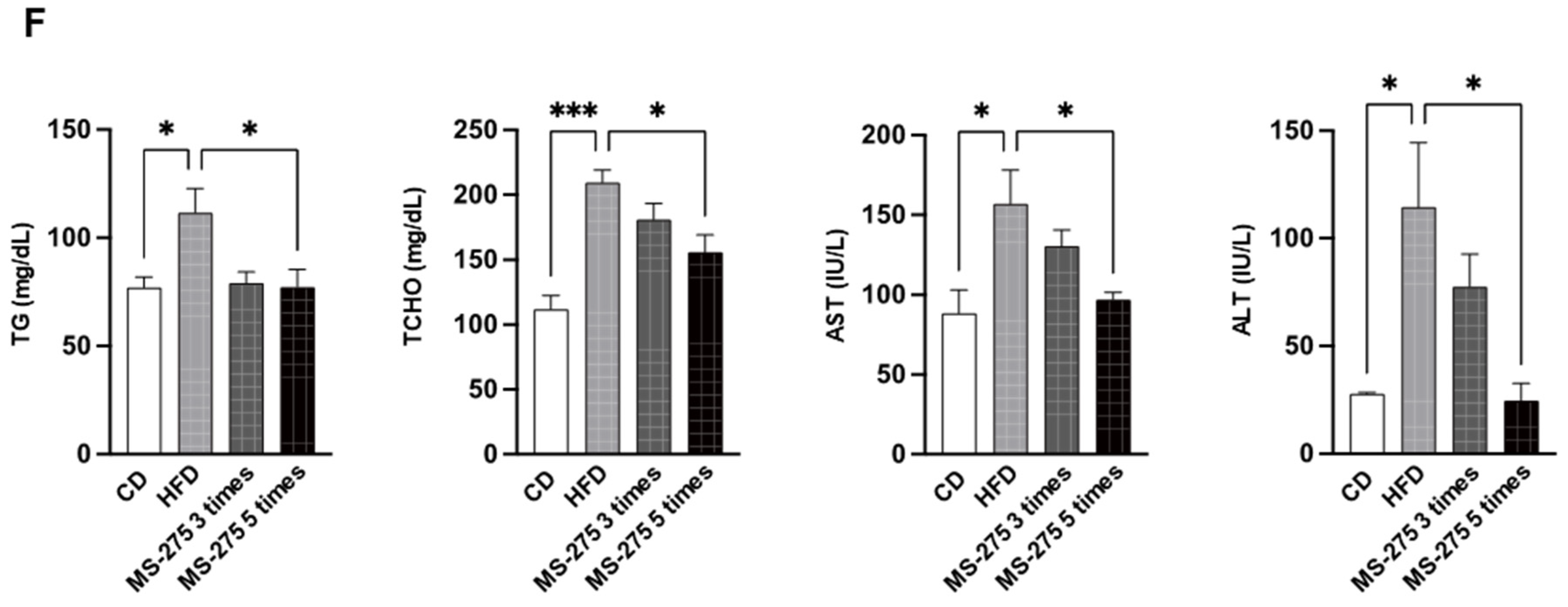
2.2. MS-275 Ameliorated Fatty Liver Disease in a Mouse Model of NAFLD
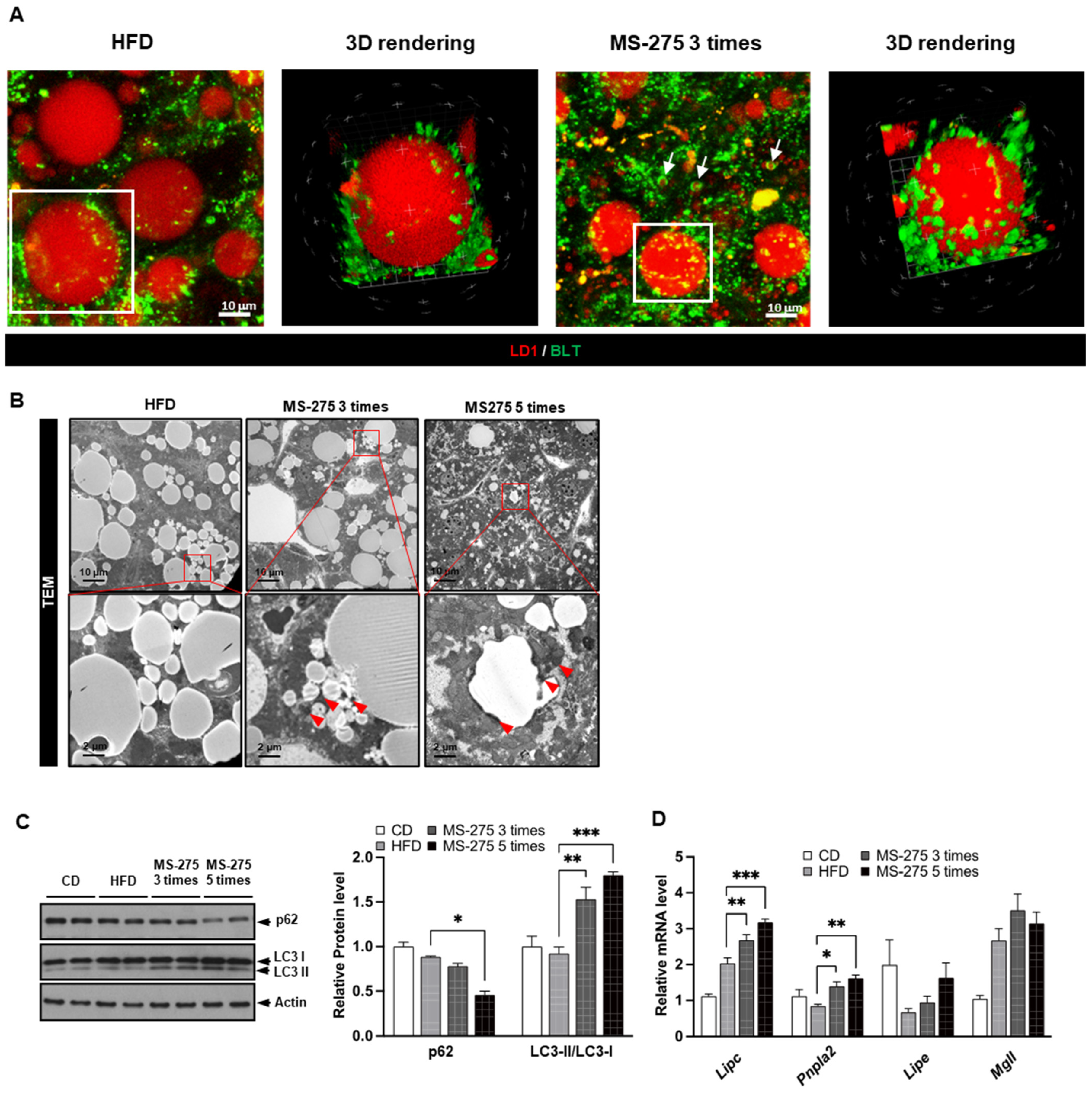
2.3. MS-275 Stimulated Dynamic Degradation of Hepatic LDs in Mouse Fatty Liver
2.4. MS-275 Increased Lipophagy- and Lipolysis-Related Gene Expression in Mouse Fatty Liver
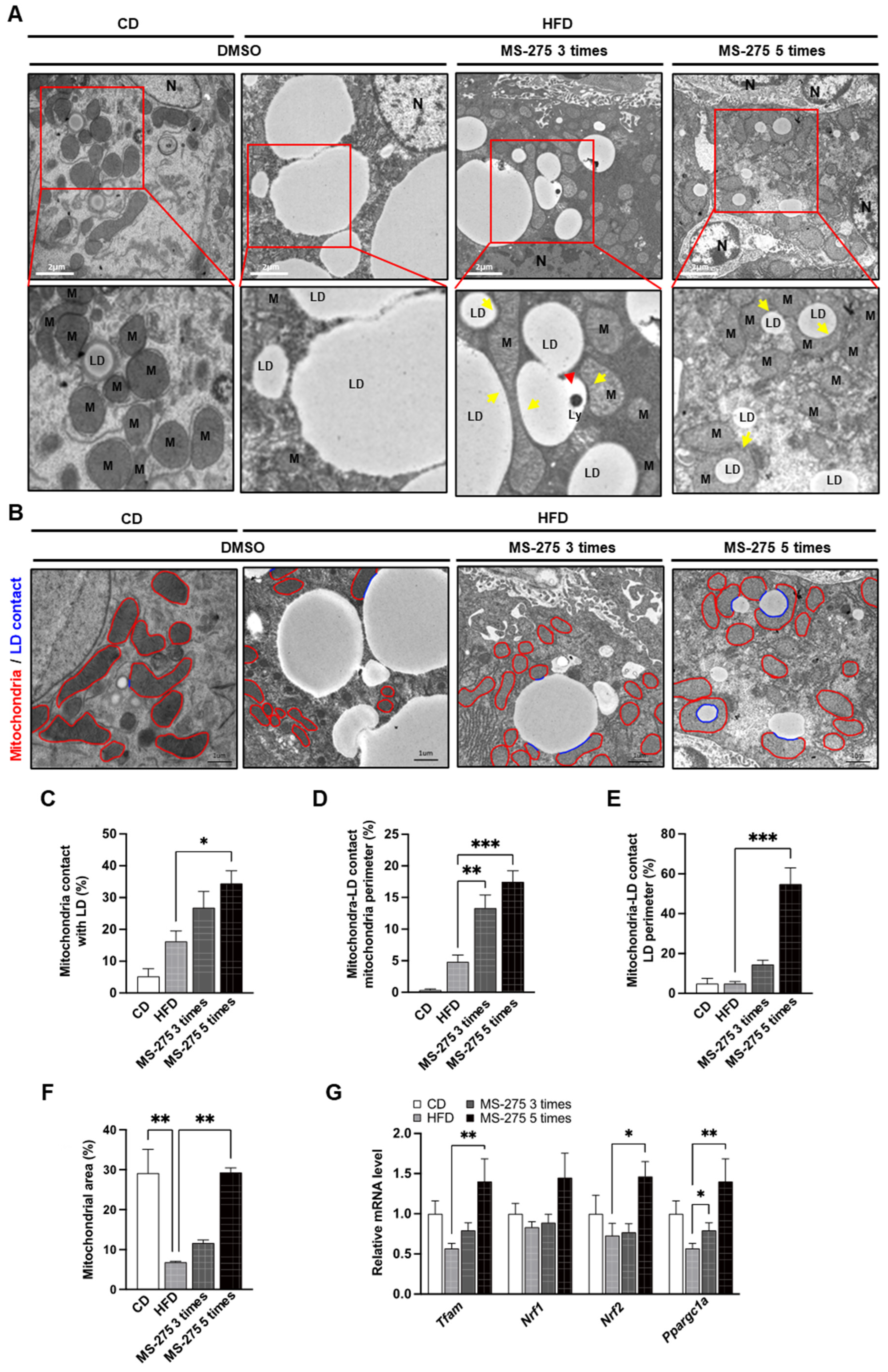
2.5. MS-275 Increased Mitochondrial Biogenesis and Content, and Peri Lipid-Droplet Mitochondria
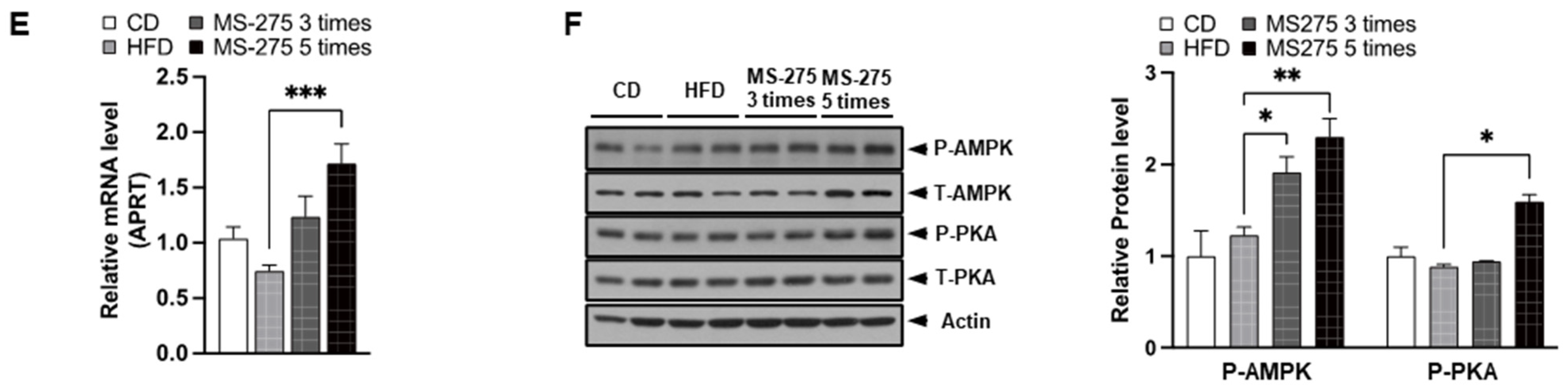
2.6. MS-275 Increased Mitochondrial Oxidation and FGF21 Expression
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Reagents
4.3. Intravital Imaging of the Liver by Two-Photon Microscopy
4.4. Image Data Analysis
4.5. TG Measurements
4.6. Histological Analysis
4.7. AST and ALT Measurements
4.8. Transmission Electron Microscopy
4.9. Isolation of Mouse primary Hepatocytes
4.10. Measurement of FAO Rate
4.11. Western Blot Analysis
4.12. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Mohanty, S.R. Nonalcoholic fatty liver disease: A review and update. Dig. Dis. Sci. 2010, 55, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Nagle, C.A.; Klett, E.L.; Coleman, R.A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009, 50, S74–S79. [Google Scholar] [CrossRef]
- Ferrari, A.; Fiorino, E.; Giudici, M.; Gilardi, F.; Galmozzi, A.; Mitro, N.; Cermenati, G.; Godio, C.; Caruso, D.; De Fabiani, E.; et al. Linking epigenetics to lipid metabolism: Focus on histone deacetylases. Mol. Membr. Biol. 2012, 29, 257–266. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Wei, C.; Xiang, Y.; Liang, X.; Phang, C.W.; Jiao, R. HDAC6 regulates lipid droplet turnover in response to nutrient deprivation via p62-mediated selective autophagy. J. Genet. Genom. 2019, 46, 221–229. [Google Scholar] [CrossRef]
- Qian, H.; Chen, Y.; Nian, Z.; Su, L.; Yu, H.; Chen, F.J.; Zhang, X.; Xu, W.; Zhou, L.; Liu, J.; et al. HDAC6-mediated acetylation of lipid droplet-binding protein CIDEC regulates fat-induced lipid storage. J. Clin. Investig. 2017, 127, 1353–1369. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, Y.E.; Chang, J.Y.; Park, K.C.; Kim, H.W.; Kim, J.T.; Kim, H.J.; Yi, H.S.; Shong, M.; Chung, H.K.; et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am. J. Transl. Res. 2016, 8, 4750–4763. [Google Scholar] [PubMed]
- Ferrari, A.; Longo, R.; Fiorino, E.; Silva, R.; Mitro, N.; Cermenati, G.; Gilardi, F.; Desvergne, B.; Andolfo, A.; Magagnotti, C.; et al. HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat. Commun. 2017, 8, 93. [Google Scholar] [CrossRef]
- Galmozzi, A.; Mitro, N.; Ferrari, A.; Gers, E.; Gilardi, F.; Godio, C.; Cermenati, G.; Gualerzi, A.; Donetti, E.; Rotili, D.; et al. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 2013, 62, 732–742. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, S.E.; Lee, H.B.; Song, M.W.; Kim, Y.H.; Jeong, J.Y.; Kang, Y.; Kim, H.J.; Kim, T.H.; Jeon, J.Y.; et al. A class I histone deacetylase inhibitor attenuates insulin resistance and inflammation in palmitate-treated C2C12 myotubes and muscle of HF/HFr diet mice. Front. Pharmacol. 2020, 11, 601448. [Google Scholar] [CrossRef]
- He, B.; Moreau, R. R-α-lipoic acid and 4-phenylbutyric acid have distinct hypolipidemic mechanisms in hepatic cells. Biomedicines 2020, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Bele, S.; Girada, S.B.; Ray, A.; Gupta, A.; Oruganti, S.; Prakash Babu, P.; Rayalla, R.S.; Kalivendi, S.V.; Ibrahim, A.; Puri, V.; et al. MS-275, a class 1 histone deacetylase inhibitor augments glucagon-like peptide-1 receptor agonism to improve glycemic control and reduce obesity in diet-induced obese mice. Elife 2020, 9, e52212. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kong, E.; Lee, J.; Jung, J.; Kim, E.; Park, S.B.; Kim, P. Intravital longitudinal imaging of hepatic lipid droplet accumulation in a murine model for nonalcoholic fatty liver disease. Biomed. Opt. Express 2020, 11, 5132–5146. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Amusa, O.A. Sodium acetate-mediated inhibition of histone deacetylase alleviates hepatic lipid dysregulation and its accompanied injury in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2020, 128, 110226. [Google Scholar] [CrossRef] [PubMed]
- Diniz, A.B.; Antunes, M.M.; Lacerda, V.A.S.; Nakagaki, B.N.; Freitas Lopes, M.A.; Castro-Oliveira, H.M.; Mattos, M.S.; Mafra, K.; de Miranda, C.D.M.; de Oliveira Costa, K.M.; et al. Imaging and immunometabolic phenotyping uncover changes in the hepatic immune response in the early phases of NAFLD. JHEP Rep. 2020, 2, 100117. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; Surewaard, B.G.J.; Turk, M.; Carestia, A.; Lee, W.-Y.; Petri, B.; Urbanski, S.J.; Coffin, C.S.; Jenne, C.N. Optimization of in vivo imaging provides a first look at mouse model of non-alcoholic fatty liver disease (NAFLD) using intravital microscopy. Front. Immunol. 2020, 10, 2988. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Li, Y.; Li, Y.; Sha, Y.; Wen, S.; You, Q.; Liu, L.; Shi, M.; Zhou, H. Intravital imaging of interactions between iNKT and kupffer cells to clear free lipids during steatohepatitis. Theranostics 2021, 11, 2149–2169. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Chong, S.Y.; Wang, X.; Chen, H.; Chen, C.; Ng, L.G.; Wang, J.-W.; Liu, B. In vivo three-photon imaging of lipids using ultrabright fluorogens with aggregation-induced emission. Adv. Mater. 2021, 33, 2007490. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Chapman, K.D.; Aziz, M.; Dyer, J.M.; Mullen, R.T. Mechanisms of lipid droplet biogenesis. Biochem. J. 2019, 476, 1929–1942. [Google Scholar] [CrossRef]
- Khawar, M.B.; Gao, H.; Li, W. Autophagy and lipid metabolism. Adv. Exp. Med. Biol. 2019, 1206, 359–374. [Google Scholar] [CrossRef]
- Grefhorst, A.; van de Peppel, I.P.; Larsen, L.E.; Jonker, J.W.; Holleboom, A.G. The role of lipophagy in the development and treatment of non-alcoholic fatty liver disease. Front. Endocrinol. 2020, 11, 601627. [Google Scholar] [CrossRef] [PubMed]
- Krumm, C.S.; Xu, X.; Bare, C.J.; Holman, C.D.; Kersten, S.; Dow, L.E.; Lee, A.H.; Cohen, D.E. Inducible hepatic expression of CREBH mitigates diet-induced obesity, insulin resistance, and hepatic steatosis in mice. J. Biol. Chem. 2021, 297, 100815. [Google Scholar] [CrossRef] [PubMed]
- Geißler, C.; Krause, C.; Neumann, A.M.; Britsemmer, J.H.; Taege, N.; Grohs, M.; Kaehler, M.; Cascorbi, I.; Lewis, A.G.; Seeley, R.J.; et al. Dietary induction of obesity and insulin resistance is associated with changes in Fgf21 DNA methylation in liver of mice. J. Nutr. Biochem. 2022, 100, 108907. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Roe, C.R.; Roe, D.S.; Wallace, M.; Garritson, B. Choice of oils for essential fat supplements can enhance production of abnormal metabolites in fat oxidation disorders. Mol. Genet. Metab. 2007, 92, 346–350. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Moon, J.; Jeon, J.; Kong, E.; Hong, S.; Lee, J.; Lee, E.K.; Kim, P. Intravital two-photon imaging and quantification of hepatic steatosis and fibrosis in a live small animal model. Biomed. Opt. Express. 2021, 12, 7918–7927. [Google Scholar] [CrossRef]
- Yu, X.D.; Wang, J.W. Ceramide de novo synthesis in non-alcoholic fatty liver disease: Pathogenic mechanisms and therapeutic perspectives. Biochem. Pharmacol. 2022, 202, 115157. [Google Scholar] [CrossRef]
- Fucho, R.; Casals, N.; Serra, D.; Herrero, L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 2017, 31, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Bikman, B.T.; Summers, S.A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Investig. 2011, 121, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Meyers-Needham, M.; Ponnusamy, S.; Gencer, S.; Jiang, W.; Thomas, R.J.; Senkal, C.E.; Ogretmen, B. Concerted functions of HDAC1 and microRNA-574-5p repress alternatively spliced ceramide synthase 1 expression in human cancer cells. EMBO Mol. Med. 2012, 4, 78–92. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Patel, M.; Chandrasekaran, S. Metabolism, HDACs, and HDAC inhibitors: A systems biology perspective. Metabolites 2021, 11, 792. [Google Scholar] [CrossRef]
- Ferrari, A.; Fiorino, E.; Longo, R.; Barilla, S.; Mitro, N.; Cermenati, G.; Giudici, M.; Caruso, D.; Mai, A.; Guerrini, U.; et al. Attenuation of diet-induced obesity and induction of white fat browning with a chemical inhibitor of histone deacetylases. Int. J. Obes. 2017, 41, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Knutson, S.K.; Chyla, B.J.; Amann, J.M.; Bhaskara, S.; Huppert, S.S.; Hiebert, S.W. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J. 2008, 27, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Miller, R.A.; Patel, R.T.; Chen, J.; Dhir, R.; Wang, H.; Zhang, D.; Graham, M.J.; Unterman, T.G.; Shulman, G.I.; et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat. Med. 2012, 18, 934–942. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Y.-W.; Zhao, Z.-H.; Yang, R.-X.; Xin, F.-Z.; Liu, X.-L.; Pan, Q.; Zhou, H.; Fan, J.-G. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Donde, H.; Ghare, S.; Joshi-Barve, S.; Zhang, J.; Vadhanam, M.V.; Gobejishvili, L.; Lorkiewicz, P.; Srivastava, S.; McClain, C.J.; Barve, S. Tributyrin inhibits ethanol-induced epigenetic repression of CPT-1A and attenuates hepatic steatosis and injury. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 569–585. [Google Scholar] [CrossRef]
- Papazyan, R.; Sun, Z.; Kim, Y.H.; Titchenell, P.M.; Hill, D.A.; Lu, W.; Damle, M.; Wan, M.; Zhang, Y.; Briggs, E.R.; et al. Physiological suppression of lipotoxic liver damage by complementary actions of HDAC3 and SCAP/SREBP. Cell Metab. 2016, 24, 863–874. [Google Scholar] [CrossRef]
- Sarver, A.E.; Wang, C.C. The adenine phosphoribosyltransferase from Giardia lamblia has a unique reaction mechanism and unusual substrate binding properties. J. Biol. Chem. 2002, 277, 39973–39980. [Google Scholar] [CrossRef] [Green Version]
- Gonneaud, A.; Turgeon, N.; Boisvert, F.M.; Boudreau, F.; Asselin, C. Loss of histone deacetylase Hdac1 disrupts metabolic processes in intestinal epithelial cells. FEBS Lett. 2015, 589, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Krenek, K.A.; Fang, C.; Minamoto, Y.; Markel, M.E.; Suchodolski, J.S.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolic derivatives from mango (Mangifera indica L.) modulate fecal microbiome, short-chain fatty acids production and the HDAC1/AMPK/LC3 axis in rats with DSS-induced colitis. J. Funct. Foods 2018, 48, 243–251. [Google Scholar] [CrossRef]
- Viollet, B.; Horman, S.; Leclerc, J.; Lantier, L.; Foretz, M.; Billaud, M.; Giri, S.; Andreelli, F. AMPK inhibition in health and disease. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Sun, X.; Chaggan, C.; Liao, Z.; In Wong, K.; He, F.; Singh, S.; Loomba, R.; Karin, M.; Witztum, J.L.; et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science 2020, 367, 652–660. [Google Scholar] [CrossRef]
- Zhao, P.; Saltiel, A.R. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J. Biol. Chem. 2020, 295, 12279–12289. [Google Scholar] [CrossRef]
- Sun, L.; Marin de Evsikova, C.; Bian, K.; Achille, A.; Telles, E.; Pei, H.; Seto, E. Programming and regulation of metabolic homeostasis by HDAC11. EBioMedicine 2018, 33, 157–168. [Google Scholar] [CrossRef]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef]
- Szczepanska, E.; Gietka-Czernel, M. FGF21: A novel regulator of glucose and lipid metabolism and whole-body energy balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef]
- Talukdar, S.; Zhou, Y.; Li, D.; Rossulek, M.; Dong, J.; Somayaji, V.; Weng, Y.; Clark, R.; Lanba, A.; Owen, B.M.; et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016, 23, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Wang, J.; Wang, Z.; Liao, H.M.; Wei, M.; Leeds, P.; Chuang, D.M. Valproic acid and other HDAC inhibitors upregulate FGF21 gene expression and promote process elongation in glia by inhibiting HDAC2 and 3. Int. J. Neuropsychopharmacol. 2016, 19, pyw035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, Q.; Deng, R.; Zhou, F.; Zhang, L.; Wang, S.; Zhu, K.; Wang, X.; Zhou, L.; Su, Q. MS-275 induces hepatic FGF21 expression via H3K18ac-mediated CREBH signal. J. Mol. Endocrinol. 2019, 62, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Park, S.K.; Lim, C.S.; Park, M.K.; Kim, H.J.; Kim, H.M.; Cho, B.R. Simultaneous imaging of mitochondria and lysosomes by using two-photon fluorescent probes. Chemistry 2012, 18, 15246–15249. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, I.J.; Lee, S.J.; Kim, Y.R.; Kim, H.M. Highly sensitive two-photon lipid droplet tracker for in vivo screening of drug induced liver injury. ACS Sens. 2022, 7, 1027–1035. [Google Scholar] [CrossRef]
- Park, S.A.; Choe, Y.H.; Lee, S.H.; Hyun, Y.M. Two-photon intravital imaging of leukocytes during the immune response in lipopolysaccharide-treated mouse liver. J. Vis. Exp. 2018, 132, 57191. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-G.; Lee, S.-J.; Park, S.; Choi, S.-E.; Song, M.-W.; Lee, H.W.; Kim, H.J.; Kang, Y.; Lee, K.W.; Kim, H.M.; et al. In Vivo Two-Photon Imaging Analysis of Dynamic Degradation of Hepatic Lipid Droplets in MS-275-Treated Mouse Liver. Int. J. Mol. Sci. 2022, 23, 9978. https://doi.org/10.3390/ijms23179978
Lee C-G, Lee S-J, Park S, Choi S-E, Song M-W, Lee HW, Kim HJ, Kang Y, Lee KW, Kim HM, et al. In Vivo Two-Photon Imaging Analysis of Dynamic Degradation of Hepatic Lipid Droplets in MS-275-Treated Mouse Liver. International Journal of Molecular Sciences. 2022; 23(17):9978. https://doi.org/10.3390/ijms23179978
Chicago/Turabian StyleLee, Chang-Gun, Soo-Jin Lee, Seokho Park, Sung-E Choi, Min-Woo Song, Hyo Won Lee, Hae Jin Kim, Yup Kang, Kwan Woo Lee, Hwan Myung Kim, and et al. 2022. "In Vivo Two-Photon Imaging Analysis of Dynamic Degradation of Hepatic Lipid Droplets in MS-275-Treated Mouse Liver" International Journal of Molecular Sciences 23, no. 17: 9978. https://doi.org/10.3390/ijms23179978





