Implication of miR-155-5p and miR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque
Abstract
:1. Introduction
2. Results
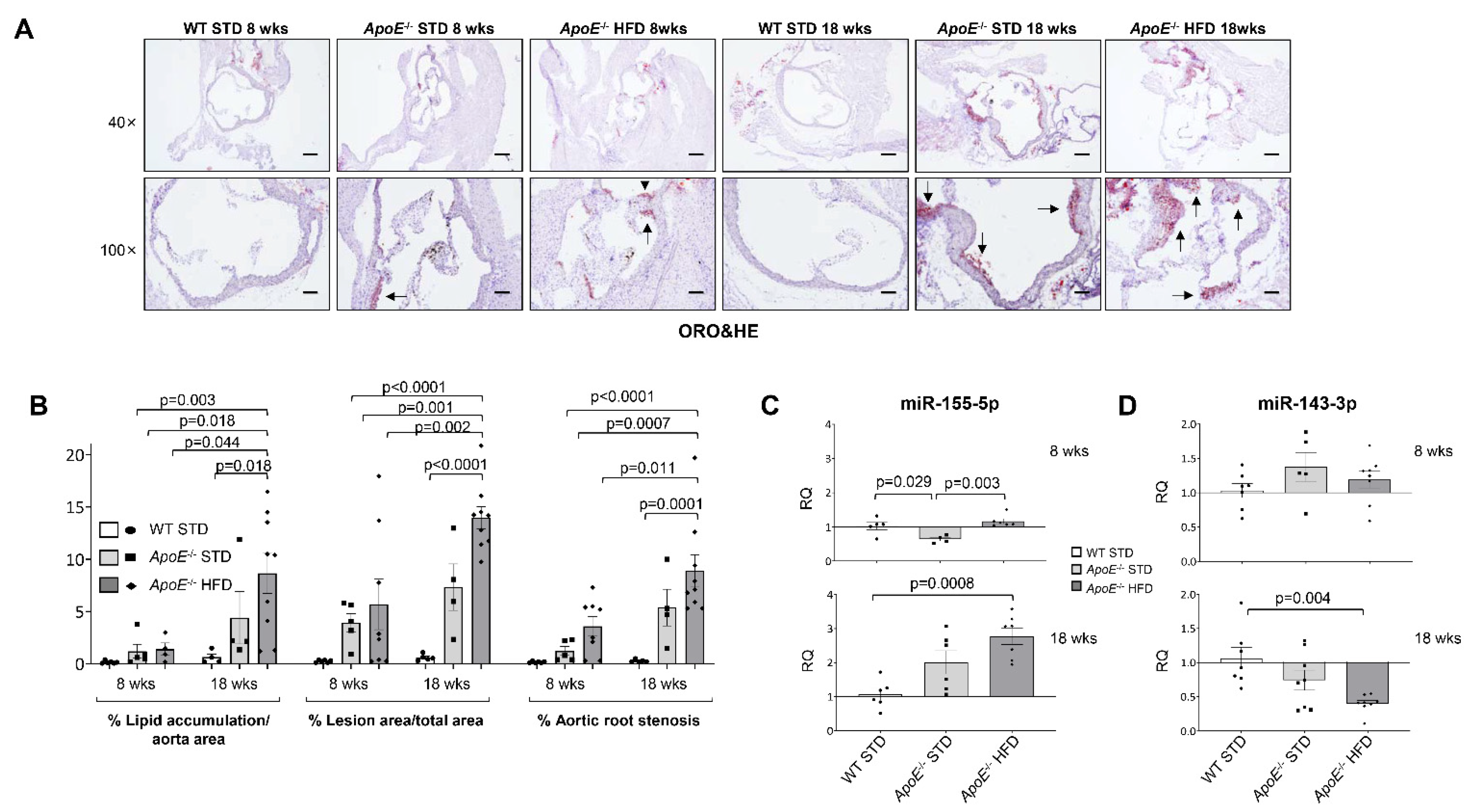
2.1. miR-143-3p and miR-155-5p Levels Are Altered in Aorta from ApoE−/− Mice
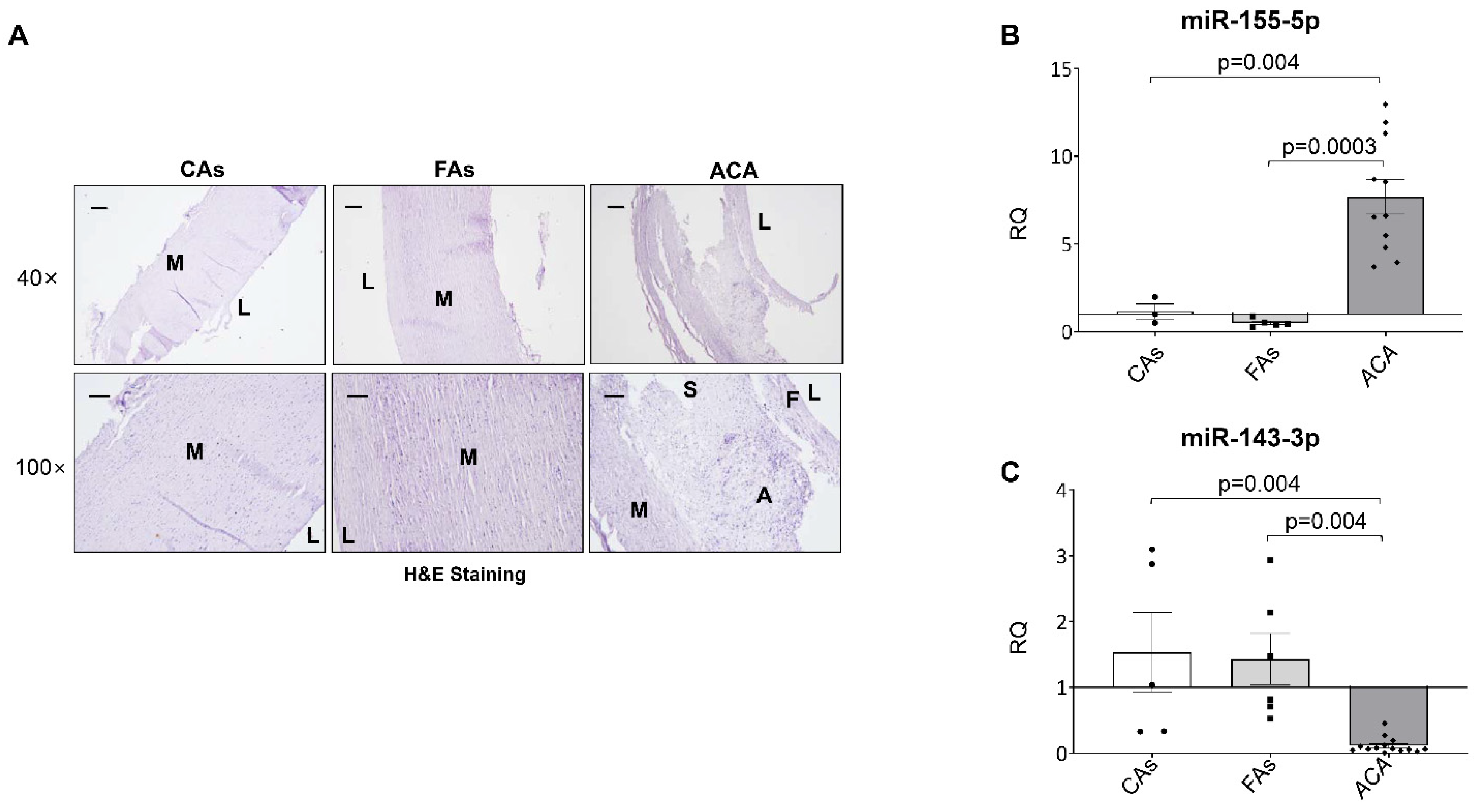
2.2. miR-143-3p and miR-155-5p Levels Are Also Altered in Human Atherosclerotic Carotid Plaque
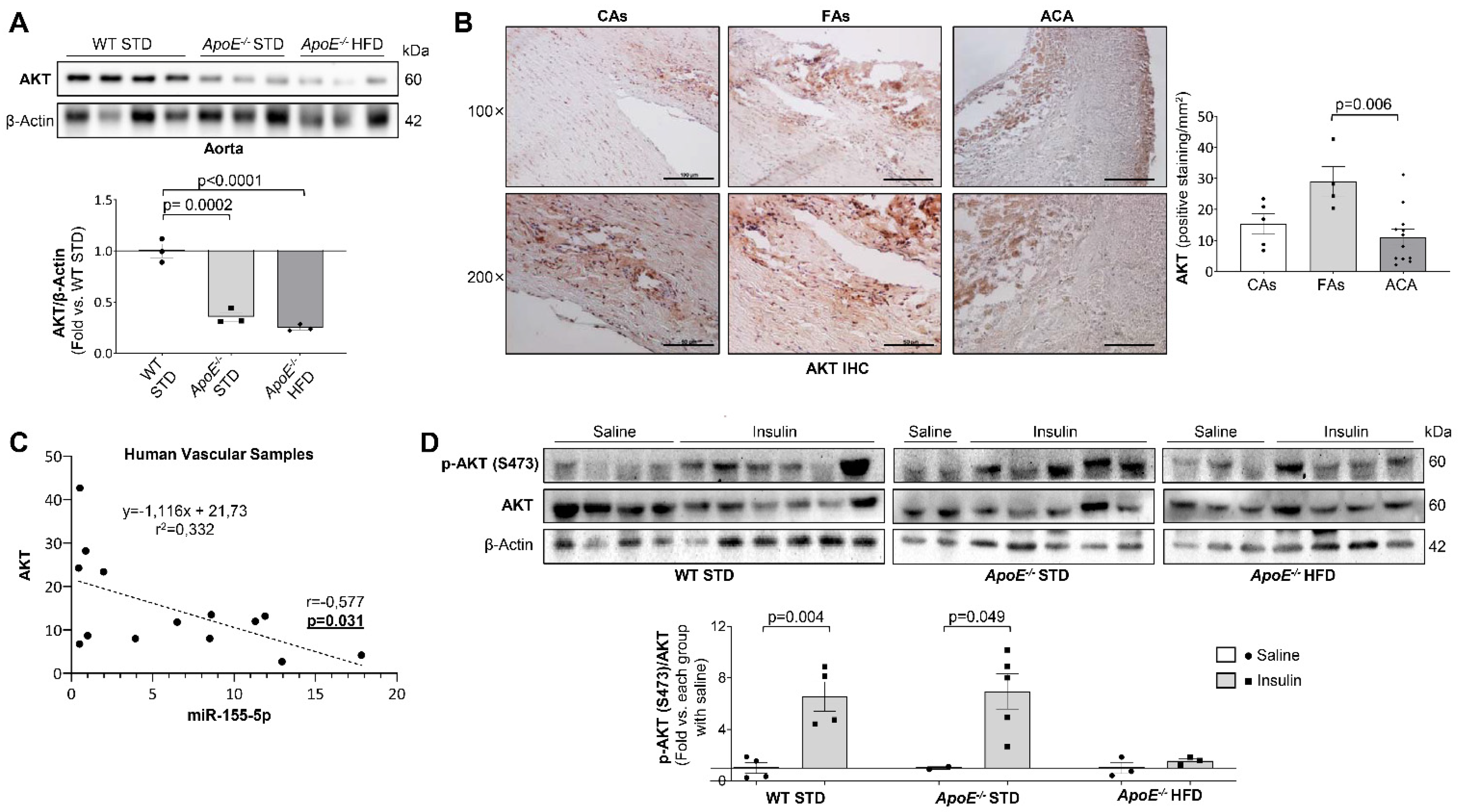
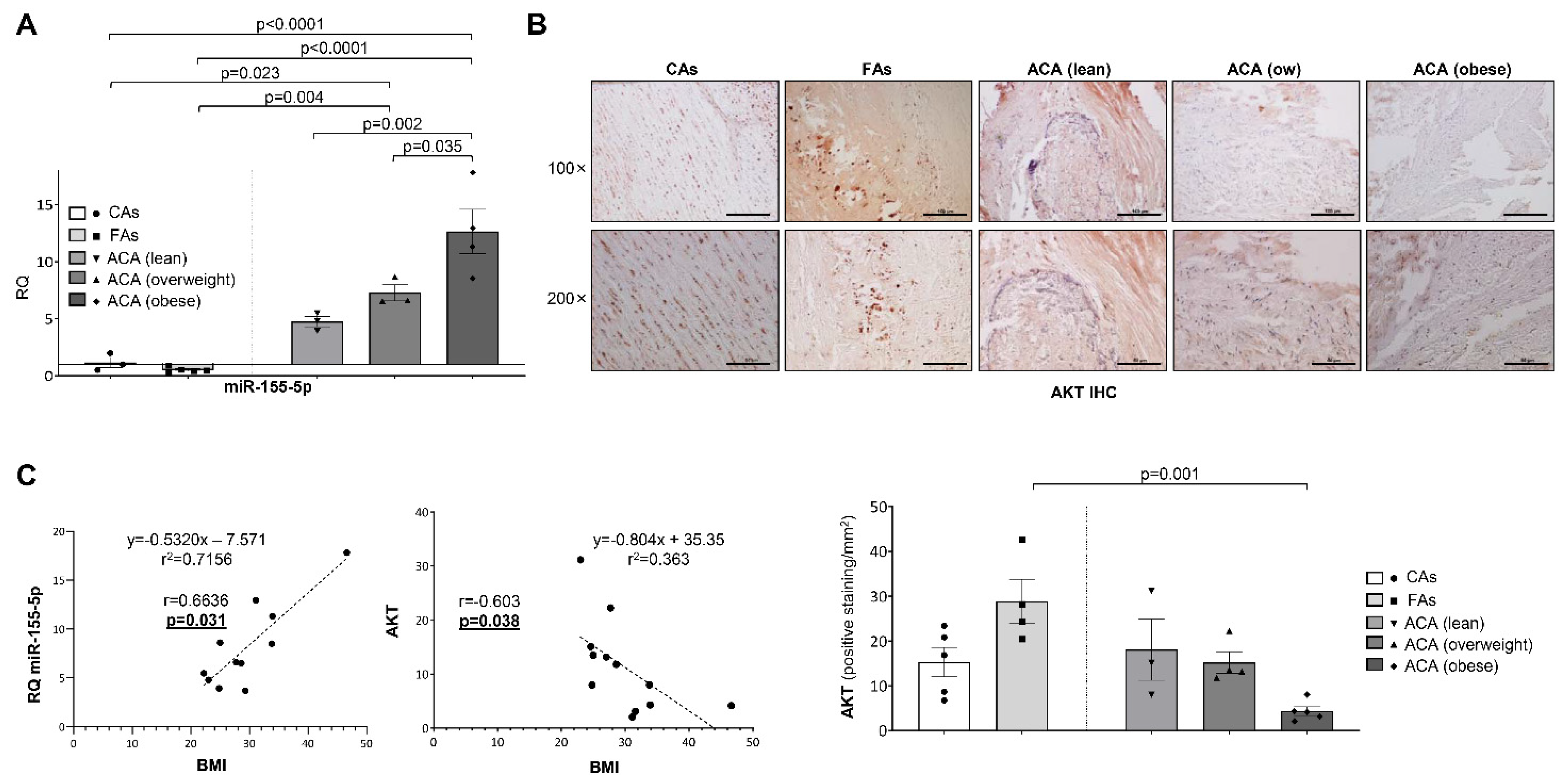
2.3. AKT Expression Is Modulated by miR-155-5p Levels in Atherosclerosis and Non-Alcoholic Fatty Liver Disease
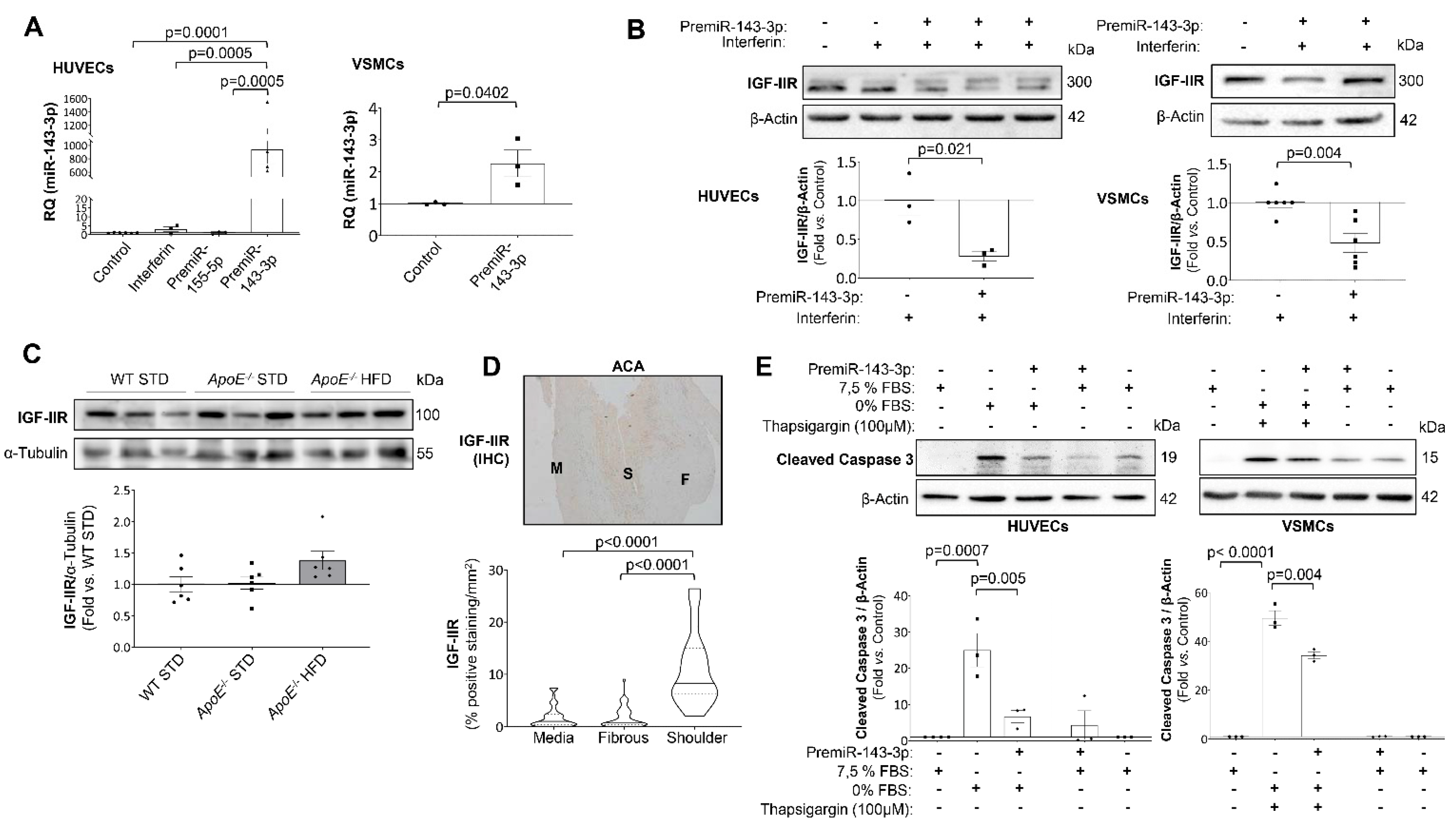
2.4. IGF-IIR Expression Is Modulated by miR-143-3p
3. Discussion
4. Materials and Methods
4.1. Human Samples
4.2. Animal Model
4.3. Cell Culture
4.4. Histological Tissue Samples
4.5. En Face Imaging of Aorta
4.6. miRNA Extraction from the Aorta, Liver, Vascular Cell Lines and Paraffin-Embedded Carotid Tissue
4.7. Cell Transfection with miRNA Precursors
4.8. RT-qPCR Analysis
4.9. Western Blot Analysis
4.10. Database Search to Find miRNAs and Their Possible Targets
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Herz 2019, 44, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Nahrendorf, M.; Swirski, F.K. Hematopoiesis and cardiovascular disease. Circ. Res. 2020, 126, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Muris, D.M.J.; Houben, A.J.H.M.; Schram, M.T.; Stehouwer, C.D.A. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: A systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3082–3094. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; de las Heras, N.; López-Pastor, A.R.; García-Gómez, G.; Infante-Menéndez, J.; González-López, P.; González-Illanes, T.; Lahera, V.; Benito, M.; Escribano, O. Severe hepatic insulin resistance induces vascular dysfunction: Improvement by liver-specific insulin receptor isoform a gene therapy in a murine diabetic model. Cells 2021, 10, 2035. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, S.; Feng, Y.; Shen, J.; Zhao, J. MicroRNA-24-3p inhibition prevents cell growth of vascular smooth muscle cells by targeting Bcl-2-like protein 11. Exp. Ther. Med. 2020, 19, 2467–2474. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Volsi, G.L.; Pitruzzella, A.; Fiore, V.; Mangiafico, M.; Vanella, L.; Parenti, R.; Rizzo, M.; Volti, G.L. Circulating miR-130a, miR-27b, and miR-210 in Patients with Peripheral Artery Disease and Their Potential Relationship with Oxidative Stress. Angiology 2016, 67, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Giglio, R.V.; Nikolic, D.; Volti, G.L.; Stoian, A.P.; Banerjee, Y.; Magan-Fernandez, A.; Castellino, G.; Patti, A.M.; Chianetta, R.; Castracani, C.C.; et al. Liraglutide Increases Serum Levels of MicroRNA-27b, -130a and -210 in Patients with Type 2 Diabetes Mellitus: A Novel Epigenetic Effect. Metabolites 2020, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shan, S.; Huo, Y.; Xie, Z.; Fang, Y.; Qi, Z.; Chen, F.; Li, Y.; Sun, B. MiR-155-5p inhibits PDK1 and promotes autophagy via the mTOR pathway in cervical cancer. Int. J. Biochem. Cell Biol. 2018, 99, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wei, Y.; Geißler, C.; Abschlag, K.; Campos, J.C.; Hristov, M.; Möllman, J.; Lehrke, M.; Karshovska, E.; Schober, A. Hyperlipidemia-induced MicroRNA-155-5p improves β-cell function by targeting Mafb. Diabetes 2017, 66, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Hu, J.; Luo, G.; Song, D.; Zhang, P.; Zhu, J.; Sun, F. MiR-155-5p Promotes Oxalate- And Calcium-Induced Kidney Oxidative Stress Injury by Suppressing MGP Expression. Oxid. Med. Cell Longev. 2020, 2020, 5863617. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, J.J.; Deng, W.Y.; Ren, K.; Yin, S.H.; Yu, X.H. CTRP12 ameliorates atherosclerosis by promoting cholesterol efflux and inhibiting inflammatory response via the miR-155-5p/LXRα pathway. Cell Death Dis. 2021, 12, 254. [Google Scholar] [CrossRef]
- Xie, F.; Li, C.; Zhang, X.; Peng, W.; Wen, T. MiR-143-3p suppresses tumorigenesis in pancreatic ductal adenocarcinoma by targeting KRAS. Biomed. Pharmacother. 2019, 119, 109424. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shen, H.; Xu, J.; Zhao, S.; Yao, S.Z.; Jiang, N. MiR-143-3p suppresses the progression of ovarian cancer. Am. J. Transl. Res. 2018, 10, 866–874. [Google Scholar]
- Ma, W.Y.; Song, R.J.; Xu, B.B.; Xu, Y.; Wang, X.X.; Sun, H.Y.; Liu, S.Z.; Yu, M.X.; Yang, F.; Ye, D.Y.; et al. Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, D.; Wang, D. LINC00528 regulates myocardial infarction by targeting the miR-143-3p/COX-2 axis. Bioengineered 2020, 11, 11–18. [Google Scholar] [CrossRef]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary atherosclerotic vulnerable plaque: Current perspectives. J. Am. Heart Assoc. 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, T.; Yang, L.; Li, Z.; Wong, M.M.; Zheng, X.; Pan, X.; Zhang, L.; Yan, H. Regulation of MicroRNA-155 in Atherosclerotic Inflammatory Responses by Targeting MAP3K10. PLoS ONE 2012, 7, e46551. [Google Scholar] [CrossRef]
- Bruen, R.; Fitzsimons, S.; Belton, O. MiR-155 in the resolution of atherosclerosis. Front. Pharmacol. 2019, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Donners, M.M.P.C.; Wolfs, I.M.J.; Stöger, L.J.; van der Vorst, E.P.C.; Pöttgens, C.C.H.; Heymans, S.; Schroen, B.; Gijbels, M.J.J.; Winther, M.P.J. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS ONE 2012, 7, 4–12. [Google Scholar] [CrossRef]
- Zhu, G.F.; Yang, L.X.; Guo, R.W.; Liu, H.; Shi, Y.K.; Wang, H.; Ye, J.S.; Yang, Z.H.; Liang, X. MiR-155 inhibits oxidized low-density lipoprotein-induced apoptosis of RAW264.7 cells. Mol. Cell Biochem. 2013, 382, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Jahantigh, M.; Wei, Y.; Noels, H.; Akhtar, S.; Zhou, Z.; Koenen, R.R.; Heyll, K.; Gremse, F.; Kiessling, F.; Grommes, J.; et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Investig. 2012, 122, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yu, F.; Wang, Y.; Hui, Y.; Carnevale, K.; Fu, M.; Lu, H.; Fan, D. MicroRNA-155 Deficiency Results in Decreased Macrophage Inflammation and Attenuated Atherogenesis in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 759–767. [Google Scholar] [CrossRef]
- Singh, S.; de Ronde, M.W.J.; Kok, M.G.M.; Beijk, M.A.; De Winter, R.J.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.M.; Creemers, E.E.; Pinto-Sietsma, S.J. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef]
- Fitzsimons, S.; Oggero, S.; Bruen, R.; McCarthy, C.; Strowitzki, M.J.; Mahon, N.G.; Ryan, N.; Brennan, E.P.; Barry, M.; Perretti, M.; et al. microRNA-155 Is Decreased During Atherosclerosis Regression and Is Increased in Urinary Extracellular Vesicles During Atherosclerosis Progression. Front. Inmunol. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, S.Y.; Yang, C.Q.; Ma, B.M.; Jiang, D. MiR-155-5p inhibits the proliferation and migration of VSMCs and HUVECs in atherosclerosis by targeting AKT1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2223–2233. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal 2018, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yan, C.; Zhang, L.; Li, Y.; Wan, Q. LncRNA ENST00113 promotes proliferation, survival, and migration by activating PI3K/Akt/mTOR signaling pathway in atherosclerosis. Medicine 2018, 97, e0473. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Kulyté, A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol 2015, 11, 276–288. [Google Scholar] [CrossRef]
- Ortega, F.J.; Moreno, M.; Mercader, J.M.; Moreno-Navarrete, J.M.; Fuentes-Batllevell, N.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin. Epigenetics 2015, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Moreno-Navarrete, J.M.; Pardo, G.; Sabater, M.; Hummel, M.; Ferrer, A.; Rodriguez-Hermosa, J.I.; Ruiz, B.; Ricart, W.; Peral, B.; et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS ONE 2010, 5, e9022. [Google Scholar] [CrossRef]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-κB signaling. J. Mol. Cell Biol 2011, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-κB Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef]
- Subedi, A.; Park, P.H. Autocrine and paracrine modulation of microRNA-155 expression by globular adiponectin in RAW 264.7 macrophages: Involvement of MAPK/NF-κB pathway. Cytokine 2013, 64, 638–641. [Google Scholar] [CrossRef]
- Martín-Ventura, J.L.; Blanco-Colio, L.M.; Muñoz-García, B.; Gómez-Hernández, A.; Arribas, A.; Ortega, L.; Tuñón, J.; Egido, J. NF-κB Activation and Fas Ligand Overexpression in Blood and Plaques of Patients with Carotid Atherosclerosis: Potential Implication in Plaque Instability. Stroke 2004, 35, 458–463. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Martín-Ventura, J.L.; Sánchez-Galán, E.; Vidal, C.; Ortego, M.; Blanco-Colio, L.M.; Ortega, L.; Tuñón, J.; Egido, J. Overexpression of COX-2, Prostaglandin E Synthase-1 and Prostaglandin E Receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: Regulation by nuclear factor-κB. Atherosclerosis 2006, 187, 139–149. [Google Scholar] [CrossRef]
- López-Franco, O.; Hernández-Vargas, P.; Ortiz-Muñoz, G.; Sanjuán, G.; Suzuki, Y.; Ortega, L.; Blanco, J.; Egido, J.; Gómez-Guerrero, C. Parthenolide modulates the NF-κB-mediated inflammatory responses in experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Scoditti, E.; Carpi, S.; Massaro, M.; Pellegrino, M.; Polini, B.; Carluccio, M.A.; Wabitsch, M.; Verri, T.; Nieri, P.; Caterina, R. Hydroxytyrosol modulates adipocyte gene and mirna expression under inflammatory condition. Nutrients 2019, 11, 2493. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K.; Gushchina, L.V.; Aubrecht, T.G.; Maurya, S.K.; Periasamy, M.; Nelson, R.J.; Popovich, P.G. MIR-155 deletion in female mice prevents diet-induced obesity. Sci. Rep. 2016, 6, 22862. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Huijsman, E.; Van De Par, C.; Economou, C.; Van Der Poel, C.; Lynch, G.S.; Schoiswohl, G.; Haemmerle, G.; Zechner, R.; Watt, M.J. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 505–513. [Google Scholar] [CrossRef]
- Li, Y.; Fromme, T.; Schweizer, S.; Schöttl, T.; Klingenspor, M. Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep. 2014, 15, 1069–1076. [Google Scholar] [CrossRef]
- Pan, M.; Deng, Y.; Zheng, C.; Nie, H.; Tang, K.; Zhang, Y.; Yang, Q. Chinese Herbal Medicine Formula Shenling Baizhu San Ameliorates High-Fat Diet-Induced NAFLD in Rats by Modulating Hepatic MicroRNA Expression Profiles. Evid. Based Complement. Alternat. Med. 2019, 2019, 8479680. [Google Scholar] [CrossRef]
- Miller, A.M.; Gilchrist, D.S.; Nijjar, J.; Araldi, E.; Ramirez, C.M.; Lavery, C.A.; Fernández-Hernando, C.; Mclnnes, I.B.; Kurowska-Stolarska, M. MiR-155 Has a Protective Role in the Development of Non-Alcoholic Hepatosteatosis in Mice. PLoS ONE 2013, 8, e72324. [Google Scholar] [CrossRef]
- Csak, T.; Bala, S.; Lippai, D.; Kodys, K.; Catalano, D.; Iracheta-Vellve, A.; Szabo, G. MicroRNA-155 Deficiency Attenuates Liver Steatosis and Fibrosis without Reducing Inflammation in a Mouse Model of Steatohepatitis. PLoS ONE 2015, 10, 1–21. [Google Scholar] [CrossRef]
- Liu, Q.; Du, G.Q.; Zhu, Z.T.; Zhang, C.; Sun, X.W.; Liu, J.J.; Li, X.; Wang, Y.S.; Du, W.J. Identification of apoptosis—Related microRNAs and their target genes in myocardial infarction post—Transplantation with skeletal myoblasts. J. Transl. Med. 2015, 13, 1–11. [Google Scholar] [CrossRef]
- Xihua, L.I.N.; Shengjie, T.; Weiwei, G.U.I.; Matro, E.; Tingting, T.A.O.; Lin, L.I.; Fang, W.; Jiaqiang, Z.; Fenping, Z.; Hong, L. Circulating miR-143-3p inhibition protects against insulin resistance in Metabolic Syndrome via targeting of the insulin-like growth factor 2 receptor. Transl. Res. 2019, 205, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, P.; Dahms, N.M.; Kornfeld, S. Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Biol. 2003, 4, 202–212. [Google Scholar] [CrossRef]
- Zaina, S.; Nilsson, J. Insulin-like growth factor II and its receptors in atherosclerosis and in conditions predisposing to atherosclerosis. Curr. Opin. Lipidol. 2003, 14, 483–489. [Google Scholar] [CrossRef]
- Beneit, N.; Luis, J.; Ventura, M.; Longás, C.R.; Escribano, Ó.; Gómez-García, G.; Fernández, S.; Sesti, G.; Hribal, M.L.; Egido, J.; et al. Potential role of insulin receptor isoforms and IGF receptors in plaque instability of human and experimental atherosclerosis. Cardiovasc. Diabetol. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, W.W.; Yeh, Y.L.; Ho, T.J.; Lin, J.Y.; Lin, D.Y.; Chu, C.H.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y. ANG II promotes IGF-IIR expression and cardiomyocyte apoptosis by inhibiting HSF1 via JNK activation and SIRT1 degradation. Cell Death Differ. 2014, 21, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, A.; Escribano, Ó.; Perdomo, L.; Otero, Y.F.; García-Gómez, G.; Fernández, S.; Beneit, N.; Benito, M. Implication of insulin receptor A isoform and IRA/IGF-IR hybrid receptors in the aortic vascular smooth muscle cell proliferation: Role of TNF-α and IGF-II. Endocrinology 2013, 154, 2352–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-López, P.; Ares-Carral, C.; López-Pastor, A.R.; Infante-Menéndez, J.; González Illaness, T.; Vega de Ceniga, M.; Esparza, L.; Beneit, N.; Martín-Ventura, J.L.; Escribano, Ó.; et al. Implication of miR-155-5p and miR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque. Int. J. Mol. Sci. 2022, 23, 10253. https://doi.org/10.3390/ijms231810253
González-López P, Ares-Carral C, López-Pastor AR, Infante-Menéndez J, González Illaness T, Vega de Ceniga M, Esparza L, Beneit N, Martín-Ventura JL, Escribano Ó, et al. Implication of miR-155-5p and miR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque. International Journal of Molecular Sciences. 2022; 23(18):10253. https://doi.org/10.3390/ijms231810253
Chicago/Turabian StyleGonzález-López, Paula, Carla Ares-Carral, Andrea R. López-Pastor, Jorge Infante-Menéndez, Tamara González Illaness, Melina Vega de Ceniga, Leticia Esparza, Nuria Beneit, José Luis Martín-Ventura, Óscar Escribano, and et al. 2022. "Implication of miR-155-5p and miR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque" International Journal of Molecular Sciences 23, no. 18: 10253. https://doi.org/10.3390/ijms231810253
APA StyleGonzález-López, P., Ares-Carral, C., López-Pastor, A. R., Infante-Menéndez, J., González Illaness, T., Vega de Ceniga, M., Esparza, L., Beneit, N., Martín-Ventura, J. L., Escribano, Ó., & Gómez-Hernández, A. (2022). Implication of miR-155-5p and miR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque. International Journal of Molecular Sciences, 23(18), 10253. https://doi.org/10.3390/ijms231810253





