Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration
Abstract
1. Introduction
2. Results
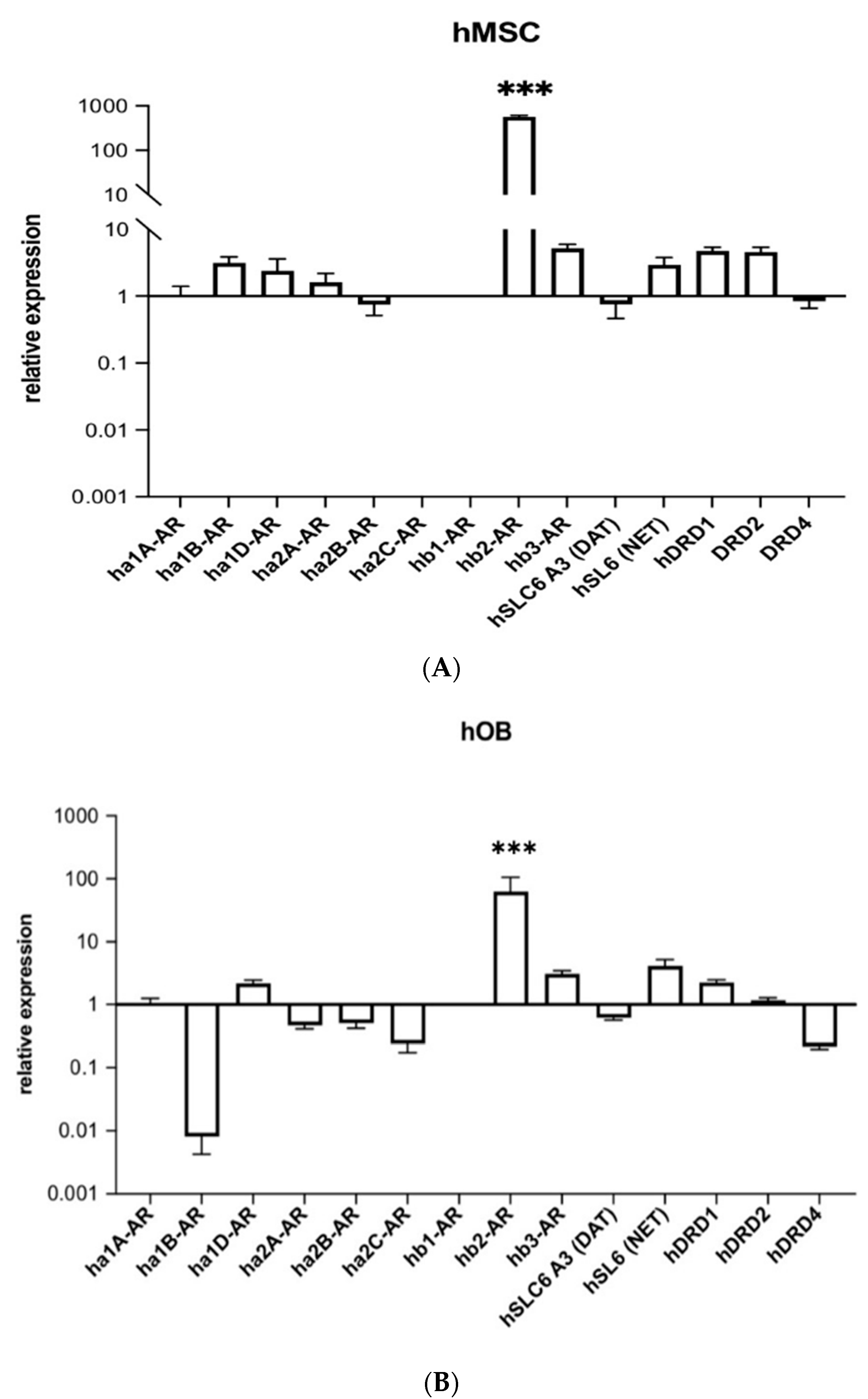
2.1. Human Beta-2 Adrenoreceptor Is Most Highly Expressed Adrenoceptor in hMSCs and Human Osteoblasts (hOBs)
2.2. ADHD-Medication Inhibits In Vitro Bone Formation
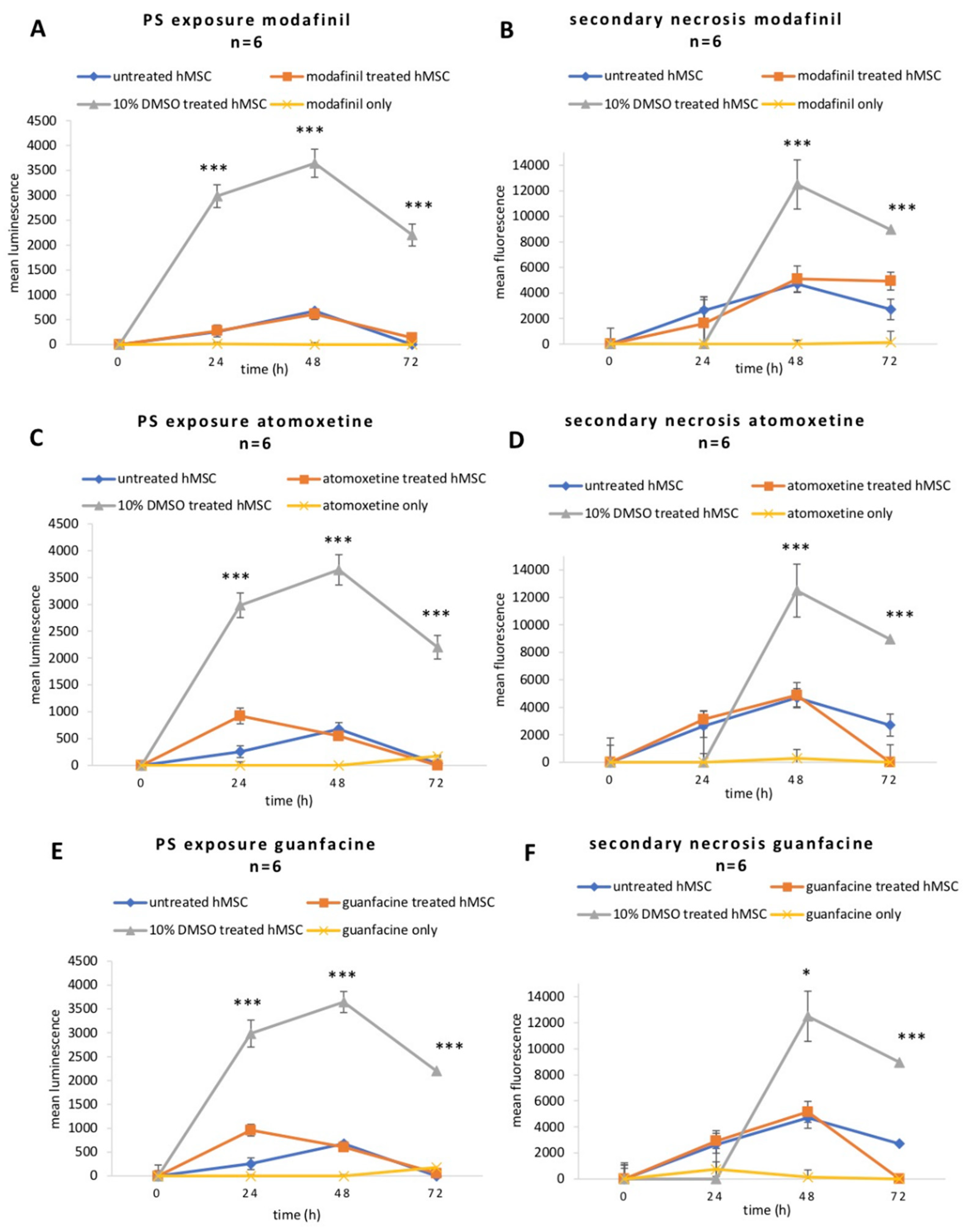
2.3. Modafinil, Atomoxetine and Guanfacine Do Not Cause Early Apoptosis and Secondary Necrosis in hMSCs
2.4. Modafinil, Atomoxetine and Guanfacine Lead to Transient Decrease in Cell Viability
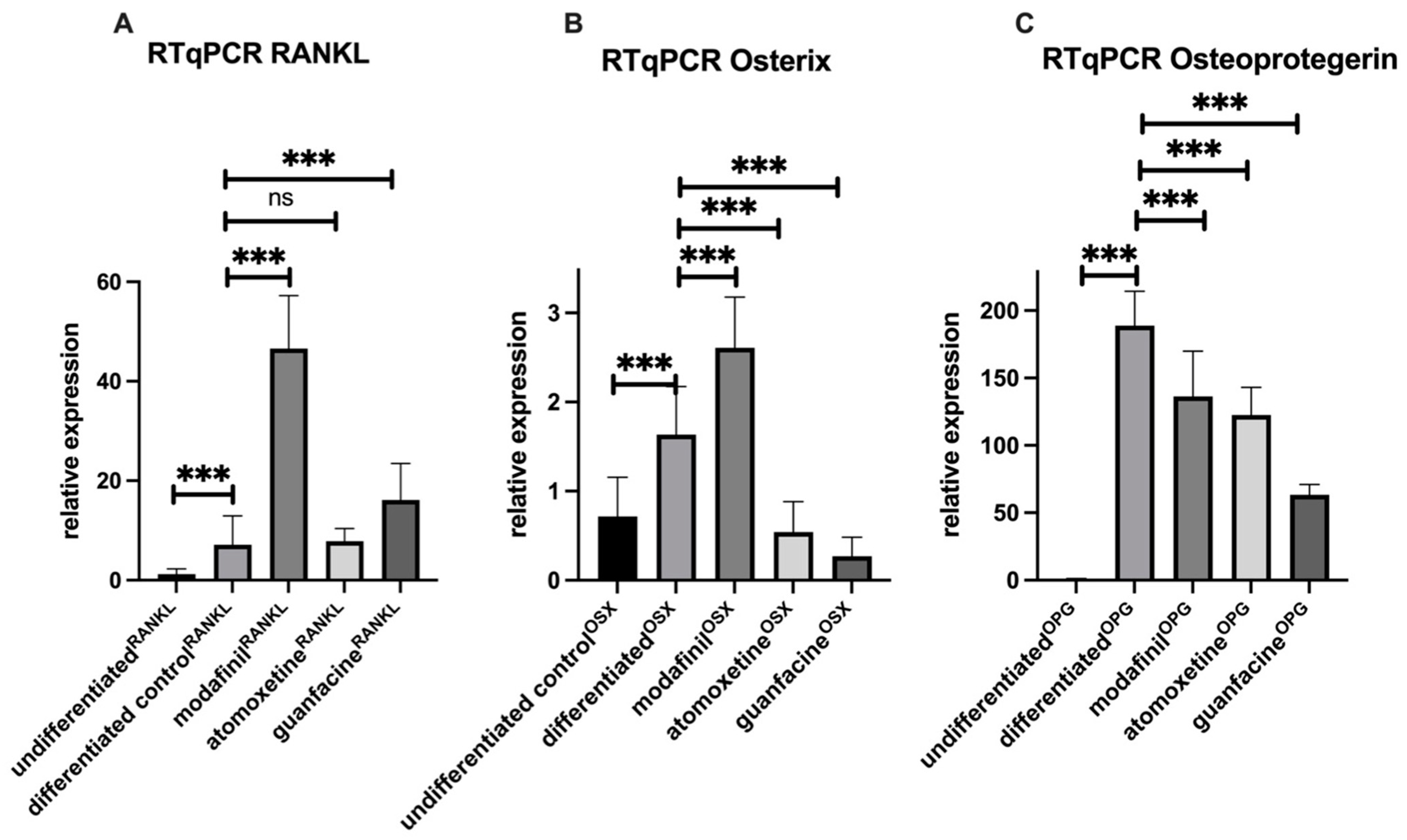
2.5. Modafinil and Guanfacine Increase the Expression of RANKL and OSX after 28 Days
2.6. Osteogenic, Adipogenic and Chondrogenic Transcripts Are Not Affected by Psychostimulants after 7 Days of Osteogenic Differentiation
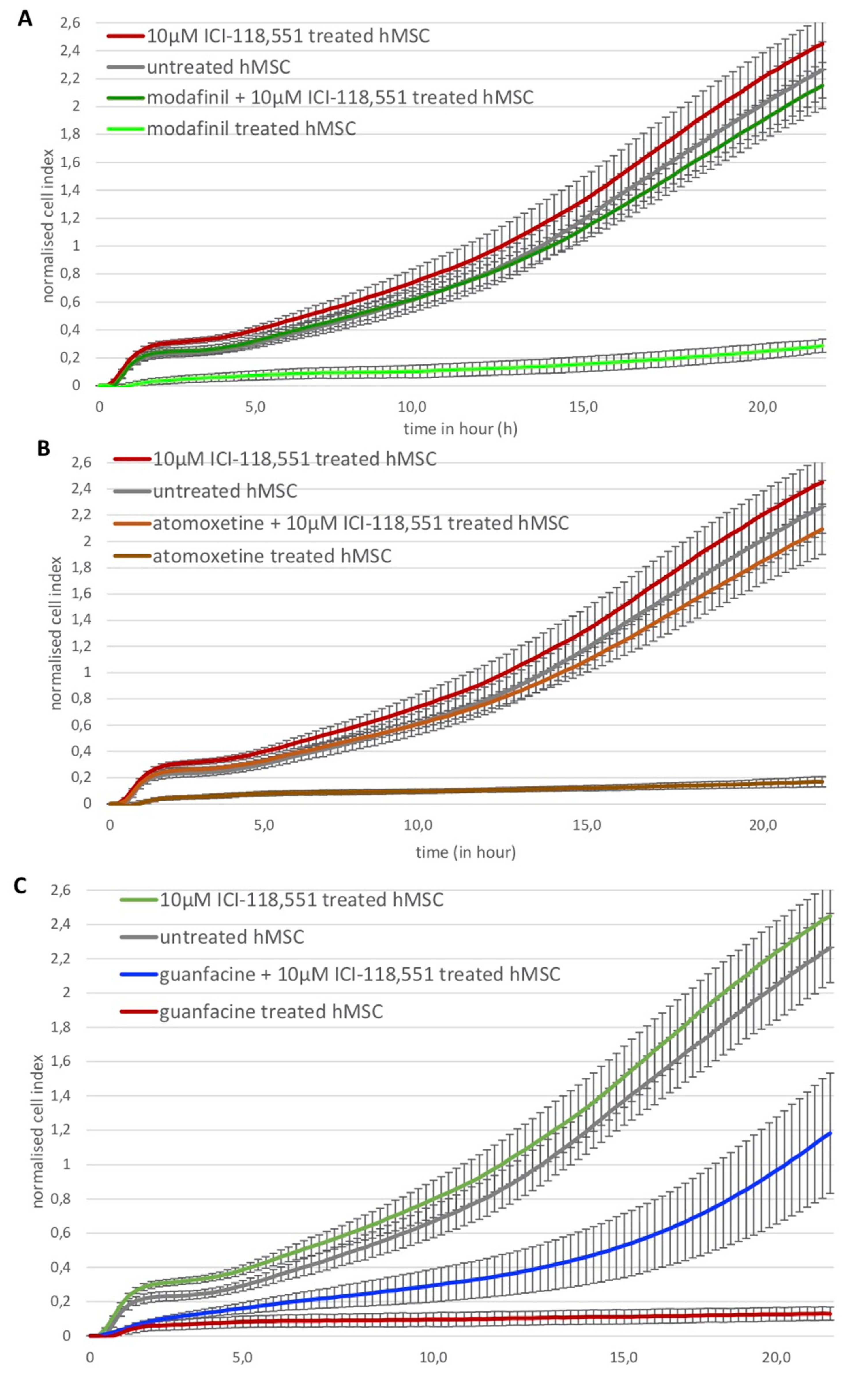
2.7. Modafinil, Atomoxetine and Guanfacine Inhibit hMSC Cell Migration
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Substances
4.3. Osteogenic Differentiation
4.4. RNA Isolation and Quantitative RT-PCR
4.5. Cell Titer-Blue Cell Viability Assay
4.6. Measurement of Apoptosis/Necrosis
4.7. Real-Time Cell Migration Analysis
4.8. Histochemical Stains
4.9. Software and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ADHD Statistics: Numbers, Facts and Information About ADD. Available online: https://www.additudemag.com/statistics-of-adhd/ (accessed on 26 March 2022).
- National Institute of Mental Health: Attention-Deficit/Hyperactivity Disorder in Children and Teens: What You Need to Know. Available online: https://www.nimh.nih.gov/health/publications/attention-deficit-hyperactivity-disorder-in-children-and-teens-what-you-need-to-know (accessed on 2 July 2022).
- Callahan, B.L.; Bierstone, D.; Stuss, D.T.; Black, S.E. Adult ADHD: Risk Factor for Dementia or Phenotypic Mimic? Front Aging Neurosci. 2017, 3, 260. [Google Scholar] [CrossRef]
- Uddin, S.M.Z.; Robinson, L.S.; Fricke, D.; Chernoff, E.; Hadjiargyrou, M.; Thanos, K.T.; Komatsu, D.E. Methylphenidate regulation of osteoclasts in a dose- and sex-dependent manner adversely affects skeletal mechanical integrity. Sci. Rep. 2018, 8, 1515. [Google Scholar] [CrossRef]
- London-Nadeau, K.; Chan, P.; Wood, S. Building Conceptions of Cognitive Enhancement: University Students’ Views on the Effects of Pharmacological Cognitive Enhancers. Subst. Use Misuse 2019, 54, 908–920. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Guanfacine’s mechanism of action in treating prefrontal cortical disorders: Successful translation across species. Neurobiol. Learn. Mem. 2020, 176, 107327. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, K.; Adams, N. Modafinil; StatPearls: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531476/ (accessed on 1 May 2022).
- Blackhall, L.; Petroni, G.; Shu, J.; Baum, L.; Farace, E. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J. Palliat. Med. 2019, 12, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E.; Barciela Veras, A.; Barbosa Rocha, N.; Budde, H.; Machado, S. An Overview of the Clinical Uses, Pharmacology, and Safety of Modafinil. ACS Chem. Neurosci. 2018, 9, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Minzenberg, M.J.; Carter, C.S. Modafinil: A Review of Neurochemical Actions and Effects on Cognition. Neuropsychopharmacology 2008, 33, 1477–1502. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, G.-F.; Markowitz, J.S. Atomoxetine: A Review of Its Pharmacokinetics and Pharmacogenomics Relative to Drug Disposition. J. Child Adolesc. Psychopharmacol. 2016, 26, 314–326. [Google Scholar] [CrossRef]
- Elefteriou, F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018, 98, 1083–1112. [Google Scholar] [CrossRef]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef]
- Kondo, H.; Nifuji, A.; Takeda, S.; Ezura, Y.; Rittling, S.R.; Denhardt, D.T.; Nakashima, K.; Karsenty, G.; Noda, M. Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J. Biol. Chem. 2005, 280, 30192–30200. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Kajimura, D.; Hinoi, E.; Ferron, M.; Kode, A.; Riley, K.J.; Zhou, B.; Guo, X.E.; Karsenty, G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J. Exp. Med. 2011, 208, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Side Effects of Methylphenidate in Children and the Young. Available online: https://pubmed.ncbi.nlm.nih.gov/29480512/ (accessed on 7 June 2022).
- Fischer, B.; O’Keefe-Markman, C.; Lee, A.M.; Daldegan-Bueno, D. Resurgent’, ‘twin’ or ‘silent’ epidemic? A select data overview and observations on increasing psycho-stimulant use and harms in North America. Subst. Abuse Treat. Prev. Policy 2021, 16, 17. [Google Scholar] [CrossRef]
- China, S.P.; Chattopadhyay, S.; Porwal, K.; Mittal, M.; Sanyal, S.; Chattopadhyay, N. The wakefulness promoting drug Modafinil causes adenosine receptor-mediated upregulation of receptor activator of nuclear factor κB ligand in osteoblasts: Negative impact of the drug on peak bone accrual in rats. Toxicol. Appl. Pharmacol. 2018, 348, 22–31. [Google Scholar] [CrossRef]
- Ma, Y.; Krueger, J.J.; Redmon, S.N.; Uppuganti, S.; Nyman, J.S.; Hahn, M.K.; Elefteriou, F. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J. Biol. Chem. 2013, 288, 30105–30113. [Google Scholar] [CrossRef]
- Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Kanareikina, M.A.; Kalinina, N.I.; Sysoeva, V.Y.; Dyikanov, D.T.; Stambolsky, D.V.; Tkachuk, V.A. Activation of β-adrenergic receptors is required for elevated α1A-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci. Rep. 2016, 6, 32835. [Google Scholar] [CrossRef]
- Huang, H.H.; Brennan, T.C.; Muir, M.M.; Mason, R.S. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J. Cell. Physiol. 2009, 220, 267–275. [Google Scholar] [CrossRef]
- Limraksasin, P.; Kondo, T.; Zhang, M.; Okawa, H.; Osathanon, T.; Pavasant, P.; Egusa, H. In Vitro Fabrication of Hybrid Bone/Cartilage Complex Using Mouse Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 581. [Google Scholar] [CrossRef]
- Ben-Ami, I.S.; Ankory, R.; Kadar, A.; Rotman, D.; Snir, N.; Schermann, H. The Effect of Previous Methylphenidate Use on Incidence of Stress Fractures in Military Recruits: A Retrospective Cohort. J. Bone Joint Surg. Am. 2018, 100, 930–935. [Google Scholar] [CrossRef]
- Stress Fracture—An Overview; ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/stress-fracture (accessed on 7 June 2022).
- Schermann, H.; Ben-Ami, I.S.; Tudor, A.; Amar, E.; Rath, E.; Yanovich, R. Past Methylphenidate Exposure and Stress Fractures in Combat Soldiers: A Case-Control Study. Am. J. Sports Med. 2018, 46, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.; Yang, Y.H.; Liao, Y.T.; Kuo, T.Y.; Liang, H.Y.; Huang, K.Y.; Huang, Y.C.; Lee, Y.; McIntyre, R.S.; Lin, T.C. The association between methylphenidate treatment and the risk for fracture among young ADHD patients: A nationwide population-based study in Taiwan. PLoS ONE 2017, 12, 0173762. [Google Scholar]
- Kong, N.; Bao, Y.; Zhao, H.; Kang, X.; Tai, X.; Shen, Y. Methylphenidate causes cytotoxicity on photoreceptor cells via autophagy. Hum. Exp. Toxicol. 2021, 40, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.V.; Miller, B.R.; Gross, G.W. Acute and sub-chronic functional neurotoxicity of methylphendate on neural networks in vitro. J. Neural Transm. 2007, 114, 1365–1375. [Google Scholar] [CrossRef]
- Kaya, Y.E.; Karaarslan, N.; Sirin, D.Y.; Ozbek, H.; Kaplan, N.; Yilmaz, I. Investigation of the Effects of Methylphenidate, an Amphetamine Derivative, on Intervertebral Disc Tissue Cell Cultures and Matrix Structures. Turk. Neurosurg. 2019, 29, 734–742. [Google Scholar] [CrossRef]
- Penna, L.M.C.; Düsman, E.; Vicentini, V.E.P. The mutagenic effect of psychostimulant modafinil in Wistar rats in vivo. Drug Chem. Toxicol. 2020, 43, 373–377. [Google Scholar] [CrossRef]
- Novotna, A.; Kamenickova, A.; Pecova, M.; Korhonova, M.; Bartonkova, I.; Dvorak, Z. Profiling of enantiopure drugs towards aryl hydrocarbon (AhR), glucocorticoid (GR) and pregnane X (PXR) receptors in human reporter cell lines. Chem. Biol. Interact. 2014, 208, 64–76. [Google Scholar] [CrossRef]
- Lee, H.; Son, J.; Yi, G.; Koo, H.; Park, J.B. Cellular viability and osteogenic differentiation potential of human gingiva-derived stem cells in 2D culture following treatment with anionic, cationic, and neutral liposomes containing doxorubicin. Exp. Ther. Med. 2018, 16, 4457–4462. [Google Scholar] [CrossRef]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef]
- Chou, I.-C.; Lin, C.-C.; Sung, F.-C.; Kao, C.-H. Attention-deficit-hyperactivity disorder increases risk of bone fracture: A population-based cohort study. Dev. Med. Child Neurol. 2014, 56, 1111–1116. [Google Scholar] [CrossRef]
- Pullar, C.E.; Isseroff, R.R. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J. Cell Sci. 2006, 119, 592–602. [Google Scholar] [CrossRef]
- Wnorowski, A.; Sadowska, M.; Paul, R.K.; Singh, N.S.; Boguszewska-Czubara, A.; Jimenez, L.; Abdelmohsen, K.; Toll, L.; Jozwiak, K.; Bernier, M.; et al. Activation of β2-adrenergic receptor by (R,R′)-4′-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells. Cell Signal. 2015, 27, 997–1007. [Google Scholar] [CrossRef][Green Version]
- Işeri, Ö.D.; Sahin, F.H.; Terzi, Y.K.; Yurtcu, E.; Erdem, S.R.; Sarialioglu, F. beta-Adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm. Biol. 2014, 52, 1374–1381. [Google Scholar] [CrossRef]
- Bravo-Calderón, D.M.; Assao, A.; Garcia, N.G.; Coutinho-Camillo, C.M.; Roffé, M.; Germano, J.N.; Oliveira, D.T. Beta adrenergic receptor activation inhibits oral cancer migration and invasiveness. Arch. Oral Biol. 2020, 118, 104865. [Google Scholar] [CrossRef]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef]
- Darwish, M.; Kirby, M.; D’Andrea, D.M.; Yang, R.; Hellriegel, E.T.; Robertson, P., Jr. Pharmacokinetics of armodafinil and modafinil after single and multiple doses in patients with excessive sleepiness associated with treated obstructive sleep apnea: A randomized, open-label, crossover study. Clin. Ther. 2010, 32, 2074–2087. [Google Scholar] [CrossRef]
- Boellner, S.W.; Pennick, M.; Fiske, K.; Lyne, A.; Shojaei, A. Pharmacokinetics of a guanfacine extended-release formulation in children and adolescents with attention-deficit-hyperactivity disorder. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1253–1262. [Google Scholar] [CrossRef]
- Functional α1- and β2-Adrenergic Receptors in Human Osteoblasts. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/jcp.21761 (accessed on 5 July 2022).
- Identification of the mRNA Expression Status of the Dopamine D2 Receptor and Dopamine Transporter in Peripheral Blood Lymphocytes of Schizophrenia Patients. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3783374/ (accessed on 5 July 2022).
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucl. Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Dragnev, S.; Thurber, T.; Seed, B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genom. 2008, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [CrossRef] [PubMed]



| Component | Volume/Concentration | Company |
|---|---|---|
| DMEM (low glucose) | 500 mL | Sigma Aldrich, Taufkirchen, Germany |
| Fetal calf serum (FCS) | 50 mL (10%) | PAN Biotech, Aidenbach, Germany |
| Penicillin/Streptomicin | 5 mL (1%) | PAN Biotech, Aidenbach, Germany |
| Ascorbic acid-2 phosphate | 200 µM | Cayman chemical company, Ann Arbor MI, USA |
| ß-glycerolphosphate | 10 mM | Carl Roth, Karlsruhe, Germany |
| Dexamethasone | 0.1 µM | Carl Roth, Karlsruhe, Germany |
| Gene | Primer Sequences |
|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | For: 5′-AGGTCGGAGTCAACGGAT-3′ |
| Rev: 5′-TCCTGGAAGATGGTGATG-3′ | |
| α1A-adrenoreceptor (ADRA1A) | For: 5′-ATCATCTCCATCGACCGCTACA-3′ |
| Rev: 5′-TCACTTGCTCCGAGTCCGACTT-3′ | |
| α1B-adrenoreceptor (ADRA1B) | For: 5′-CCATTCCAAGAACTTTCACGA-3′ |
| Rev: 5′-CCAGAACACCACCTTGAACAC-3′ | |
| α1D-adrenoreceptor (ADRA1D) | For: 5′-TCTTTTCGGGGTGCTGGGTAA-3′ |
| Rev: 5′-TGGGTGACGATGGTTGGGTAG-3′ | |
| α2A-adrenoreceptor (ADRA2A) | For: 5′-TCGTCATCATCGCCGTGTTCA-3′ |
| Rev: 5′-GCCGCCGCCGCCCTTCTTCTC-3′ | |
| α2B-adrenoreceptor (ADRA2B) | For: 5′-GGGAGACCCCTGAAGATACTG-3′ |
| Rev: 3′-ACAAAAACGCCAATGACCACA-5′ | |
| α2C-adrenoreceptor (ADR2C) | For: 5′-GTGGTGATCGCCGTGCTGAC-3′ |
| Rev: 5′-CGTTTTCGGTAGTCGGGGAC-3′ | |
| b1-adrenoreceptor (ADRB1) | For: 5′-GCCATCGCCTCGTCCGTAGTC-3′ |
| Rev: 5′-CGTAGCCCAGCCAGTTGAAGA-3′ | |
| b2-adrenoreceptor (ADRB2) | For: 5′-TCTGATGGTGTGGATTGTGTC-3′ |
| Rev: 5′-ACGTCTTGAGGGCTTTGTGCT-3′ | |
| b3-adrenoreceptor (ADRB3) | For: 5′-CCCAATACCGCCAACACCAGT-3′ |
| Rev: 5′-CGACCCACACCAGGACCACAG-3′ | |
| Dopamine transporter 1 (SLC6A3) | For: 5′-CGGCCAGACCAAGAGGGAAGAAGCA 3′ |
| Rev: 5′-TGGGCACACTGGGAGTTGAGGAA-3′ | |
| Norepinephrine transporter (SLC6A2) | For: 5′-GGCGTTGGCTATGCTGTCAT-3′ |
| Rev: 5′-AGCTTGGGGTCGGTACAGTT-3′ | |
| Dopamine receptor 1 (DRD1) | For: 5′-TGGTCTGTGCTGCCGTTATCAG-3′ |
| Rev: 5′-CAATCTCAGCCACTGCCTTCCA-3′ | |
| Dopamine receptor 2 (DRD2) | For: 5′-CAATACGCGCTACAGCTCCAAG-3′ |
| Rev: 5′-GGCAATGATGCACTCGTTCTGG-3′ | |
| Dopamine transporter 4 (DRD4) | For: 5′-TGCTGCCGCTCTTCGTCTACTC-3′ |
| Rev: 5′-ACAGGTTGAAGATGGAGGCGGT-3′ |
| Gene | Primer Sequences |
|---|---|
| GAPDH | For: 5′-AGGTCGGAGTCAACGGAT-3′ |
| Rev: 5′-TCCTGGAAGATGGTGATG-3′ | |
| Osteoprotegerin (TNFRSF11B) | For: 5′-GCGCTCGTGTTTCTGGACA-3′ |
| Rev: 5′-AGTATAGACACTCGTCACTGGTG-3′ | |
| RANKL (TNFSF11) | For: 5′-CAACATATCGTTGGATCACAGCA-3′ |
| Rev: 5′-GACAGACTCACTTTATGGGAACC-3′ | |
| Osterix (SP7) | For: 5′-CCTCTGCGGGACTCAACAAC-3′ |
| Rev: 5′-AGCCCATTAGTGCTTGTAAAGG -3′ | |
| Nanog Homeobox(NANOG) | For: 5′-ACCTCAGCCTCCAGCAGATG-3′ |
| Rev: 5′-TGCACCAGGTCTGAGTGTTC-3′ | |
| Octamer binding transcription factor 4(OCT-4) | For: 5′-GATCACCCTGGGTATACAC-3′ |
| Rev: 5′-GCTTTGCATATCTCCTGAAG-3′ | |
| Sex-determining region Y (SRY)-box 2 (SOX2) | For: 5′-ATGGGTTCGGTGGTGGTCAAG-3′ |
| Rev: 5′-GGCAGTGTGCCGTTAATG-3′ | |
| Fatty acid-binding protein 4 (FABP4) | For: 5′-ACTGGGCCAGGAATTTGACG-3′ |
| Rev: 5′-CTCGTGGAAGTGACGCCTT-3′ | |
| Lipoprotein lipase (LPL) | For: 5′-TCATTCCCGGAGTAGCAGAGT-3′ |
| Rev: 5′-GGCCACAAGTTTTGGCACC-3′ | |
| Adiponectin, C1Q And Collagen Domain Containing(ADIPOQ) | For: 5′-AACATGCCCATTCGCTTTACC-3′ |
| Rev: 5′-TAGGCAAAGTAGTACAGCCCA-3′ | |
| ACAN (Aggrecan) | For: 5′-GTGCCTATCAGGACAAGGTCT-3′ |
| Rev: 5′-GATGCCTTTCACCACGACTTC-3′ | |
| Collagen Type II Alpha 1 Chain (COL2A1) | For: 5′-CCAGATGACCTTCCTACGCC-3′ |
| Rev: 5′-TTCAGGGCAGTGTACGTGAAC-3′ | |
| SRY-Box Transcription Factor9(SOX9) | For: 5′-AGCGAACGCACATCAAGA-3′ |
| Rev: 5′-CTGTAGGCGATCTGTTGGGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagener, N.; Lehmann, W.; Weiser, L.; Jäckle, K.; Di Fazio, P.; Schilling, A.F.; Böker, K.O. Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration. Int. J. Mol. Sci. 2022, 23, 10257. https://doi.org/10.3390/ijms231810257
Wagener N, Lehmann W, Weiser L, Jäckle K, Di Fazio P, Schilling AF, Böker KO. Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration. International Journal of Molecular Sciences. 2022; 23(18):10257. https://doi.org/10.3390/ijms231810257
Chicago/Turabian StyleWagener, Nele, Wolfgang Lehmann, Lukas Weiser, Katharina Jäckle, Pietro Di Fazio, Arndt F. Schilling, and Kai O. Böker. 2022. "Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration" International Journal of Molecular Sciences 23, no. 18: 10257. https://doi.org/10.3390/ijms231810257
APA StyleWagener, N., Lehmann, W., Weiser, L., Jäckle, K., Di Fazio, P., Schilling, A. F., & Böker, K. O. (2022). Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration. International Journal of Molecular Sciences, 23(18), 10257. https://doi.org/10.3390/ijms231810257








