Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum africanum with Potential Application in Urology
Abstract
1. Introduction
2. Results and Discussion
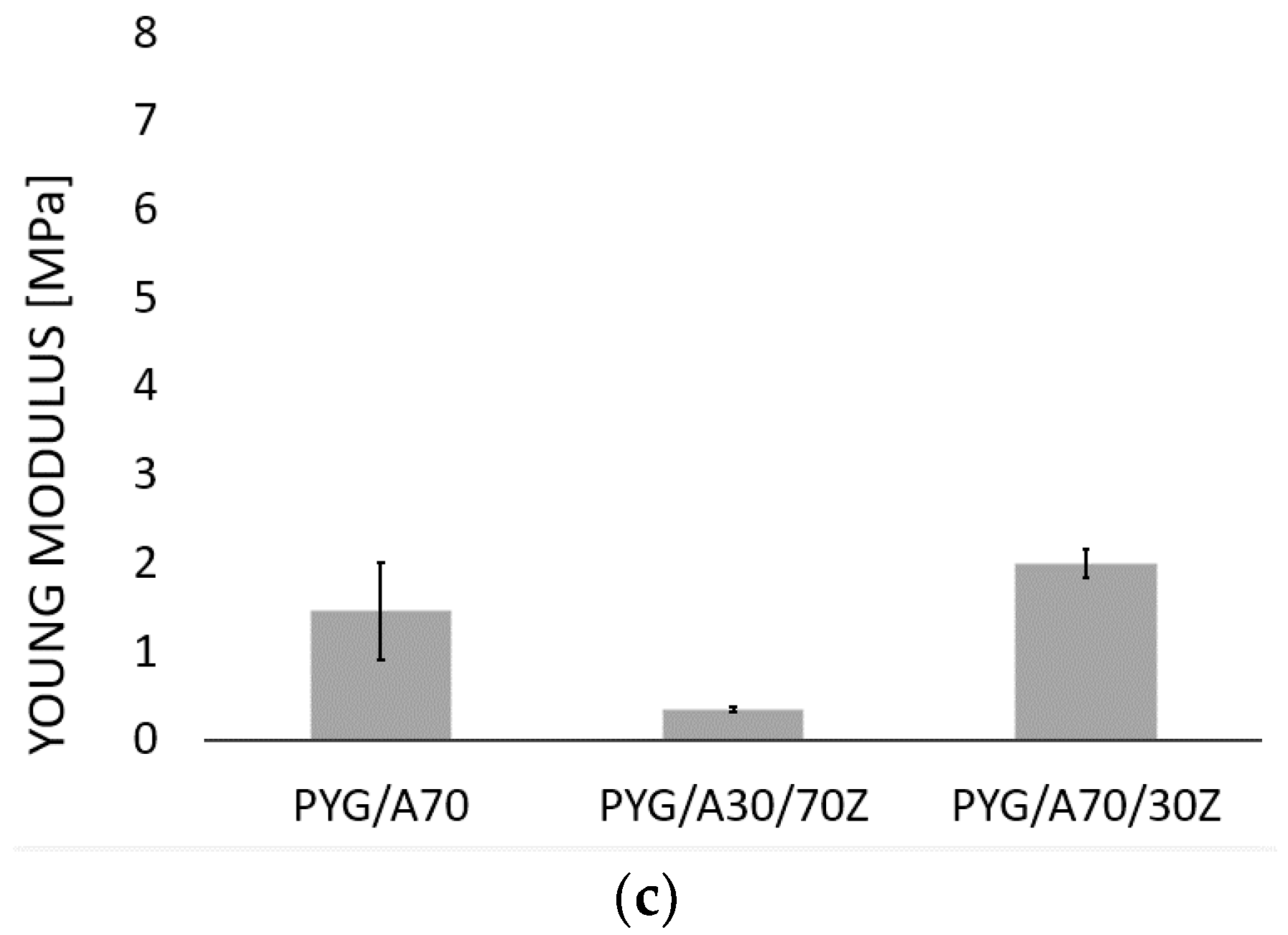
2.1. Results of Mechanical Properties Tests
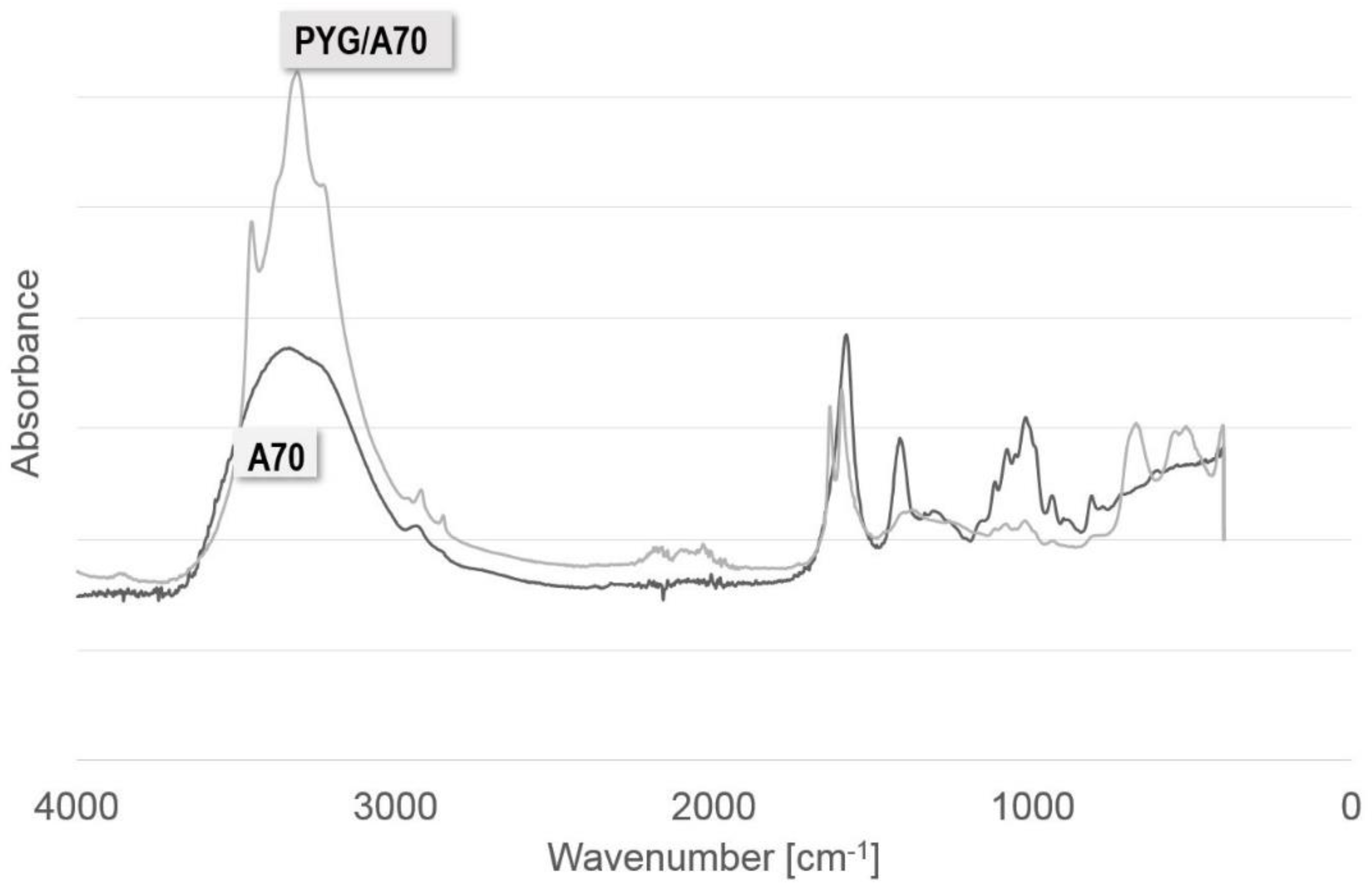
2.2. FTIR-ATR
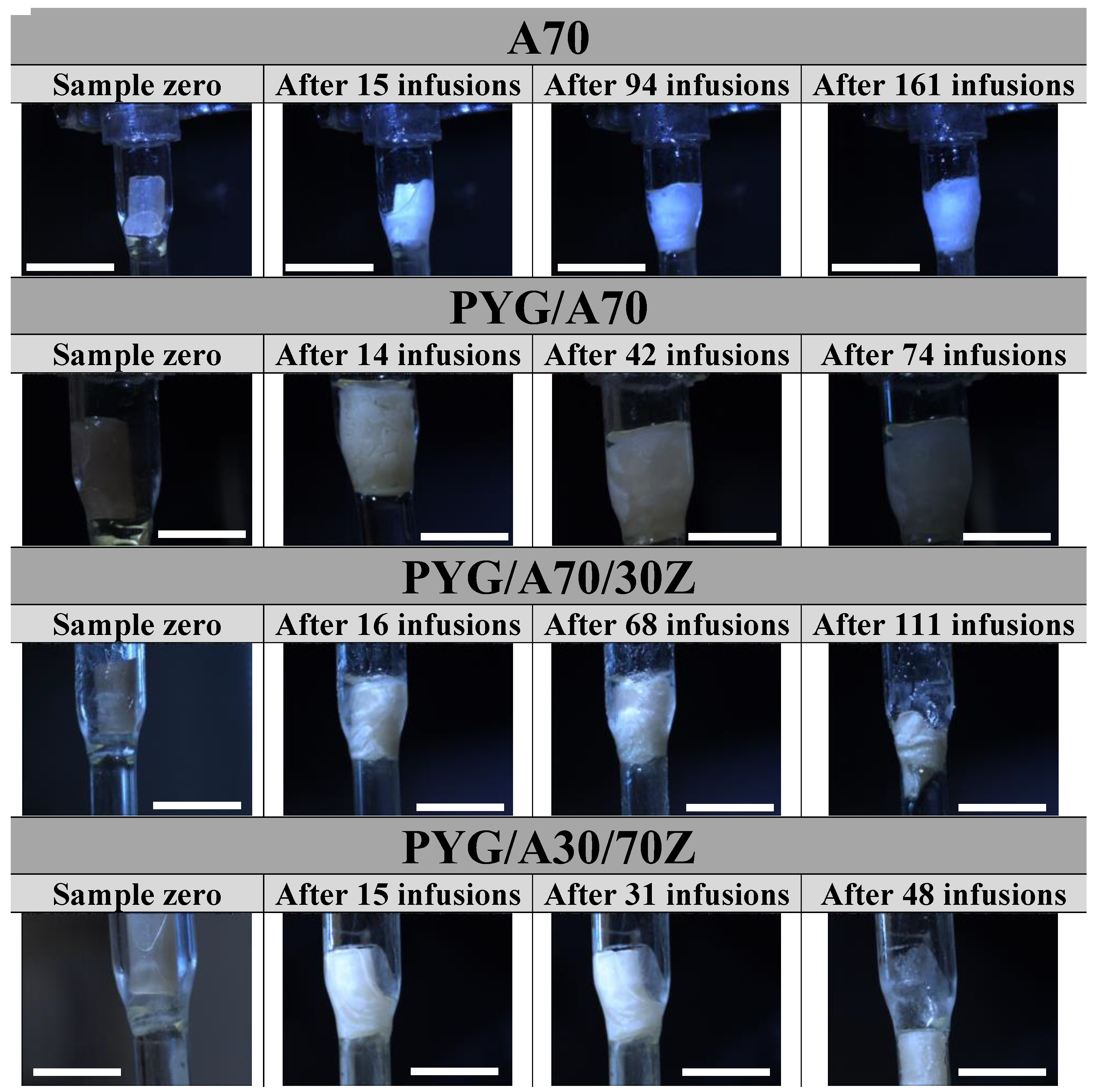
2.3. Resorption
2.4. Antibacterial Tests
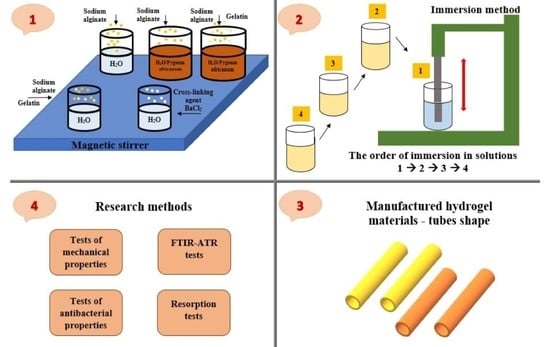
3. Materials and Methods
3.1. Materials
3.2. Samples Preparation
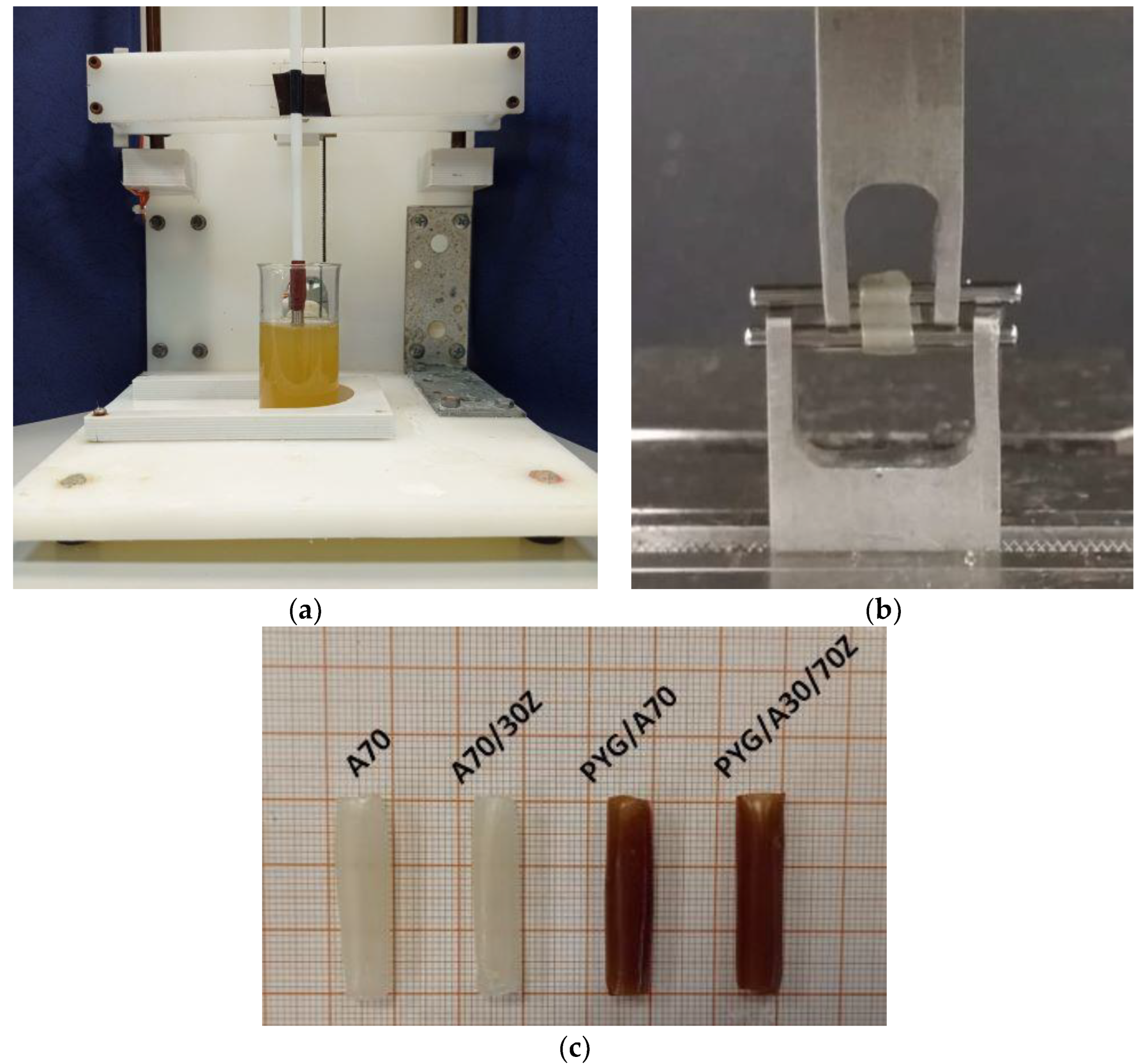
3.3. Tests of Mechanical Properties
3.4. FTIR-ATR Tests
3.5. Tests of Antibacterial Properties
3.6. Resorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Dai, G.; Hong, Y. Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater. 2019, 95, 50–59. [Google Scholar] [CrossRef]
- Cao, Z.; Yuan, Z.; Wu, R.; Wu, H.; Jin, B.; Zheng, J.; Wu, J. Tough and Resilient Hydrogels Enabled by a Multifunctional Initiating and Cross-Linking Agent. Gels 2021, 7, 177. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Taaca, K.L.M.; Prieto, E.I.; Vasquez, M.R., Jr. Current Trends in Biomedical Hydrogels: From Traditional Crosslinking to Plasma-Assisted Synthesis. Polymers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Izquierdo-Alvarez, A.; Bhattacharya, P.; Meerts, M.; Moldenaers, P.; Ramon, H.; Van Oosterwyck, H. The Influence of Swelling on Elastic Properties of Polyacrylamide Hydrogels. Front. Mater. 2020, 7, 212. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Brørnøy, S.H.; Bassett, D.C.; Ucar, S.; Strand, B.L.; Andreassen, J.-P.; Sikorski, P. A corrective spatiotemporal microscale study of calcium phosphate formation and transformation within an alginate hydrogels. Acta Biomater. 2016, 44, 254–266. [Google Scholar] [CrossRef]
- Chalanqui, M.J.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H.O. Influence of alginate backbone on efficacy of thermos-responsive alginate-g-P(NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 409–421. [Google Scholar] [CrossRef]
- Saul, J.M.; Williams, D.F. 12-Hydrogels in Regenerative Medicine. In Handbook of Polymer Applications in Medicine and Medical Devices, 2nd ed.; Modjarrad, K., Ebnesajjad, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; William Andrew: San Diego, CA, USA, 2013; pp. 279–302. [Google Scholar]
- Wang, Y. Programmable hydrogels. Biomaterials 2018, 178, 663–680. [Google Scholar] [CrossRef]
- Xia, B.; Chen, G. Research progress of natural tissue-derived hydrogels for tissue repair and reconstruction. Int. J. Biol. Macromol. 2022, 214, 480–491. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.; Oliveira, C.; Lima, E.; Rita, A.; Duarte, C.; Reis, R.L. Gelatin-based biodegradable ureteral stents with enhanced mechanical properties. Appl. Mater. Today 2016, 5, 9–18. [Google Scholar] [CrossRef]
- Somo, S.I.; Langert, K.; Yang, C.-Y.; Vaicik, M.K.; Ibarra, V.; Appel, A.A.; Akar, B.; Cheng, M.-H.; Brey, E.M. Synthesis and evaluation of dual crosslinked alginate microbeads. Acta Biomater. 2018, 65, 53–65. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Varshney, L.; Francis, S.; Rajneesh. Designing sterile biocompatible moxifloxacin loaded trgacanth-PVA-Alginate wound dressing by radiation crosslinking method. Wound Med. 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Spang, M.T.; Christman, K.L. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 2018, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- da Silva Fernandes, R.; de Moura, M.R.; Glenn, G.M.; Aouada, F.A. Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Hirakendu, B.; Singhal, R.K.; Pimple, M.V.; Sudeshna, S. Graphene oxide encapsulated in alginate beads for enhanced sorption of uranium from different aquatic environments. J. Environ. Chem. Eng. 2018, 6, 1625–1633. [Google Scholar]
- Eqbal, D.; Gundabala, V. Controlled fabrication of multi-core alginate microcapsules. J. Colloid Interface Sci. 2017, 507, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing- based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.H.; Mazlam, M.I.; Ahmad, N. Fabrication and characterization of porous biphasic β-tricalcium phosphate/carbonate apatite alginate coated scaffolds. Ceram. Int. 2018, 44, 9499–9505. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Carvalho, L.; Silva-Correia, J.; Vieira, S.; Majchrzak, M.; Lukomska, B.; Stanaszek, L.; Strymecka, P.; Malysz-Cymborska, J.; Golubczyk, D.; et al. Hydrogels-based scaffolds to support intrathecal stem cell transplantation as a geteway to the spinal cord: Clinical needs, biomaterials, and imaging technologies. NPJ Regen. Med. 2018, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H.; Nam, Y.S. Study on gelatin-containing artificial skin: I. Preparation and characteristics of novel gelatin-alginate sponge. Biomaterials 1999, 20, 409–417. [Google Scholar] [CrossRef]
- Sun, T.; Shi, Q.; Huang, Q.; Wang, H.; Xiong, X.; Hu, C.; Fukuda, T. Magnetic alginate microfibers as scaffolding elements for the fabrication of microvascular-like structures. Acta Biomater. 2018, 66, 272–281. [Google Scholar] [CrossRef]
- Cabrera, M.S.; Sanders, B.; Goor, O.J.G.M.; Driessen-Mol, A.; Oomens, C.W.J.; Baaijens, F.P.T. Computationally Designed 3D Printed Self-Expandable Polymer Stents with Biodegradation Capacity for Minimally Invasive Heart Valve Implantation: A Proof-of-Concept Study. 3D Print. Addit. Manuf. 2017, 4, 19–29. [Google Scholar] [CrossRef]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef]
- Majumdar, T.; Cooke, M.E.; Lawless, B.M.; Bellier, F.; Hughes, E.A.B.; Grover, L.M.; Jones, S.W.; Cox, S.C. Formulation and viscoelasticity of mineralised hydrogels for use in bone-cartilage interfacial reconstruction. J. Mech. Behav. Biomed. Mater. 2018, 80, 33–41. [Google Scholar] [CrossRef]
- Kanca, Y.; Milner, P.; Dini, D.; Amis, A.A. Tribological properties of PVA/PVP blend hydrogels against articular cartilage. J. Mech. Behav. Biomed. Mater. 2018, 78, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidi, Y. Hydrogel-based scaffolds for bone and cartilage tissue engineering and regeneration. React. Funct. Polym. 2022, 177, 105313. [Google Scholar] [CrossRef]
- Barisón, M.J.; Nogoceke, R.; Josino, R.; Horinouchi, C.D.d.S.; Marcon, B.H.; Correa, A.; Stimamiglio, M.A.; Robert, A.W. Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling. Int. J. Mol. Sci. 2022, 23, 6010. [Google Scholar] [CrossRef]
- Zimoch, J.; Padial, J.S.; Klar, A.S.; Vallmajo-Martin, Q.; Meuli, M.; Biedermann, T.; Wilson, C.J.; Rowan, A.; Reichmann, E. Polyisocyanopeptide hydrogels: A novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 2018, 70, 129–139. [Google Scholar] [CrossRef]
- Fitzsimmons, R.E.B.; Aquilino, M.S.; Quigley, J.; Chebotarev, O.; Tarlan, F.; Simmons, C.A. Generating vascular channels within hydrogel constructs using an economical open-source 3D bioprinter and thermoreversible gels. Bioprinting 2018, 9, 7–18. [Google Scholar] [CrossRef]
- Post, A.; Kishan, A.P.; Diaz-Rodriquez, P.; Tuzun, E.; Hahn, M.; Cosgriff-Hernandez, E. Introduction of sacrificial bonds to hydrogel to increase defect tolerance during suturing of multilayer vascular grafts. Acta Biomater. 2018, 69, 313–322. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Hsieh, F.-Y.; Theato, P.; Wei, Y.; Hsu, S.-H. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized construct. Biomaterials 2017, 133, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Z.; Chen, A.; He, H.; He, C.; Shuai, X.; Li, X.; Chen, S.; Zhang, Y.; Ren, B.; et al. Sulfated zwitterionic poly(sulfobetaine methacrylate) hydrogels promote complete skin regeneration. Acta Biomater. 2018, 71, 293–305. [Google Scholar] [CrossRef]
- Lei, Z.; Singh, G.; Min, Z.; Shixuan, C.; Xu, K.; Pengcheng, X.; Xueer, W.; Yinghua, C.; Lu, Z.; Lin, Z. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for management of serve skin wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 159–167. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Melnik, T.; Ben Ameur, S.; Kanfar, N.; Vinet, L.; Delie, F.; Jordan, O. Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking. Int. J. Mol. Sci. 2022, 23, 5706. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Korc, M.; Lin, C.-C. Biomimetic and enzyme-responsive dynamic hydrogel for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials 2018, 160, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Greene, T.; Lin, T.-Y.; Dawes, C.S.; Korc, M.; Lin, C.-L. Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells. Acta Biomater. 2017, 48, 258–269. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Q.; Gao, Y.; Yang, C.; Hu, L. Combination of coating and injectable hydrogel depot to improve the sustained delivery of insulin. J. Drug Deliv. Sci. Technol. 2018, 45, 415–421. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Fernandez, L.; Ochoa, I.; Saenz del Burgo, L.; Pedraz, J.L. Tunable injectable alginate-based hydrogel for cell therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018, 107, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.A.; Bartkowiak-Jowsa, M.; Bedzinski, R. Experimental and constitutive modeling approaches for a study of the biomechanical properties of human coronary arteries. J. Mech. Behav. Biomed. Mater. 2015, 50, 1–12. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Nava, A.; Mazza, E.; Kleinermann, F.; Avis, N.J.; McClure, J.; Bajka, M. Evaluation of the mechanical properties of human liver and kidney through aspiration experiments. Technol. Health Care 2004, 12, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Tan, S.A.; Onesto, V.; Law, J.X.; Agrawal, G.; Pal, S.; Lim, W.L.; Sharifi, E.; Moghaddam, F.D.; Maiti, T.K. Engineered herbal scaffolds for tissue repair and regeneration: Recent trends and technologies. Biomed. Eng. Adv. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Alaribe, F.N.; Motaung, K.S.C.M. Medicinal Plants in Tissue Engineering and Regenerative Medicine in the African Continent. Tissue Eng. Part A 2019, 25, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Komakech, R.; Kang, Y. Ethnopharmacological Potential of African Cherry [Prunus africana]. J. Herb. Med. 2019, 17, 100283. [Google Scholar] [CrossRef]
- Das, K.; Buchholz, N. Benign prostate hyperplasia and nutrition. Clin. Nutri. ESPEN 2019, 33, 5–11. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Pan, Y.; Liu, T. Investigating the Multi-Target Pharmacological Mechanism of Hedyotis diffusa Willd Acting on Prostate Cancer: A Network Pharmacology Approach. Biomolecules 2019, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Csikós, E.; Horváth, A.; Ács, K.; Papp, N.; Balázs, V.L.; Dolenc, M.S.; Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Protti, M.; et al. Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules 2021, 26, 7141. [Google Scholar] [CrossRef]
- Thompson, R.Q.; Katz, D.; Sheehan, B. Chemical comparison of Prunus africana bark and pygeum products marketed for prostate health. J. Pharm. Biomed. Anal. 2019, 163, 162–169. [Google Scholar] [CrossRef]
- Basati, G.; Ghanadi, P.; Abbaszadeh, S. A review of the most important natural antioxidants and effective medicinal plants in traditional medicine on prostate cancer and its disorders. J. Herbmed. Pharmacol. 2020, 9, 112–120. [Google Scholar] [CrossRef]
- Roell, D.; Baniahmad, A. The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth. Mol. Cell. Endocrinol. 2011, 332, 1–8. [Google Scholar] [CrossRef]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Wang, L.; Wang, L.; Zhu, M.; Ming, Y.; Zhao, S.; Fan, J.; Lai, E.Y. Ethanol extract of root of Prunus persica inhibited the growth of liver cancer cell HepG2 by inducing cell cycle arrest and migration suppression. Evid. Bas Complementary Altern. Med. 2017, 2017, 8231936. [Google Scholar] [CrossRef]
- Mwitari, P.G.; Ayeka, P.A.; Ondicho, J.; Matu, E.N.; Bii, C.C. Antimicrobial Activity and Probable Mechanisms of Action of Medicinal Plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS ONE 2013, 8, e65619. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Maina, E.G.; Kairigo, P.K.; Murigi, M.K.; Ogilo, J.K.; Nyangau, J.O.; Kimani, P.K.; Kipyegon, C. In vitro antioxidant and antimicrobial activity of Prunus africana (Hook. F.) Kalkman (bark extracts) and Oliv. Extracts (bark extracts): A comparative study. J. Med. Plants Econ. Dev. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- McLellan, L.K.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef]
- Kolasiński, W. Surgical site infections—Review of current knowledge, methods of prevention. Pol. Przegl. Chir. 2018, 91, 41–47. [Google Scholar] [CrossRef]
- Mackiewicz, A.; Klekiel, T.; Kurowiak, J.; Piasecki, T.; Bedzinski, R. Determination of Stent Load Conditions in New Zealand White Rabbit Urethra. J. Funct. Biomater. 2020, 11, 70. [Google Scholar] [CrossRef]
- Klekiel, T.; Mackiewicz, A.; Kaczmarek-Pawelska, A.; Kurowiak, J.; Piasecki, T.; Noszczyk-Nowak, A.; Będziński, R. Novel design of sodium alginate based absorbable stent for the use in urethral stricture disease. J. Mater. Res. Technol. 2020, 9, 9004–9015. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Ratajczak, M.; Ptak, M.; Chybowski, L.; Gawdzińska, K.; Będziński, R. Material and Structural Modeling Aspects of Brain Tissue Deformation under Dynamic Loads. Materials 2019, 12, 271. [Google Scholar] [CrossRef]
- Stevens, M.M.; Qanadilo, H.F.; Langer, R.; Shastri, P.V. A rapid-curing alginate gel system: Utility in periosteum-derived cartilage tissue engineering. Biomaterials 2004, 25, 887–894. [Google Scholar] [CrossRef]
- Liling, G.; Di, Z.; Jiachao, X.; Xin, G.; Xiaoting, F.; Qing, Z. Effects of ionic crosslinking on physical and mechanical properties of alginate mulching films. Carbohydr. Polym. 2016, 136, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D printing. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Investigation of morphological, mechanical and biological properties of cellulose nanocrystal reinforced electrospun gelatin nanofibers. Int. J. Biol. Macromol. 2019, 124, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Negrini, C.N.; Tarsini, P.; Tanzi, M.C.; Faré, S. Chemically crosslinked gelatin hydrogels as scaffolding materials for adipose tissue engineering. J. Appl. Polym. Sci. 2019, 136, 47104. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M. Material properties in unconfined compression of gelatin hydrogel for skin tissue engineering applications. Biomed. Tech. 2014, 59, 479–486. [Google Scholar] [CrossRef]
- Yao, F.; Laudano, M.A.; Seklehner, S.; Chughtai, B.; Lee, R.K. Image-Based Simulation of Urethral Dispensability and Flow Resistance as a Function of Pelvic Floor Anatomy. Neurourol. Urodyn. 2015, 34, 664–668. [Google Scholar] [CrossRef]
- Spirka, T.; Kenton, K.; Brubaker, L.; Damaser, M. Effect of Material Properties on Predicted Vesical Pressure During a Cough in a Simplified Computational Model of the Bladder and Urethra. Ann. Biomed. Eng. 2013, 41, 185–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Krupa, I. Alginate-Mediated Synthesis of Hetero-Shaped Silver Nanoparticles and Their Hydrogen Peroxide Sensing Ability. Molecules 2020, 25, 435. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.A.; Sanad, M.F.; Shalan, A.E. Synthesis, characterization and antimicrobial activity applications of grafted copolymer alginateg-poly(N-vinyl imidazole). RSC Adv. 2021, 11, 11541. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Ali, N.S.M.; Fuzlin, A.F.A.; Samsudin, A.S. Ionic Conductiviy of Alginate-NH4Cl Polymer Electrolyte. Makara J. Technol. 2020, 24, 125–130. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Deng, X.; Hao, L.; Wang, W. Modification of Alginate Hydrogel Films for Delivering Hydrophobic Kaempferol. J. Nanomater. 2019, 2019, 9170732. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Merijs Meri, R.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterization of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, M.; Chen, J.; Zhang, X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stab. 2009, 94, 1405–1410. [Google Scholar] [CrossRef]
- Rosellini, E.; Cristallini, C.; Barbani, N.; Vozzi, G.; Paolo, G. Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2008, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Asefnejad, A.; Daliri-Joupari, M.; Joughehdoust, S. The Physicochemical and Mechanical Investigation of Siloxane Modified Gelatin/Sodium alginate Injectable Hydrogels Loaded by Ascorbic Acid and β-Glycerophosphate. Mater. Today Commun. 2020, 26, 101914. [Google Scholar] [CrossRef]
- Kaczmarek-Pawelska, A.; Winiarczyk, K.; Mazurek, J. Alginate based hydrogel for tissue regeneration: Optimization, antibacterial activity and mechanical properties. J. Achiev. Mater. Manuf. Eng. 2017, 81, 35–40. [Google Scholar] [CrossRef]
- Deepa, T.; Latha, M.S.; Kurien, T.K. Evaluation of the antibacterial activity of calcium alginate beads modified with ethanolic extract of Adhatoda Vasica leaf extract on Staphylococcus Aureus and Escherichia Coli. Asian J. Pharm. Clin. Res. 2018, 11, 68–70. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Li, X.; Xia, Y.; Wang, B.; Zhao, Z. Antibacterial activity and in vitro cytotoxicity evaluation of alginate-AgNP fibers. Text. Res. J. 2016, 87, 1377–1386. [Google Scholar] [CrossRef]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Assob, J.C.N.; Kamga, H.L.F.; Nsagha, D.S.; Njunda, A.L.; Nde, P.F.; Asongalem, E.A.; Njouendou, A.J.; Sandjon, B.; Penlap, V.B. Antimicrobial and toxicological activities of five medicinal plant species from Cameroon Traditional Medicine. BMC Complement. Altern. Med. 2011, 11, 70. [Google Scholar] [CrossRef]
- Bii, C.; Korir, K.R.; Rugutt, J.; Mutai, C. The potential use of Prunus africana for the control, treatment and management of common fungal and bacterial infections. J. Med. Plant Res. 2010, 4, 995–998. [Google Scholar] [CrossRef]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, A. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Ardiles, C.S.; Rodríguez, C.C. Theoretical study for determining the type of interactions between a GG block of an alginate chain with metals Cu2+, Mn2+, Ca2+ and Mg2+. Arab. J. Chem. 2021, 14, 103325. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horsky, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Özen, I.; von Klitzing, R.; Erim, F.B. Antimicrobial cerium ion-chitosan crosslinked alginate biopolymer films: A novel and potential wound dressing. Int. J. Biol. Macromol. 2017, 105, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, M.; Zheng, X.; Liang, X.; Su, X.; Zhang, Y.; Lu, A.; Zhang, L. Effects of Chitin Whiskers on Physical Properties and Osteoblast Culture of Alginate Based Nanocomposite Hydrogels. Biomacromolecules 2015, 16, 3499–3507. [Google Scholar] [CrossRef]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelation microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 274–284. [Google Scholar] [CrossRef]
- Drenseikiene, D.; Schrüfer, S.; Schubert, D.W.; Reakasame, S.; Boccaccini, A.R. Cell-laden alginate dialdehyde-gelatin hydrogel formed in 3D printed sacrificial gel. J. Mater. Sci. Mater. Med. 2020, 31, 31. [Google Scholar] [CrossRef]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.G.; Bedzinski, R. Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries. Processes 2020, 8, 304. [Google Scholar] [CrossRef]
- Kurowiak, J.; Mackiewicz, A.; Klekiel, T.; Będziński, R. Evaluation of Selected Properties of Sodium Alginate-Based Hydrogel Material-Mechanical Strength? DIC Analysis and Degradation. Materials 2022, 15, 1225. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Torri, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression. What Does it Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Thongboonkerd, V. Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal. Biochem. 2010, 402, 110–112. [Google Scholar] [CrossRef] [PubMed]

| Sodium Alginate Concentration [wt.%] | Gelatin Concentration [wt.%] | Young’s Modulus | Sample Name |
|---|---|---|---|
| 7 | 0 | 4.93 MPa | A70 |
| 8 | 0 | 2.08 MPa | A80 |
| 9 | 0 | 6.09 MPa | A90 |
| 7 | 3 | 2.04 MPa | A70/30Z |
| 3 | 7 | 0.83 MPa | A30/70Z |
| 7 | 0 | 1.47 MPa | PYG/A70 |
| 7 | 3 | 2.00 MPa | PYG/A70/30Z |
| 3 | 7 | 0.36 MPa | PYG/A30/70Z |
| 0 | 15 | 45.56 kPa | Nergini et al. [73] |
| 0 | 25 | 76.55 kPa | |
| 0 | 10 | 70 kPa | Karimi et al. [74] |
| 0 | 15 | 80 kPa | |
| 30 | 65 | 1000–1200 MPa | Barros et al. [14] |
| Human urethra | 2.4 MPa | Yao et al. [75] | |
| 5 MPa | Spirka et al. [76] | ||
| Wavenumber [cm−1] | Assignment | Reference |
|---|---|---|
| 3000–3600 | characteristic of the tensile (valence) bands of the O-H bond | [77,78,79,80,81] |
| 1400 and 1600 | characteristic for the asymmetrical and symmetrical tensile vibrations of the carboxylic groups (COO-) and bivalent cross-linking cations | |
| 1000–1200 | characteristic of the vibrations of the C-O bond | |
| 800 | characteristic of mannuronic acid | |
| 700 | characteristic of guluronic acid-binding of crosslinking ions |
| Type of Sample | E. coli Optical Density Values (OD 600) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | ||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 8 | 24 | |
| PYG/A70 | 0.034 ± 0.002 * | 0.037 ± 0.002 | 0.045 ± 0.004 | 0.066 ± 0.003 | 0.073 ± 0.002 | 0.083 ± 0.002 | 0.787 ± 0.061 | 2.336 ± 0.066 |
| A70 | 0.032 ± 0.002 | 0.045 ± 0.002 | 0.063 ± 0.002 | 0.084 ± 0.004 | 0.106 ± 0.005 | 0.128 ± 0.011 | 0.767 ± 0.111 | 2.159 ± 0.089 |
| Control | 0.032 ± 0.002 | 0.064 ± 0.005 | 0.102 ± 0.009 | 0.130 ± 0.001 | 0.304 ± 0.027 | 0.613 ± 0.115 | 1.232 ± 0.059 | 3.236 ± 0.028 |
| Type of Sample | S. aureus Optical Density Values (OD 600) | |||||||
| Time (h) | ||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 8 | 24 | |
| PYG/A70 | 0.034 ± 0.004 | 0.056 ± 0.006 | 0.082 ± 0.003 | 0.117 ± 0.015 | 0.223 ± 0.02 | 0.443 ± 0.088 | 0.781 ± 0.042 | 3.269 ± 0.171 |
| A70 | 0.034 ± 0.004 | 0.058 ± 0.009 | 0.097 ± 0.004 | 0.141 ± 0.014 | 0.333 ± 0.028 | 0.643 ± 0.033 | 1.187 ± 0.021 | 2.842 ± 0.045 |
| Control | 0.034 ± 0.004 | 0.074 ± 0.005 | 0.116 ± 0.005 | 0.171 ± 0.012 | 0.57 ± 0.054 | 0.856 ± 0.037 | 1.366 ± 0.028 | 4.09 ± 0.065 |
| Sodium Alginate Concentration [wt.%] | Gelatin Concentration [wt.%] | Content of Pygeum | Sample Name |
|---|---|---|---|
| 7 | 0 | - | A70 |
| 8 | 0 | - | A80 |
| 9 | 0 | - | A90 |
| 7 | 3 | - | A70/30Z |
| 3 | 7 | - | A30/70Z |
| 7 | 0 | + | PYG/A70 |
| 7 | 3 | + | PYG/A70/30Z |
| 3 | 7 | + | PYG/A30/70Z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.; Baldy-Chudzik, K.; Mazurek-Popczyk, J.; Zaręba, Ł.; Klekiel, T.; Będziński, R. Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum africanum with Potential Application in Urology. Int. J. Mol. Sci. 2022, 23, 10324. https://doi.org/10.3390/ijms231810324
Kurowiak J, Kaczmarek-Pawelska A, Mackiewicz A, Baldy-Chudzik K, Mazurek-Popczyk J, Zaręba Ł, Klekiel T, Będziński R. Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum africanum with Potential Application in Urology. International Journal of Molecular Sciences. 2022; 23(18):10324. https://doi.org/10.3390/ijms231810324
Chicago/Turabian StyleKurowiak, Jagoda, Agnieszka Kaczmarek-Pawelska, Agnieszka Mackiewicz, Katarzyna Baldy-Chudzik, Justyna Mazurek-Popczyk, Łukasz Zaręba, Tomasz Klekiel, and Romuald Będziński. 2022. "Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum africanum with Potential Application in Urology" International Journal of Molecular Sciences 23, no. 18: 10324. https://doi.org/10.3390/ijms231810324
APA StyleKurowiak, J., Kaczmarek-Pawelska, A., Mackiewicz, A., Baldy-Chudzik, K., Mazurek-Popczyk, J., Zaręba, Ł., Klekiel, T., & Będziński, R. (2022). Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum africanum with Potential Application in Urology. International Journal of Molecular Sciences, 23(18), 10324. https://doi.org/10.3390/ijms231810324