Applications of Metabolomics in Calcium Metabolism Disorders in Humans
Abstract
:1. Introduction
2. Methods
3. Metabolomics in Calcium Metabolism Disorders
4. Primary Hyperparathyroidism
5. Secondary Hyperparathyroidism
6. Calcium Deficiency
7. Osteoporosis
8. Vitamin D Supplementation
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, M.; Wu, X.; Yu, Y.; Wang, L.; Xie, D.; Zhang, Z.; Chen, L.; Lu, A.; Zhang, G.; Li, F. Disorders of Calcium and Phosphorus Metabolism and the Proteomics/Metabolomics-Based Research. Front. Cell Dev. Biol. 2020, 8, 576110. [Google Scholar] [CrossRef]
- Yu, E.; Sharma, S. Physiology, Calcium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Şenkal, S.; Doğan, A. Parathyroid Cell Differentiation from Progenitor Cells and Stem Cells: Development, Molecular Mechanism, Function, and Tissue Engineering. Adv. Exp. Med. Biol. 2022, 16, 13–24. [Google Scholar] [CrossRef]
- Conigrave, A.D.; Mun, H.C.; Delbridge, L.; Quinn, S.J.; Wilkinson, M.; Brown, E.M. L-amino acids regulate parathyroid hormone secretion. J. Biol. Chem. 2004, 279, 38151–38159. [Google Scholar] [CrossRef]
- Gogusev, J.; Duchambon, P.; Hory, B.; Giovannini, M.; Goureau, Y.; Sarfati, E.; Drüeke, T.B. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997, 51, 328–336. [Google Scholar] [CrossRef]
- Kifor, O.; Moore, F.D., Jr.; Wang, P.; Goldstein, M.; Vassilev, P.; Kifor, I.; Hebert, S.C.; Brown, E.M. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J. Clin. Endocrinol. Metab. 1996, 81, 1598–1606. [Google Scholar] [CrossRef]
- Conigrave, A.D.; Quinn, S.J.; Brown, E.M. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4814–4819. [Google Scholar] [CrossRef]
- Jüppner, H. Phosphate and FGF-23. Kidney Int. Suppl. 2011, 79, S24–S27. [Google Scholar] [CrossRef]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal. Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Bhadada, S.K.; Arya, A.K.; Sharma, S.; Behera, A.; Bhansali, A.; Rao, S.D. Changes in parathyroid proteome in patients with primary hyperparathyroidism due to sporadic parathyroid adenomas. Clin. Endocrinol. (Oxf.) 2014, 81, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Hogue, J.A.; Roman, S.A.; Scheri, R.P.; Fradin, H.; Corcoran, D.L.; Sosa, J.A. Transcriptional profiling reveals distinct classes of parathyroid tumors in PHPT. Endocr.-Relat. Cancer 2018, 25, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.J.O. Hypercalcaemia—presentation and management. Clin. Med. (Lond.) 2017, 17, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Pathophysiology of Hypercalcemia. Endocrinol. Metab. Clin. N. Am. 2021, 50, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Wielogórska, M.; Podgórska, B.; Niemira, M.; Szelachowska, M.; Krętowski, A.; Siewko, K. MicroRNA Profile Alterations in Parathyroid Carcinoma: Latest Updates and Perspectives. Cancers 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wägele, B.; Altmaier, E.; Deloukas, P.; Erdmann, J.; Grundberg, E.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Hollywood, K.; Brison, D.R.; Goodacre, R. Metabolomics: Current technologies and future trends. Proteomics 2006, 6, 4716–4723. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Armitage, E.G.; Southam, A.D. Monitoring cancer prognosis, diagnosis and treatment efficacy using metabolomics and lipidomics. Metabolomics 2016, 12, 146. [Google Scholar] [CrossRef]
- Armitage, E.G.; Ciborowski, M. Applications of Metabolomics in Cancer Studies. Adv. Exp. Med. Biol. 2017, 965, 209–234. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Ciborowski, M.; Kisluk, J.; Kretowski, A.; Barbas, C. Mass spectrometry based proteomics and metabolomics in personalized oncology. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165690. [Google Scholar] [CrossRef]
- Want, E.J.; Nordström, A.; Morita, H.; Siuzdak, G. From exogenous to endogenous: The inevitable imprint of mass spectrometry in metabolomics. J. Proteome Res. 2007, 6, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Battini, S.; Imperiale, A.; Taïeb, D.; Elbayed, K.; Cicek, A.E.; Sebag, F.; Brunaud, L.; Namer, I.J. High-resolution magic angle spinning (1)H nuclear magnetic resonance spectroscopy metabolomics of hyperfunctioning parathyroid glands. Surgery 2016, 160, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, D.; Matsukawa, N.; Hishinuma, E.; Koshiba, S. Identification of biomarkers to diagnose diseases and find adverse drug reactions by metabolomics. Drug Metab. Pharm. 2021, 37, 100373. [Google Scholar] [CrossRef]
- Jamshidi, N.; Miller, F.J.; Mandel, J.; Evans, T.; Kuo, M.D. Individualized therapy of HHT driven by network analysis of metabolomic profiles. BMC Syst. Biol. 2011, 5, 200. [Google Scholar] [CrossRef]
- Hu, X.; Saunders, N.; Safley, S.; Smith, M.R.; Liang, Y.; Tran, V.; Sharma, J.; Jones, D.P.; Weber, C.J. Environmental chemicals and metabolic disruption in primary and secondary human parathyroid tumors. Surgery 2021, 169, 102–108. [Google Scholar] [CrossRef]
- Wu, Q.; Lai, X.; Zhu, Z.; Hong, Z.; Dong, X.; Wang, T.; Wang, H.; Lou, Z.; Lin, Q.; Guo, Z.; et al. Evidence for Chronic Kidney Disease-Mineral and Bone Disorder Associated With Metabolic Pathway Changes. Medicine (Baltimore) 2015, 94, e1273. [Google Scholar] [CrossRef]
- Shen, Q.; Xiang, W.; Ye, S.; Lei, X.; Wang, L.; Jia, S.; Shao, X.; Weng, C.; Shen, X.; Wang, Y.; et al. Plasma metabolite biomarkers related to secondary hyperparathyroidism and parathyroid hormone. J. Cell Biochem. 2019, 120, 15766–15775. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Wang, F.; Li, R.; Ning, H.; Na, L.; Huang, Y.; Song, Y.; Liu, L.; Pan, H.; et al. Calcium-deficiency assessment and biomarker identification by an integrated urinary metabonomics analysis. BMC Med. 2013, 11, 86. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Ren, L.; Li, S.; He, X.; Wu, X.; Liu, T.; Lin, L.; Li, Y.; Sun, C. Biomarkers identified by urinary metabonomics for noninvasive diagnosis of nutritional rickets. J. Proteome Res. 2014, 13, 4131–4142. [Google Scholar] [CrossRef]
- Elnenaei, M.O.; Chandra, R.; Mangion, T.; Moniz, C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation. Br. J. Nutr. 2011, 105, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Aleidi, S.M.; Alnehmi, E.A.; Alshaker, M.; Masood, A.; Benabdelkamel, H.; Al-Ansari, M.M.; Abdel Rahman, A.M. A Distinctive Human Metabolomics Alteration Associated with Osteopenic and Osteoporotic Patients. Metabolites 2021, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- You, Y.S.; Lin, C.Y.; Liang, H.J.; Lee, S.H.; Tsai, K.S.; Chiou, J.M.; Chen, Y.C.; Tsao, C.K.; Chen, J.H. Association between the metabolome and low bone mineral density in Taiwanese women determined by (1)H NMR spectroscopy. J. Bone Miner. Res. 2014, 29, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016, 12, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Miyamoto, H.; Mori, T.; Yoshida, S.; Fujie, A.; et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 2017, 95, 1–4. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Fujie, A.; Morita, M.; Watanabe, R.; Tando, T.; et al. Metabolomics-based profiles predictive of low bone mass in menopausal women. Bone Rep. 2018, 9, 11–18. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, H.; Su, K.J.; Zhang, J.G.; Tian, Q.; Zhao, L.J.; Qiu, C.; Zhang, Q.; Garrett, T.J.; Liu, J.; et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr. Metab. (Lond.) 2018, 15, 57. [Google Scholar] [CrossRef]
- Liang, W.D.; Huang, P.J.; Xiong, L.H.; Zhou, S.; Ye, R.Y.; Liu, J.R.; Wei, H.; Lai, R.Y. Metabolomics and its application in the mechanism analysis on diabetic bone metabolic abnormality. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9591–9600. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Li, G.H.; Long, M.T.; Cheung, C.L.; Vasan, R.S.; Hsu, Y.H.; Kiel, D.P.; Liu, C.T. Metabolomics Insights into Osteoporosis Through Association With Bone Mineral Density. J. Bone Miner. Res. 2021, 36, 729–738. [Google Scholar] [CrossRef]
- Ling, C.W.; Miao, Z.; Xiao, M.L.; Zhou, H.; Jiang, Z.; Fu, Y.; Xiong, F.; Zuo, L.S.; Liu, Y.P.; Wu, Y.Y.; et al. The Association of Gut Microbiota With Osteoporosis Is Mediated by Amino Acid Metabolism: Multiomics in a Large Cohort. J. Clin. Endocrinol. Metab. 2021, 106, e3852–e3864. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Allen, R.; Charoenngam, N.; Lewanczuk, R.; Holick, M.F. Variable Genomic and Metabolomic Responses to Varying Doses of Vitamin D Supplementation. Anticancer Res. 2020, 40, 535–543. [Google Scholar] [CrossRef]
- Bislev, L.S.; Sundekilde, U.K.; Kilic, E.; Dalsgaard, T.K.; Rejnmark, L.; Bertram, H.C. Circulating Levels of Muscle-Related Metabolites Increase in Response to a Daily Moderately High Dose of a Vitamin D3 Supplement in Women with Vitamin D Insufficiency-Secondary Analysis of a Randomized Placebo-Controlled Trial. Nutrients 2020, 12, 1310. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Vaira, V.; Verdelli, C.; Forno, I.; Corbetta, S. MicroRNAs in parathyroid physiopathology. Mol. Cell Endocrinol. 2017, 456, 9–15. [Google Scholar] [CrossRef]
- Mackenzie-Feder, J.; Sirrs, S.; Anderson, D.; Sharif, J.; Khan, A. Primary hyperparathyroidism: An overview. Int. J. Endocrinol. 2011, 2011, 251410. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Anastasilakis, A.D.; Panagiotakou, A.; Kassi, E.; Makras, P. Gender Predilection in Sporadic Parathyroid Adenomas. Int. J. Mol. Sci. 2020, 21, 2964. [Google Scholar] [CrossRef]
- Walker, M.D.; Silverberg, S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018, 14, 115–125. [Google Scholar] [CrossRef]
- Zavatta, G.; Clarke, B.L. Normocalcemic Primary Hyperparathyroidism: Need for a Standardized Clinical Approach. Endocrinol. Metab. (Seoul) 2021, 36, 525–535. [Google Scholar] [CrossRef]
- Yener Ozturk, F.; Erol, S.; Canat, M.M.; Karatas, S.; Kuzu, I.; Dogan Cakir, S.; Altuntas, Y. Patients with normocalcemic primary hyperparathyroidism may have similar metabolic profile as hypercalcemic patients. Endocr. J. 2016, 63, 111–118. [Google Scholar] [CrossRef]
- Palermo, A.; Fosca, M.; Tabacco, G.; Marini, F.; Graziani, V.; Santarsia, M.C.; Longo, F.; Lauria, A.; Cesareo, R.; Giovannoni, I.; et al. Raman Spectroscopy Applied to Parathyroid Tissues: A New Diagnostic Tool to Discriminate Normal Tissue from Adenoma. Anal. Chem. 2018, 90, 847–854. [Google Scholar] [CrossRef]
- di Masi, A.; Leboffe, L.; Sodo, A.; Tabacco, G.; Cesareo, R.; Sbroscia, M.; Giovannoni, I.; Taffon, C.; Crucitti, P.; Longo, F.; et al. Metabolic profile of human parathyroid adenoma. Endocrine 2020, 67, 699–707. [Google Scholar] [CrossRef]
- Cetani, F.; Pardi, E.; Marcocci, C. Parathyroid carcinoma: A clinical and genetic perspective. Minerva Endocrinol. 2018, 43, 144–155. [Google Scholar] [CrossRef]
- Bargagli, M.; Arena, M.; Naticchia, A.; Gambaro, G.; Mazzaferro, S.; Fuster, D.; Ferraro, P.M. The Role of Diet in Bone and Mineral Metabolism and Secondary Hyperparathyroidism. Nutrients 2021, 13, 2328. [Google Scholar] [CrossRef]
- Habas, E., Sr.; Eledrisi, M.; Khan, F.; Elzouki, A.Y. Secondary Hyperparathyroidism in Chronic Kidney Disease: Pathophysiology and Management. Cureus 2021, 13, e16388. [Google Scholar] [CrossRef]
- Steinl, G.K.; Kuo, J.H. Surgical Management of Secondary Hyperparathyroidism. Kidney Int. Rep. 2021, 6, 254–264. [Google Scholar] [CrossRef]
- Favero, C.; Carriazo, S.; Cuarental, L.; Fernandez-Prado, R.; Gomá-Garcés, E.; Perez-Gomez, M.V.; Ortiz, A.; Fernandez-Fernandez, B.; Sanchez-Niño, M.D. Phosphate, Microbiota and CKD. Nutrients 2021, 13, 1273. [Google Scholar] [CrossRef]
- Rahbari, R.; Holloway, A.K.; He, M.; Khanafshar, E.; Clark, O.H.; Kebebew, E. Identification of differentially expressed microRNA in parathyroid tumors. Ann. Surg. Oncol. 2011, 18, 1158–1165. [Google Scholar] [CrossRef]
- Mun, H.C.; Leach, K.M.; Conigrave, A.D. L-Amino Acids Promote Calcitonin Release via a Calcium-Sensing Receptor: Gq/11-Mediated Pathway in Human C-Cells. Endocrinology 2019, 160, 1590–1599. [Google Scholar] [CrossRef]
- Dobrowolski, S.F.; Tourkova, I.L.; Sudano, C.R.; Larrouture, Q.C.; Blair, H.C. A New View of Bone Loss in Phenylketonuria. Organogenesis 2021, 17, 50–55. [Google Scholar] [CrossRef]
- Schwahn, B.; Mokov, E.; Scheidhauer, K.; Lettgen, B.; Schönau, E. Decreased trabecular bone mineral density in patients with phenylketonuria measured by peripheral quantitative computed tomography. Acta Paediatr. 1998, 87, 61–63. [Google Scholar] [CrossRef]
- Tan, R.; Li, J.; Liu, F.; Liao, P.; Ruiz, M.; Dupuis, J.; Zhu, L.; Hu, Q. Phenylalanine induces pulmonary hypertension through calcium-sensing receptor activation. Am. J. Physiol. Lung. Cell Mol. Physiol. 2020, 319, L1010–L1020. [Google Scholar] [CrossRef]
- Srinivasan, A.; Wong, F.K.; Karponis, D. Calcitonin: A useful old friend. J. Musculoskelet. Neuronal. Interact 2020, 20, 600–609. [Google Scholar]
- Walker, M.D.; Bilezikian, J.P. Primary Hyperparathyroidism. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Meng, F.; Fan, L.; Sun, L.; Yu, Q.; Wang, M.; Sun, C. Serum biomarkers of the calcium-deficient rats identified by metabolomics based on UPLC/Q-TOF MS/MS. Nutr. Metab. (Lond.) 2020, 17, 99. [Google Scholar] [CrossRef]
- Kamprom, W.; Tawonsawatruk, T.; Mas-Oodi, S.; Anansilp, K.; Rattanasompattikul, M.; Supokawej, A. P-cresol and Indoxyl Sulfate Impair Osteogenic Differentiation by Triggering Mesenchymal Stem Cell Senescence. Int. J. Med. Sci. 2021, 18, 744–755. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Xie, H.; Luo, X.H.; Wu, X.P.; Zhou, H.D.; Lu, Y.; Liao, E.Y. Taurine transporter is expressed in osteoblasts. Amino Acids 2006, 31, 157–163. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Lu, Y.; Luo, X.H.; Xie, H.; Wu, X.P.; Liao, E.Y. Taurine promotes connective tissue growth factor (CTGF) expression in osteoblasts through the ERK signal pathway. Amino Acids 2007, 32, 425–430. [Google Scholar] [CrossRef]
- Fan, J.; Jahed, V.; Klavins, K. Metabolomics in Bone Research. Metabolites 2021, 11, 434. [Google Scholar] [CrossRef]
- Tian, L.; Yang, R.; Wei, L.; Liu, J.; Yang, Y.; Shao, F.; Ma, W.; Li, T.; Wang, Y.; Guo, T. Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine (Baltimore) 2017, 96, e8294. [Google Scholar] [CrossRef]
- Mao, H.; Wang, W.; Shi, L.; Chen, C.; Han, C.; Zhao, J.; Zhuo, Q.; Shen, S.; Li, Y.; Huo, J. Metabolomics and physiological analysis of the effect of calcium supplements on reducing bone loss in ovariectomized rats by increasing estradiol levels. Nutr. Metab. (Lond.) 2021, 18, 76. [Google Scholar] [CrossRef]
- Lv, H.; Jiang, F.; Guan, D.; Lu, C.; Guo, B.; Chan, C.; Peng, S.; Liu, B.; Guo, W.; Zhu, H.; et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. Int. J. Mol. Sci. 2016, 17, 2018. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, D.; Chen, H.; Chao, Y.; Wu, K.; Dong, X.; Su, J. Integrative Bone Metabolomics-Lipidomics Strategy for Pathological Mechanism of Postmenopausal Osteoporosis Mouse Model. Sci. Rep. 2018, 8, 16456. [Google Scholar] [CrossRef]
- Wei, Z.; Ge, F.; Che, Y.; Wu, S.; Dong, X.; Song, D. Metabolomics Coupled with Pathway Analysis Provides Insights into Sarco-Osteoporosis Metabolic Alterations and Estrogen Therapeutic Effects in Mice. Biomolecules 2021, 12, 41. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Gao, M.; Bao, B.; Cao, Y.; Cheng, F.; Zhang, L.; Li, Z.; Shan, J.; Yao, W. Metabolomics-driven of relationships among kidney, bone marrow and bone of rats with postmenopausal osteoporosis. Bone 2022, 156, 116306. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Zhou, S.; Shen, Z.; Luan, F. High-Throughput Metabolomics Discovers Metabolic Biomarkers and Pathways to Evaluating the Efficacy and Exploring Potential Mechanisms of Osthole Against Osteoporosis Based on UPLC/Q-TOF-MS Coupled With Multivariate Data Analysis. Front. Pharmacol. 2020, 11, 741. [Google Scholar] [CrossRef]
- Jones, G.; Kaufmann, M. Diagnostic Aspects of Vitamin D: Clinical Utility of Vitamin D Metabolite Profiling. JBMR Plus 2021, 5, e10581. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Morishima, Y.; Inaba, M.; Nishizawa, Y.; Morii, H.; Hasuma, T.; Matsui-Yuasa, I.; Otani, S. The involvement of polyamines in the activation of vitamin D receptor from porcine intestinal mucosa. Eur. J. Biochem. 1994, 219, 349–356. [Google Scholar] [CrossRef]
- Shinki, T.; Takahashi, N.; Kadofuku, T.; Sato, T.; Suda, T. Induction of spermidine N1-acetyltransferase by 1 alpha,25-dihydroxyvitamin D3 as an early common event in the target tissues of vitamin D. J. Biol. Chem. 1985, 260, 2185–2190. [Google Scholar] [CrossRef]
- Chailurkit, L.; Nimitphong, H.; Saetung, S.; Ongphiphadhanakul, B. Urinary metabolic profiles after vitamin D(2) versus vitamin D(3) supplementation in prediabetes. J. Clin. Transl. Endocrinol. 2019, 16, 100194. [Google Scholar] [CrossRef]
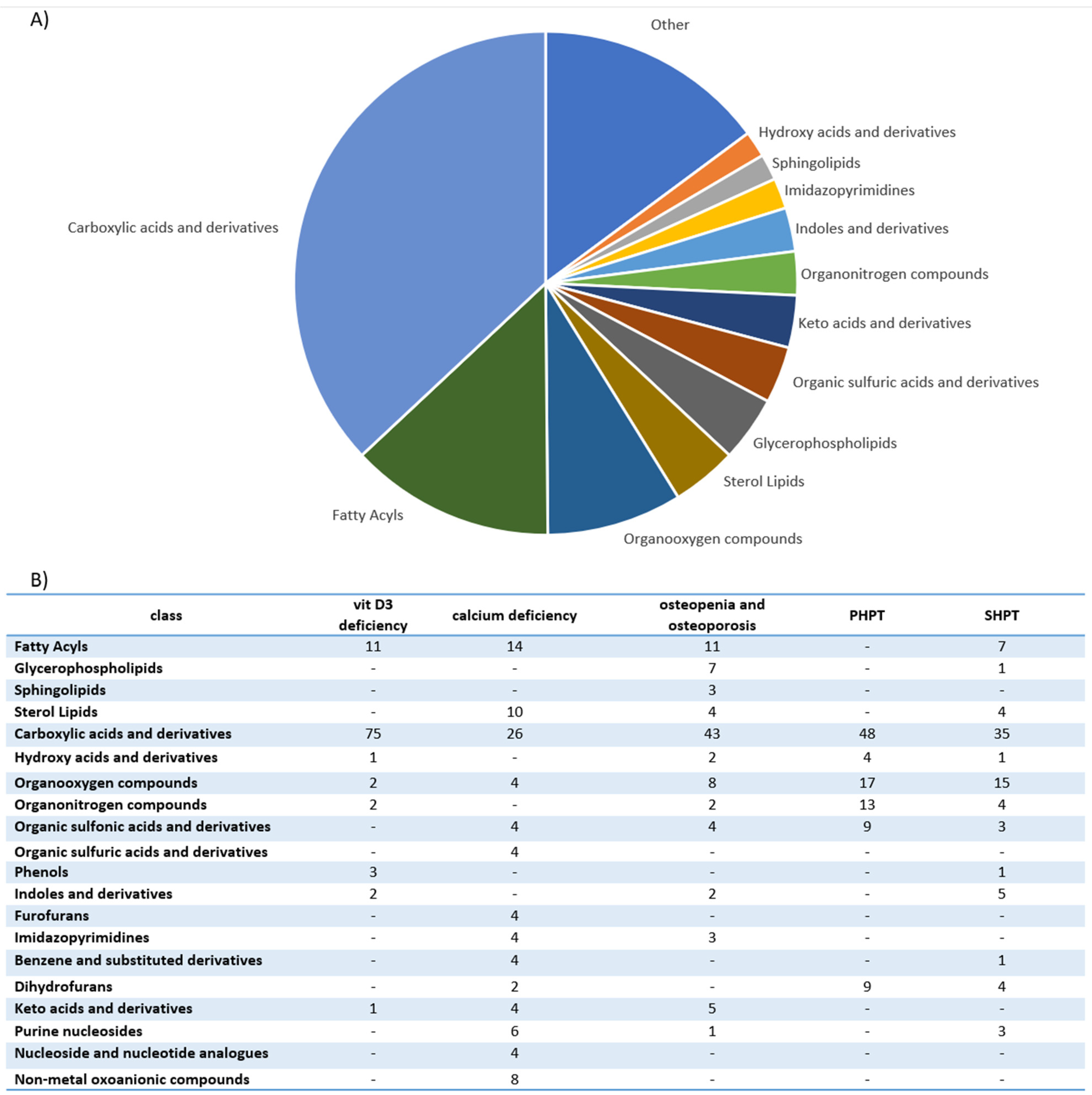

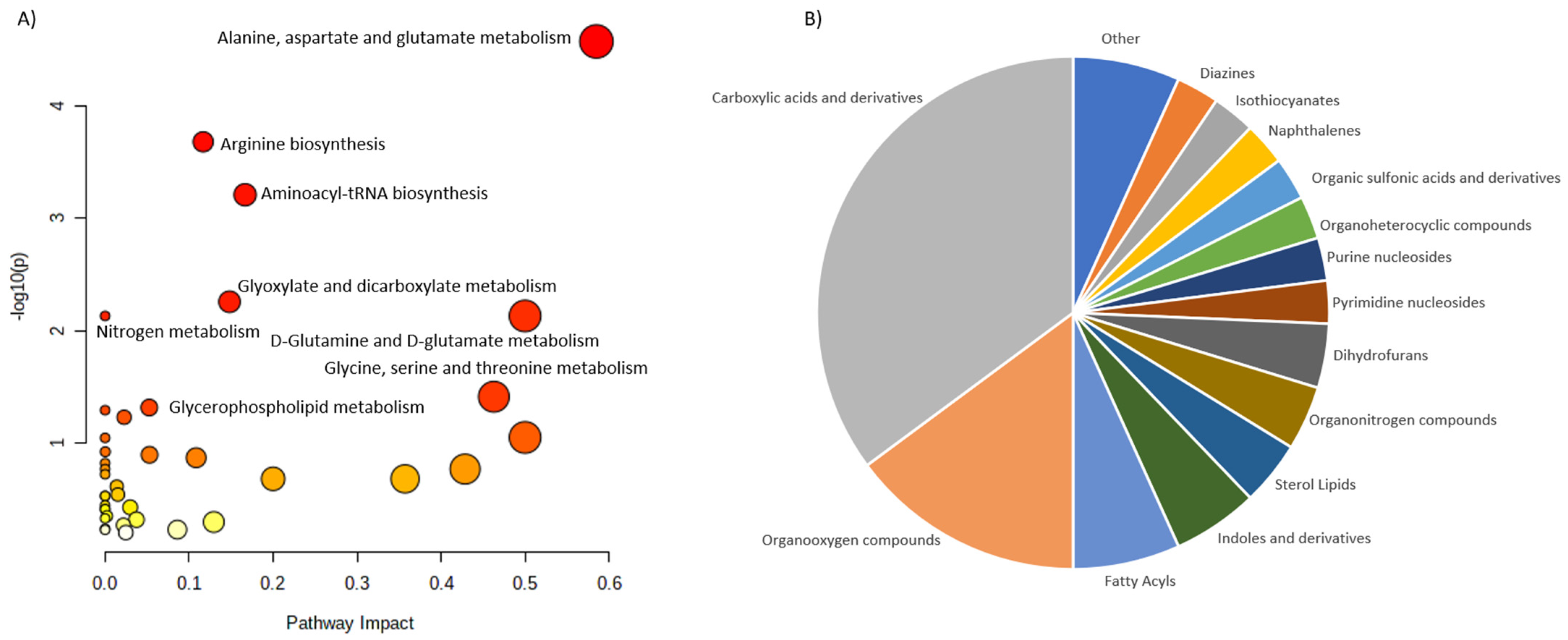

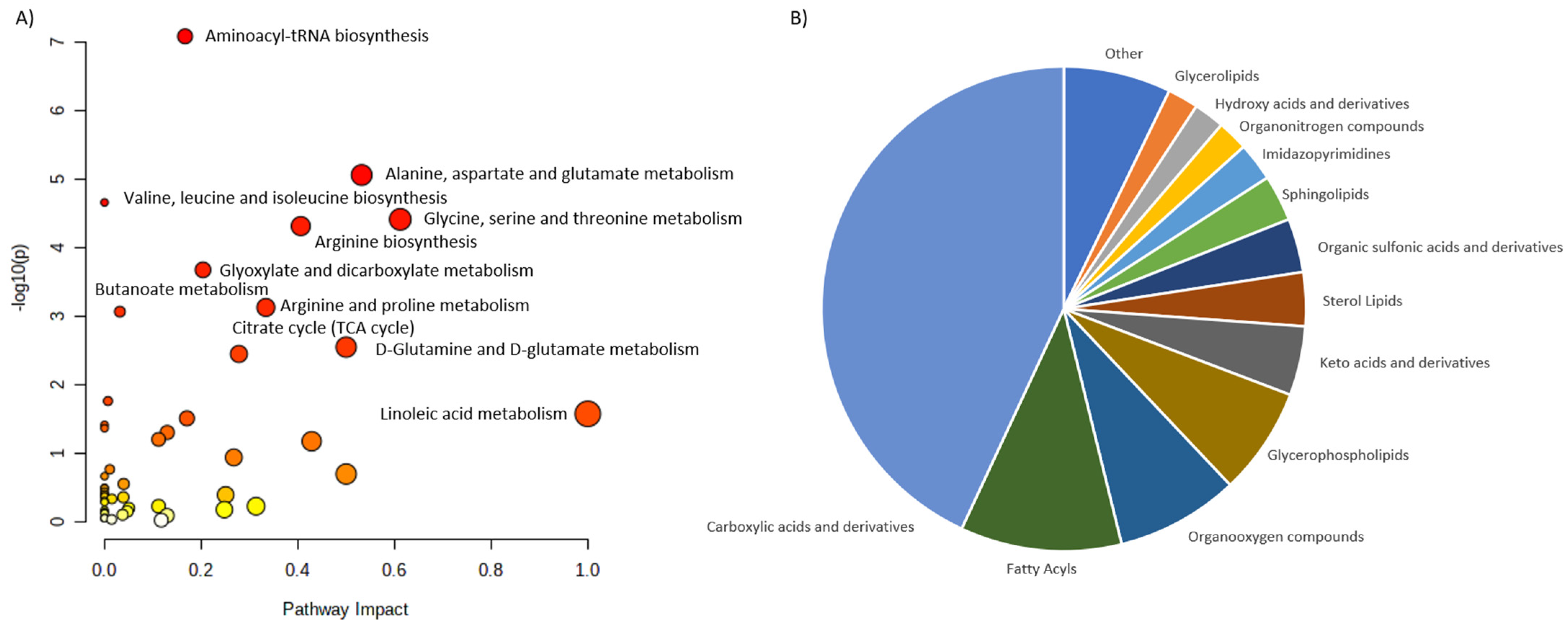

| Disorders | Approaches | Study Groups | Sample Type | Metabolite Change | Authors |
|---|---|---|---|---|---|
| PHPT SHPT THPT | HRMAS NMR spectroscopy | -18 patients with PHPT; -11 patients with SHPT; -3 patients with THPT. | Parathyroid tissue | Single adenoma and multiglandular disease could be distinguished with metabolomic profiling. Single adenoma: ↑ fumarate, choline, β-glucose, myo-inositol, ascorbate, glycine, scyllo-inositol. Multiglandular disease: ↑ glutamate, glutamine, taurine, aspartate, lactate, GSH. PHPT and SHPT could be distinguished with metabolomic profiling. PHPT: ↑ β-glucose, GSH, ascorbate, myo-inositol glutamate, phosphorylcholine, taurine. SHPT: ↑ fumarate, serine, choline, aspartate, glycerophosphocholine glutamine. | Battini et al., 2016 [23] |
| PHPT SHPT | GC-MS LC-MS | -40 patients with PHPT; -40 patients with SHPT; -4 patients with normal parathyroid gland. | Parathyroid tissue |
Polychlorinated biphenyls, dichloro-diphenyl-trichloroethane derivatives and polybrominated diphenyl ethers were detected in parathyroid tumors. Polychlorinated biphenyl-49 and polychlorinated biphenyl-28 and levels were positively correlated with parathyroid tumor mass. The levels of calcium serum were inversely correlated with concentrations of polybrominated diphenyl ether-47. Polychlorinated biphenyl-49 and p,p’-dichlorodiphenyldichloroethylene were not detected in normal parathyroid gland tissue. | Hu et al., 2021 [26] |
| SHPT | UPLC-Q- TOF/MS | Maintenance- peritoneal-dialysis patients: -19 disease controls with PTH 150–300 pg/mL; -19 patients with PTH > 300 pg/mL. | Serum | 32 unique metabolites were identified in the high-PTH group: ↑ N-(1-Deoxy-1-fructosyl)-tryptophan, N-acetylserotonin glucuronide, dopamine glucuronide, prolyl-tyrosine, glycylprolylhydroxyproline, aminohippuric acid, 2-phenylglycine, 4-carboxyphenylglycine, N-(3-Indolylacetyl)- L-isoleucine, glutaminyl-hydroxyproline, diethyl fumarate, isopropyl citrate, (R)-2-methylmalate, glutamyl-glutamate, N-acetylaspartylglutamic acid. Only 2 of 32 found metabolites were downregulated: ↓ cytidine and L-phenylalanine. Most identified metabolites were not primary metabolites. | Wu et al., 2015 [27] |
| SHPT | UPLC-MS | -15 patients in the preoperative group with SHPT with PTH level > 600 pg/mL; -15 patients in postoperative group, after parathyroidectomy plus forearm transplantation due to SHPT with PTH level < 150 pg/mL; -5 healthy controls. | Serum | 5 metabolites were highly correlated with SHPT. Biomarker group with SHPT: ↑ allyl isothiocyanate, D-aspartic acid, L-phenylalanine. ↓ D-galactose, indoleacetaldehyde. -preoperative group vs. healthy controls (AUC = 0.947); -postoperative group vs. healthy controls (AUC = 0.6). | Shen et al., 2019 [28] |
| Calcium deficiency | UPLC-Q-TOF MS/MS | Phase I: male rats. Phase II: 70 postmenopausal women. | Urine | Biomarkers (Phase I): glycine, sebacic acid, oxoglutaric acid, pyrophosphoric acid, pseudouridine, taurine, phenylacetylglycine, indoxyl sulfate. 2 biomarkers (pseudouridine and citrate) were further confirmed in 70 women. | Wang et al., 2013 [29] |
| Nutritional rickets | UPLC−MS/MS | -115 children with rickets; -85 healthy children. | Urine | 31 biomarkers of nutritional rickets were identified. 5 candidate biomarkers for clinical diagnosis were screened (phosphate, pyrophosphate, citric acid, cAMP, sebacic acid). The combination of sebacic acid and phosphate was selected as the candidate biomarker with high sensitivity (94.0%) and specificity (71.2%) (AUC = 0.85). | Wang et al., 2014 [30] |
| Osteopenia and osteoporosis | NMR spectroscopy | 56 postmenopausal women: -36 with low bone mass (T-score < −1); -20 with normal bone mass. | Serum and Urine | Disparities between individuals’ responses to vitamin D and calcium supplementation in patients with genetic variations in oestrogen receptor 1 gene and vitamin D receptor gene were found. NMR studies indicated unique patterns of metabolites, separating responders from non-responders and controls. | Elnenaei et al., 2010 [31] |
| Osteopenia and osteoporosis | LC-MS | 69 patients: -25 patients with osteoporosis; -22 patients with osteopenia; -22 patients with normal bone mineral density. | Serum | 116 metabolites were associated with low bone mineral density compared to controls (94 metabolites were dysregulated: ↑52, ↑42). The most frequently dysregulated metabolic pathways in low bone mineral density: histidine metabolism, glyoxylate, aminoacyl-tRNA biosynthesis, dicarboxylate metabolism and unsaturated fatty acid biosynthesis. 35 metabolites were dysregulated between patients with osteopenia and osteoporosis: ↑11(3-carboxy-4-methyl-5-propyl-2-2furanopropionic acid, carnitine derivatives) and ↓24(phosphatidylcholine, sphingomyelin, palmitic acid) in patients with osteopenia compared to patients with osteoporosis). | Aleidi et al., 2021 [32] |
| Osteoporosis | HRMAS NMR spectroscopy | 601 healthy Taiwanese women (40–55 years old). | Plasma | 7 metabolites characterizing low BMD were identified. Elevated glutamine was significantly associated with low BMD. Elevated lactate, lipids, acetone and very-low-density lipoprotein protected against low BMD. Metabolomic profiling may improve the risk prediction of osteoporosis. | You et al., 2014 [33] |
| Osteoporosis | GC-MS | 364 women: -Premenopausal women with normal BMD; -Postmenopausal women with normal BMD; -Postmenopausal women with osteopenia; -Postmenopausal women with osteoporosis. | Serum | 12 metabolites were able to differentiate low-BMD groups from normal-BMD groups. 5 free fatty acids (11,14-eicosadienoic acid, oleic acid, LA and AA) had the greatest potential to be used as osteoporosis biomarkers. ↑ Arachidonic acid, eicosadienoic acid, lysine, linoleic acid, tryptophan, allose, oleic acid. ↓ 3-hydroxy-l-proline, homoserine, pyruvic acid. | Qi et al., 2016 [34] |
| Osteoporosis | CE-TOFMS | Women (39–64 years old). | Serum | 57 metabolites differed significantly among various groups. Diglycine and cystine were lower in the low-BMD group. Hydroxyproline was higher in the low-BMD group. Metabolomic profiling may improve the risk prediction of osteoporosis. | Miyamoto et al., 2017 [35] |
| Osteoporosis | CE-TOFMS | Women (31–69 years old): -30 premenopausal and normal BMD; -46 postmenopausal and normal BMD; -33 postmenopausal and low BMD. | Serum | 52 metabolites differed significantly among various groups. Metabolomic profiling may improve the risk prediction of osteoporosis. Ornithine, arginine, citrulline, creatine and urea levels were increased in postmenopausal groups. The level of guanidinoacetate was decreased in postmenopausal groups. The levels of most amino acids, except branched-chain amino acids, were increased in postmenopausal women with low BMD. | Miyamoto et al., 2018 [36] |
| Osteoporosis | LC-MS | 136 women (20–40 years old): -65 with low hip BMD; -71 with high hip BMD. | Serum | 14 metabolites (7 amino acids and amino acid derivatives and 5 lipids: 3 bile acids and 2 organic acids) were associated with a risk of low BMD. Glutamic acid, threonine, taurine, GABA and cysteine were significantly associated with BMD. Metabolomic profiling may improve the risk prediction of osteoporosis. | Zhao et al., 2018 [37] |
| Osteoporosis | (1)H-NMR | -18 healthy volunteers; -18 diabetic patients with disordered bone metabolism. | Plasma | Metabolomic profiling may improve the risk prediction of diabetic osteoporosis. ↑ Isoleucine valine, glutamine, alanine, inositol, proline, leucine, glucose, 1-methyl-histidine, tyrosine, N-acetylglycoprotein. ↓ O-acetylglycoprotein, creatine, α-ketoglutaric acid, citrate. | Liang et al., 2020 [38] |
| Osteoporosis | LC-MS/MS | Discovery cohort: 1552 participants. Replication cohort: 634 participants. | Serum | 27 metabolites were associated with femoral-neck BMD or lumbar-spine BMD. Glycine, triacylglycerol and phosphatidylcholine were negatively associated with femoral-neck BMD. Phosphatidylcholine and triacylglycerol were negatively associated with lumbar-spine BMD. Authors replicated improvement of fracture prediction with selected metabolites in 634 participants. Metabolomic profiling may improve the risk prediction of osteoporosis. | Zhang et al., 2021 [39] |
| Osteoporosis | UPLC−MS/MS | 971 adults. | Serum and feces | Isoleucine, valine and leucine degradation was associated with osteoporosis. Strong evidence linking gut dysbiosis, fecal metabolomics and serum metabolomics with osteoporosis was reported. | Ling et al., 2021 [40] |
| Vitamin D3 deficiency | DI-LC/MS/MS GC-MS | 30 healthy adults were given 600, 4000 or 10,000 IUs of vitamin D3/day for 6 months. | Serum and urine | Statistically significant changes in 11 metabolites (7 from serum and 4 from urine) after 6 months of vitamin D3 supplementation were found. There was a distinct difference in the targeted metabolites between the more and less vitamin D3 responsive participants. Targeted analysis included 83 metabolites from serum and 36 metabolites from urine. | Shirvani et al., 2020 [41] |
| Vitamin D3 deficiency | (1)H-NMR | 76 postmenopausal women with Vitamin D insufficiency (<50 nmol/l) were given 2800 IUs of vitamin D3/day or placebo for 12 weeks | Serum | Supplementation of vitamin D significantly increased serum levels of carnitine, choline and urea and trimethylamine-N-oxide tendency to rise. | Bislev et al., 2020 [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgórska, B.; Wielogórska-Partyka, M.; Godzień, J.; Siemińska, J.; Ciborowski, M.; Szelachowska, M.; Krętowski, A.; Siewko, K. Applications of Metabolomics in Calcium Metabolism Disorders in Humans. Int. J. Mol. Sci. 2022, 23, 10407. https://doi.org/10.3390/ijms231810407
Podgórska B, Wielogórska-Partyka M, Godzień J, Siemińska J, Ciborowski M, Szelachowska M, Krętowski A, Siewko K. Applications of Metabolomics in Calcium Metabolism Disorders in Humans. International Journal of Molecular Sciences. 2022; 23(18):10407. https://doi.org/10.3390/ijms231810407
Chicago/Turabian StylePodgórska, Beata, Marta Wielogórska-Partyka, Joanna Godzień, Julia Siemińska, Michał Ciborowski, Małgorzata Szelachowska, Adam Krętowski, and Katarzyna Siewko. 2022. "Applications of Metabolomics in Calcium Metabolism Disorders in Humans" International Journal of Molecular Sciences 23, no. 18: 10407. https://doi.org/10.3390/ijms231810407
APA StylePodgórska, B., Wielogórska-Partyka, M., Godzień, J., Siemińska, J., Ciborowski, M., Szelachowska, M., Krętowski, A., & Siewko, K. (2022). Applications of Metabolomics in Calcium Metabolism Disorders in Humans. International Journal of Molecular Sciences, 23(18), 10407. https://doi.org/10.3390/ijms231810407








