Metabolomic Profiling of End-Stage Heart Failure Secondary to Chronic Chagas Cardiomyopathy
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
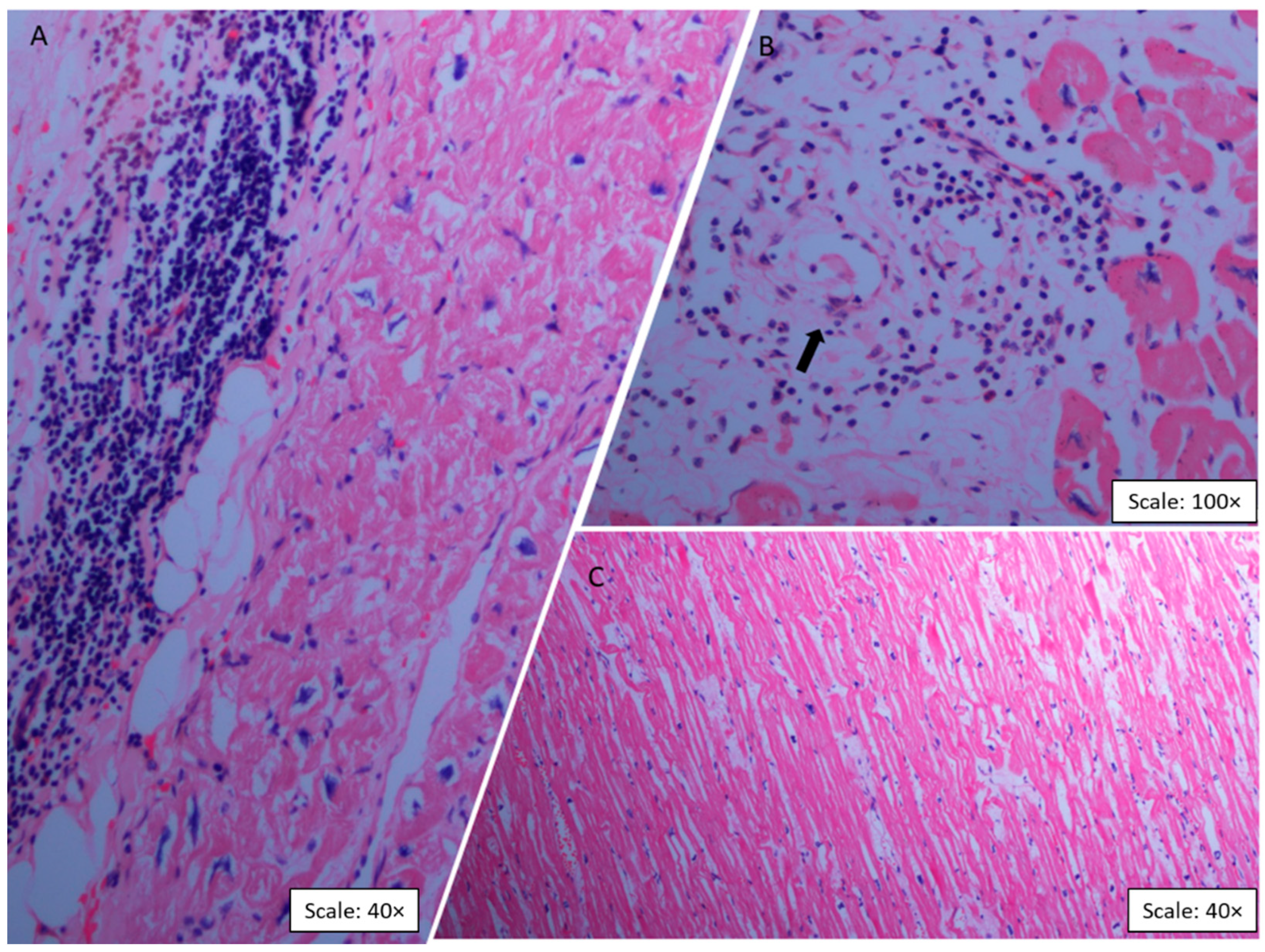
2.2. Histopathologic Findings
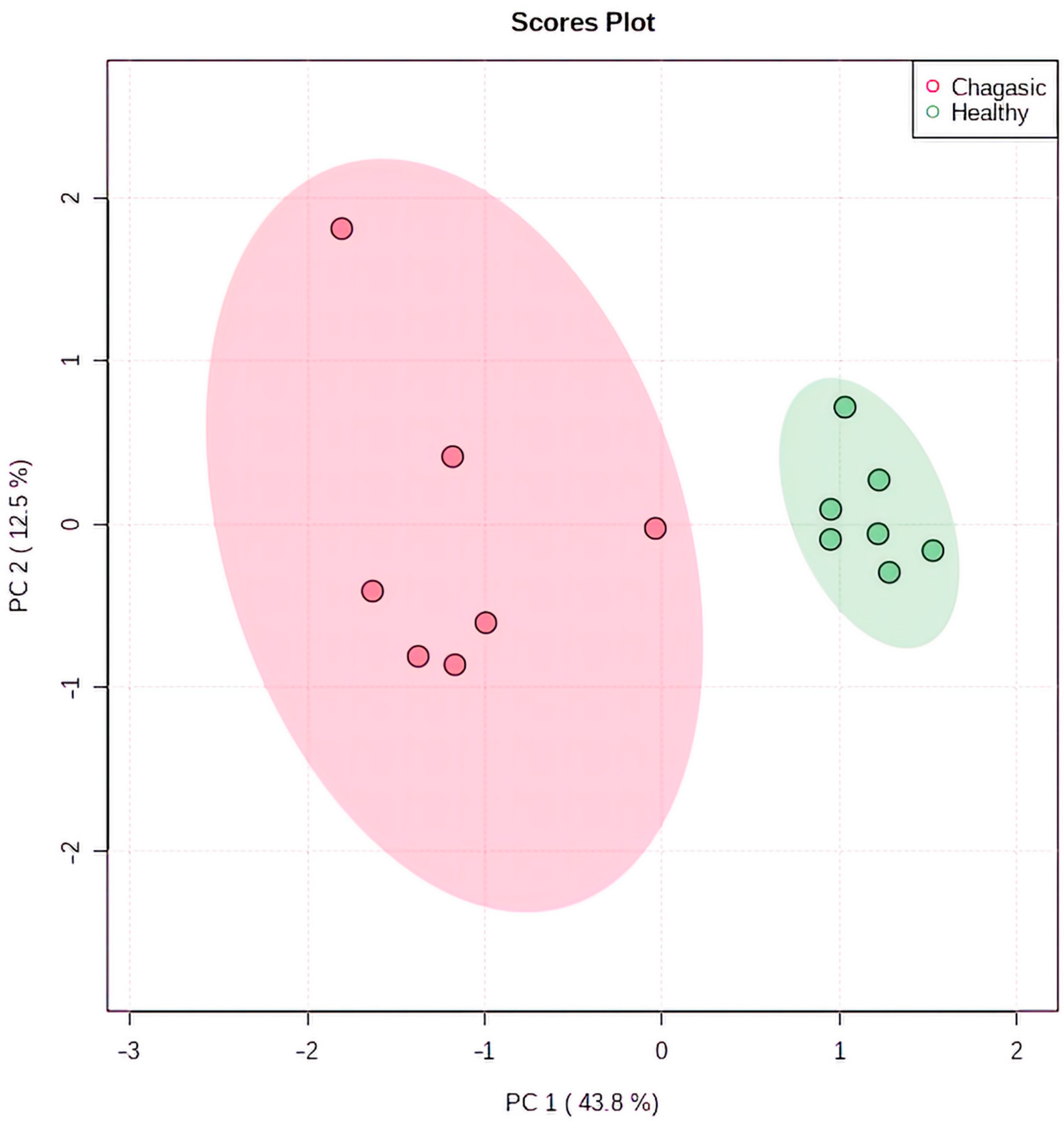
2.3. Metabolomic Profiling in Myocardial Tissue from CCC Patients and Healthy Donors
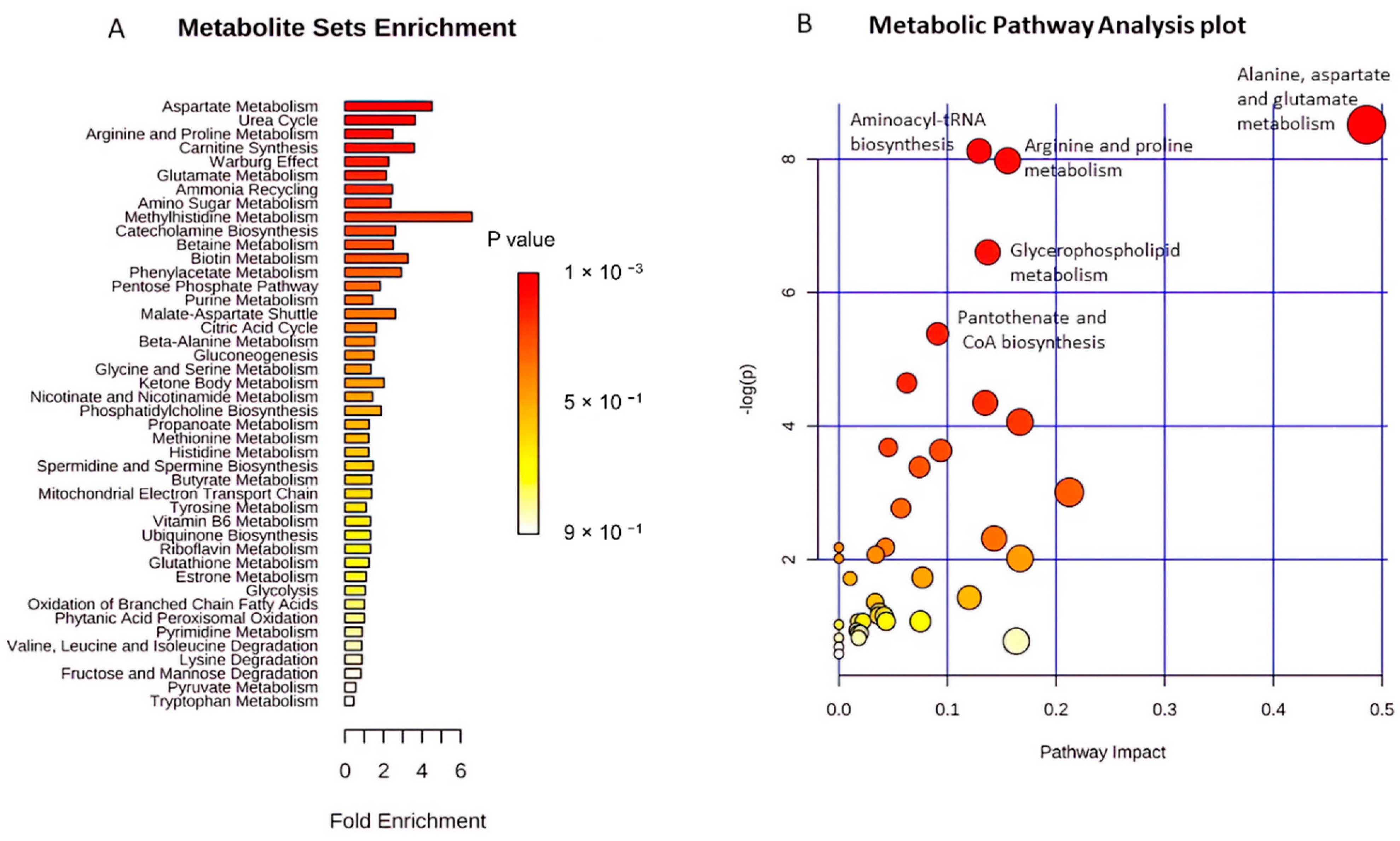
2.4. Functional Pathway Analyses
3. Discussion
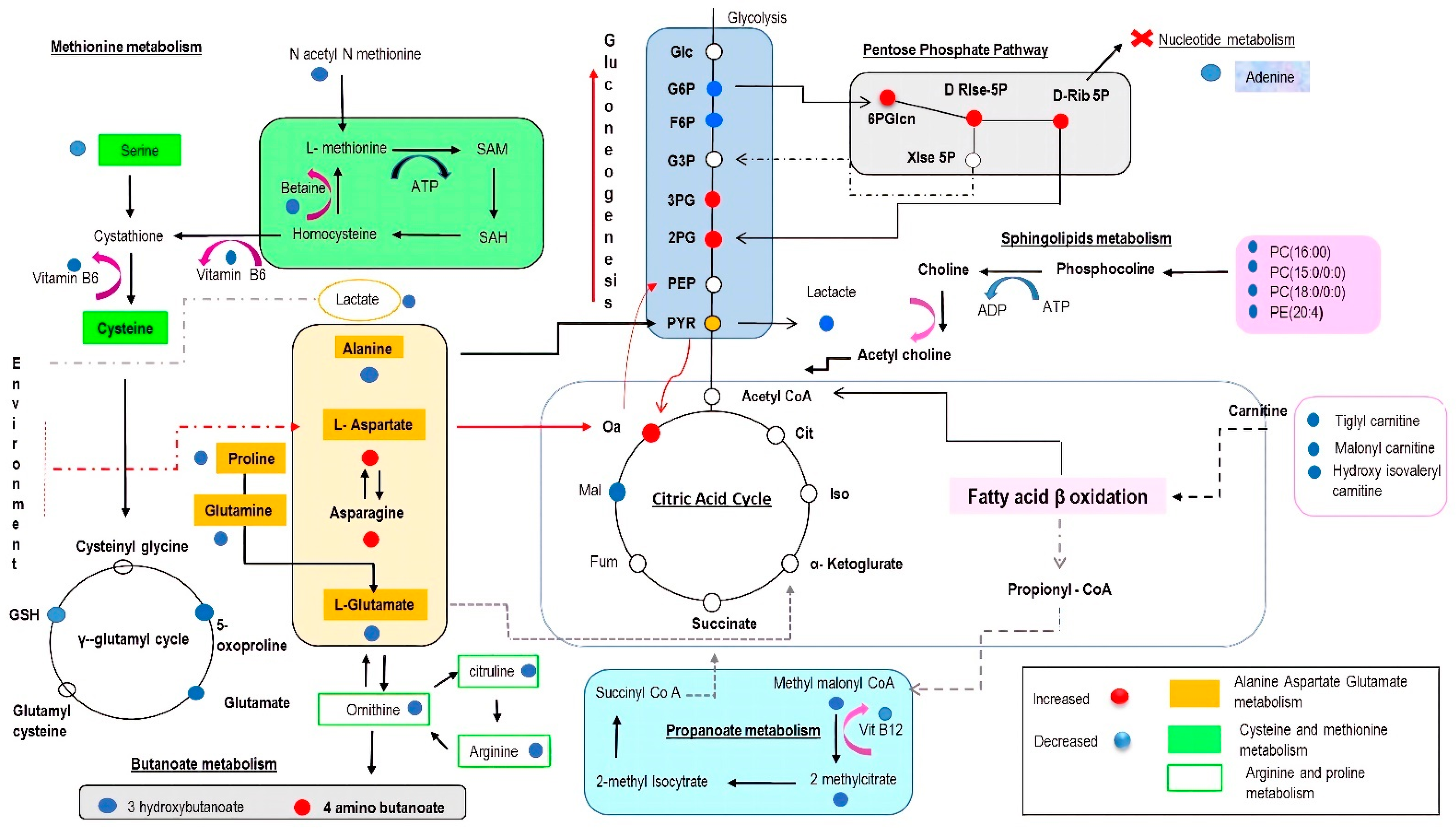
3.1. Amino Acids as Metabolic Substrates
3.2. Significance of Acylcarnitines and Glycerophospholipids Biosynthesis
3.3. Significance of Glycolysis
3.4. The Impact on the Tricarboxylic Acid (TCA) Cycle
3.5. The Potential Impact of Age on Metabolomic Profiling
3.6. Strengths and Limitations
4. Materials and Methods
4.1. Chemical Reagents and Chromatography Column
4.2. Explanted Hearts
4.3. Tissue Preparation
4.4. Extraction of Metabolites from Heart Tissue
LC/MS Analytical Platform and Methods
4.5. Data Acquisition and Processing
Metabolite Identification and Pathway Analysis
4.6. Statistical Analysis
4.7. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Chagas disease in Latin America: An epidemiological update based on 2010 estimates. In Weekly Epidemiological Record; World Health Organization: Geneva, Switzerland, 2015; Volume 90. Available online: https://www.who.int/wer/2015/wer9006.pdf?ua=1 (accessed on 28 March 2022).
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ramires, F.; Martinez, F.; Bodanese, L.C.; Echeverría, L.E.; Gómez, E.A.; Abraham, W.T.; Dickstein, K.; Køber, L.; Packer, M.; et al. Contemporary Characteristics and Outcomes in Chagasic Heart Failure Compared with Other Nonischemic and Ischemic Cardiomyopathy. Circ. Heart Fail. 2017, 10, e004361. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, L.E.; Morillo, C.A. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2019, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Bestetti, R.B.; Cardinalli-Neto, A. Device therapy in Chagas disease heart failure. Expert Rev. Cardiovasc. Ther. 2012, 10, 1307–1317. [Google Scholar] [CrossRef]
- Atik, F.A.; Cunha, C.R.; Chaves, R.B.; Ulhoa, M.B.; Barzilai, V.S. Left Ventricular Assist Device as a Bridge to Candidacy in End-stage Chagas Cardiomyopathy. Arq. Bras. Cardiol. 2018, 111, 112–114. [Google Scholar] [CrossRef]
- Moreira, L.F.P.; Galantier, J.; Benício, A.; Leirner, A.A.; Cestari, I.A.; Stolf, N.A. Left Ventricular Circulatory Support as Bridge to Heart Transplantation in Chagas’ Disease Cardiomyopathy. Artif. Organs 2007, 31, 253–258. [Google Scholar] [CrossRef]
- Echeverría, L.E.; Rojas, L.Z.; Rueda-Ochoa, O.L.; Gómez-Ochoa, S.A.; Rugeles, C.I.G.; Díaz, M.L.; Marcus, R.; Morillo, C.A. Circulating Trypanosoma cruzi load and major cardiovascular outcomes in patients with chronic Chagas cardiomyopathy: A prospective cohort study. Trop. Med. Int. Health 2020, 25, 1534–1541. [Google Scholar] [CrossRef]
- Echeverría, L.E.; Rojas, L.Z.; Gómez-Ochoa, S.A. Coagulation disorders in Chagas disease: A pathophysiological systematic review and meta-analysis. Thromb. Res. 2021, 201, 73–83. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Beaton, A.; Acquatella, H.; Bern, C.; Bolger, A.F.; Echeverría, L.E.; Dutra, W.O.; Gascon, J.; Morillo, C.A.; Oliveira-Filho, J.; et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation 2018, 138, e169–e209. [Google Scholar] [CrossRef]
- Palomino, S.A.P.; Aiello, V.D.; Higuchi, M.L. Systematic mapping of hearts from chronic chagasic patients: The association between the occurrence of histopathological lesions and Trypanosoma cruzi antigens. Ann. Trop. Med. Parasitol. 2000, 94, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.; Silva-Mota, K.N.; Lima, R.S.; Bellintani, M.C.; Pontes-De-Carvalho, L.; Ribeiro-Dos-Santos, R. Modulation of Chagasic Cardiomyopathy by Interleukin-4: Dissociation between Inflammation and Tissue Parasitism. Am. J. Pathol. 2001, 159, 703–709. [Google Scholar] [CrossRef]
- Zhang, L.; Tarleton, R.L. Parasite Persistence Correlates with Disease Severity and Localization in Chronic Chagas’ Disease. J. Infect. Dis. 1999, 180, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Gironès, N.; Carbajosa, S.; Guerrero, N.A.; Poveda, C.; Chillón-Marinas, C.; Fresno, M. Global Metabolomic Profiling of Acute Myocarditis Caused by Trypanosoma cruzi Infection. PLoS Negl. Trop. Dis. 2014, 8, e3337. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac Metabolism in Heart Failure: Implications Beyond ATP Production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Müller, J.; Bertsch, T.; Volke, J.; Schmid, A.; Klingbeil, R.; Metodiev, Y.; Karaca, B.; Kim, S.-H.; Lindner, S.; Schupp, T.; et al. Narrative review of metabolomics in cardiovascular disease. J. Thorac. Dis. 2021, 13, 2532–2550. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Benchimol-Barbosa, P. Cardiac remodeling and predictors for cardiac death in long-term follow-up of subjects with chronic Chagas’ heart disease: A mathematical model for progression of myocardial damage. Int. J. Cardiol. 2009, 131, 435–438. [Google Scholar] [CrossRef]
- De Bona, E.; Lidani, K.C.F.; Bavia, L.; Omidian, Z.; Gremski, L.; Sandri, T.L.; Reason, I.J.D.M. Autoimmunity in Chronic Chagas Disease: A Road of Multiple Pathways to Cardiomyopathy? Front. Immunol. 2018, 9, 1842. [Google Scholar] [CrossRef] [Green Version]
- Tanowitz, H.B.; Machado, F.S.; Spray, D.C.; Friedman, J.M.; Weiss, O.S.; Lora, J.N.; Nagajyothi, J.; Moraes, D.N.; Garg, N.J.; Nunes, M.C.P.; et al. Developments in the management of Chagas cardiomyopathy. Expert Rev. Cardiovasc. Ther. 2015, 13, 1393–1409. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, K.; Ayyappan, J.P.; Ganapathi, U.; Dutra, W.O.; Qiu, Y.; Weiss, L.M.; Nagajyothi, J.F. Diet Alters Serum Metabolomic Profiling in the Mouse Model of Chronic Chagas Cardiomyopathy. Dis. Markers 2019, 2019, 4956016. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Gautham, G.; Siqueira-Neto, J.L.; McKerrow, J.H.; Dorrestein, P.C.; McCall, L.-I. Spatial metabolomics identifies localized chemical changes in heart tissue during chronic cardiac Chagas Disease. PLoS Negl. Trop. Dis. 2021, 15, e0009819. [Google Scholar] [CrossRef]
- Tsang, H.G.; Rashdan, N.A.; Whitelaw, C.B.A.; Corcoran, B.M.; Summers, K.M.; MacRae, V.E. Large animal models of cardiovascular disease. Cell Biochem. Funct. 2016, 34, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.R.; Brahma, M.K.; McGinnis, G.R.; Young, M.E. Metabolic Origins of Heart Failure. JACC Basic Transl. Sci. 2017, 2, 297–310. [Google Scholar] [CrossRef]
- Drake, K.J.; Sidorov, V.Y.; Mcguinness, O.P.; Wasserman, D.H.; Wikswo, J.P. Amino acids as metabolic substrates during cardiac ischemia. Exp. Biol. Med. 2012, 237, 1369–1378. [Google Scholar] [CrossRef]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma Amino Acid Profiling Identifies Specific Amino Acid Associations with Cardiovascular Function in Patients with Systolic Heart Failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic Syndrome: Molecular Basis and Effects of Nutritional Supplements with Amino Acids. Am. J. Cardiol. 2008, 101, S11–S15. [Google Scholar] [CrossRef]
- Lai, L.; Leone, T.C.; Keller, M.P.; Martin, O.J.; Broman, A.T.; Nigro, J.; Kapoor, K.; Koves, T.R.; Stevens, R.; Ilkayeva, O.R.; et al. Energy Metabolic Reprogramming in the Hypertrophied and Early Stage Failing Heart: A multisystems approach. Circ. Heart Fail. 2014, 7, 1022–1031. [Google Scholar] [CrossRef]
- Aquilani, R.; La Rovere, M.T.; Corbellini, D.; Pasini, E.; Verri, M.; Barbieri, A.; Condino, A.M.; Boschi, F. Plasma Amino Acid Abnormalities in Chronic Heart Failure. Mechanisms, Potential Risks and Targets in Human Myocardium Metabolism. Nutrients 2017, 9, 1251. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.; Olson, E. Cardiac Hypertrophy: The Good, the Bad, and the Ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, M.; Sun, H.; Wang, Y. Branched-chain amino acid metabolism in heart disease: An epiphenomenon or a real culprit? Cardiovasc. Res. 2011, 90, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, H.; Katano, S.; Yano, T.; Ohori, K.; Nagaoka, R.; Inoue, T.; Takamura, Y.; Ishigo, T.; Watanabe, A.; Koyama, M.; et al. Plasma amino acid profiling improves predictive accuracy of adverse events in patients with heart failure. ESC Heart Fail. 2021, 8, 5045–5056. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.; McGregor, G.; Al-Mohammad, A.; Ali, A.N.; Tew, G.; O’Doherty, A.F. The effect of protein and essential amino acid supplementation on muscle strength and performance in patients with chronic heart failure: A systematic review. Eur. J. Nutr. 2020, 59, 1785–1801. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Sevostjanovs, E.; Vilks, K.; Volska, K.; Antone, U.; Kuka, J.; Makarova, E.; Pugovics, O.; Dambrova, M.; Liepinsh, E. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci. Rep. 2017, 7, 17528. [Google Scholar] [CrossRef]
- Fornasini, G.; Upton, R.N.; Evans, A.M. A pharmacokinetic model for L-carnitine in patients receiving haemodialysis. Br. J. Clin. Pharmacol. 2007, 64, 335–345. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Ono-Moore, K.D.; Oort, P.J.; Adams, S.H. Long-chain acylcarnitines activate cell stress and myokine release in C2C12myotubes: Calcium-dependent and -independent effects. Am. J. Physiol. Metab. 2015, 308, E990–E1000. [Google Scholar] [CrossRef]
- Chintapalli, S.V.; Jayanthi, S.; Mallipeddi, P.L.; Gundampati, R.; Kumar, S.T.; van Rossum, D.; Anishkin, A.; Adams, S.H. Novel Molecular Interactions of Acylcarnitines and Fatty Acids with Myoglobin. J. Biol. Chem. 2016, 291, 25133–25143. [Google Scholar] [CrossRef] [Green Version]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Tuunanen, H.; Engblom, E.; Naum, A.; Scheinin, M.; Någren, K.; Airaksinen, J.; Nuutila, P.; Iozzo, P.; Ukkonen, H.; Knuuti, J. Decreased Myocardial Free Fatty Acid Uptake in Patients with Idiopathic Dilated Cardiomyopathy: Evidence of Relationship with Insulin Resistance and Left Ventricular Dysfunction. J. Card. Fail. 2006, 12, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Obrzut, S.; Tiongson, J.; Jamshidi, N.; Phan, H.M.; Hoh, C.; Birgersdotter-Green, U. Assessment of Metabolic Phenotypes in Patients with Non-ischemic Dilated Cardiomyopathy Undergoing Cardiac Resynchronization Therapy. J. Cardiovasc. Transl. Res. 2010, 3, 643–651. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, J.; Ganapathy, V.; Longo, N. Mutations in the organic cation/carnitine transporter OCTN2 in primary carnitine deficiency. Proc. Natl. Acad. Sci. USA 1999, 96, 2356–2360. [Google Scholar] [CrossRef]
- Hossain, E.; Khanam, S.; Dean, D.A.; Wu, C.; Lostracco-Johnson, S.; Thomas, D.; Kane, S.S.; Parab, A.R.; Flores, K.; Katemauswa, M.; et al. Mapping of host-parasite-microbiome interactions reveals metabolic determinants of tropism and tolerance in Chagas disease. Sci. Adv. 2020, 6, eaaz2015. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.-I.; Morton, J.T.; Bernatchez, J.A.; de Siqueira-Neto, J.L.; Knight, R.; Dorrestein, P.C.; McKerrow, J.H. Mass Spectrometry-Based Chemical Cartography of a Cardiac Parasitic Infection. Anal. Chem. 2017, 89, 10414–10421. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Long-Chain Acylcarnitine Metabolites are Associated with Adverse Outcomes and Reversible with Mechanical Circulatory Support in Systolic Heart Failure. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef]
- Truby, L.K.; Regan, J.A.; Giamberardino, S.N.; Ilkayeva, O.; Bain, J.; Newgard, C.B.; O’Connor, C.M.; Felker, G.M.; Kraus, W.E.; McGarrah, R.W.; et al. Circulating long chain acylcarnitines and outcomes in diabetic heart failure: An HF-ACTION clinical trial substudy. Cardiovasc. Diabetol. 2021, 20, 161. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Sack, M.N.; Rader, T.A.; Park, S.; Bastin, J.; McCune, S.A.; Kelly, D.P. Fatty Acid Oxidation Enzyme Gene Expression Is Downregulated in the Failing Heart. Circulation 1996, 94, 2837–2842. [Google Scholar] [CrossRef]
- Badolia, R.; Ramadurai, D.K.; Abel, E.D.; Ferrin, P.; Taleb, I.; Shankar, T.S.; Krokidi, A.T.; Navankasattusas, S.; McKellar, S.H.; Yin, M.; et al. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure. Circulation 2020, 142, 259–274. [Google Scholar] [CrossRef]
- Zimmer, H.-G. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol. Cell. Biochem. 1996, 160–161, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Meerson, F.Z.; Spiritchev, V.B.; Pshennikova, M.G.; Djachkova, L.V. The role of the pentose-phosphate pathway in adjustment of the heart to a high load and the development of myocardial hypertrophy. Experientia 1967, 23, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, C.; Qanud, K.; Mitacchione, G.; Sosnowska, D.; Ungvari, Z.; Sarnari, R.; Mania, D.; Patel, N.; Hintze, T.H.; Gupte, S.A.; et al. Beneficial effects of acute inhibition of the oxidative pentose phosphate pathway in the failing heart. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H709–H717. [Google Scholar] [CrossRef] [PubMed]
- Gerin, I.; Ury, B.; Breloy, I.; Bouchet-Seraphin, C.; Bolsée, J.; Halbout, M.; Graff, J.; Vertommen, D.; Muccioli, G.G.; Seta, N.; et al. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto α-dystroglycan. Nat. Commun. 2016, 7, 11534. [Google Scholar] [CrossRef]
- Cataldi, M.P.; Lu, P.; Blaeser, A.; Lu, Q.L. Ribitol restores functionally glycosylated α-dystroglycan and improves muscle function in dystrophic FKRP-mutant mice. Nat. Commun. 2018, 9, 3448. [Google Scholar] [CrossRef]
- Michele, D.E.; Kabaeva, Z.; Davis, S.L.; Weiss, R.M.; Campbell, K.P. Dystroglycan Matrix Receptor Function in Cardiac Myocytes Is Important for Limiting Activity-Induced Myocardial Damage. Circ. Res. 2009, 105, 984–993. [Google Scholar] [CrossRef]
- Brancaccio, A. Increased levels of expression of dystroglycan may protect the heart. Neuromuscul. Disord. 2013, 23, 867–870. [Google Scholar] [CrossRef]
- West, J.A.; Beqqali, A.; Ament, Z.; Elliott, P.; Pinto, Y.M.; Arbustini, E.; Griffin, J.L. A targeted metabolomics assay for cardiac metabolism and demonstration using a mouse model of dilated cardiomyopathy. Metabolomics 2016, 12, 59. [Google Scholar] [CrossRef]
- Pietersen, H.; Langenberg, C.; Geskes, G.; Soeters, P.; Wagenmakers, A. Glutamate metabolism of the heart during coronary artery bypass grafting. Clin. Nutr. 1998, 17, 73–75. [Google Scholar] [CrossRef]
- Jessen, M.E.; Kovarik, T.E.; Jeffrey, F.M.; Sherry, A.D.; Storey, C.J.; Chao, R.Y.; Ring, W.S.; Malloy, C.R. Effects of amino acids on substrate selection, anaplerosis, and left ventricular function in the ischemic reperfused rat heart. J. Clin. Investig. 1993, 92, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.J.; Pietersen, H.G.; Geskes, G.; Wagenmakers, A.; De Lange, S.; Schouten, H.J.; Soeters, P.B. The effect of glutamate infusion on cardiac performance is independent of changes in metabolism in patients undergoing routine coronary artery bypass surgery. Clin. Sci. 2001, 101, 573–578. [Google Scholar] [CrossRef]
- Durante, W. The Emerging Role of l-Glutamine in Cardiovascular Health and Disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef]
- Yan, M.; Sun, S.; Xu, K.; Huang, X.; Dou, L.; Pang, J.; Tang, W.; Shen, T.; Li, J. Cardiac Aging: From Basic Research to Therapeutics. Oxidative Med. Cell. Longev. 2021, 2021, 9570325. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, a025148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chong, S.Y.; Lim, A.; Singh, B.K.; Sinha, R.A.; Salmon, A.B.; Yen, P.M. Changes in macroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging 2017, 9, 583–599. [Google Scholar] [CrossRef]
- Dai, D.-F.; Karunadharma, P.P.; Chiao, Y.A.; Basisty, N.; Crispin, D.; Hsieh, E.J.; Chen, T.; Gu, H.; Djukovic, D.; Raftery, D.; et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 2014, 13, 529–539. [Google Scholar] [CrossRef]
- Latimer, M.N.; Sonkar, R.; Mia, S.; Frayne, I.R.; Carter, K.J.; Johnson, C.A.; Rana, S.; Xie, M.; Rowe, G.C.; Wende, A.R.; et al. Branched chain amino acids selectively promote cardiac growth at the end of the awake period. J. Mol. Cell. Cardiol. 2021, 157, 31–44. [Google Scholar] [CrossRef]
- Portero, V.; Nicol, T.; Podliesna, S.; Marchal, G.A.; Baartscheer, A.; Casini, S.; Tadros, R.; Treur, J.L.; Tanck, M.W.T.; Cox, I.J.; et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc. Res. 2022, 118, 1742–1757. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef] [Green Version]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- Czibik, G.; Mezdari, Z.; Altintas, D.M.; Bréhat, J.; Pini, M.; D’Humières, T.; Delmont, T.; Radu, C.; Breau, M.; Liang, H.; et al. Dysregulated Phenylalanine Catabolism Plays a Key Role in the Trajectory of Cardiac Aging. Circulation 2021, 144, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Marijianowski, M.M.; Teeling, P.; Mann, J.; Becker, A.E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: A quantitative assessment. J. Am. Coll. Cardiol. 1995, 25, 1263–1272. [Google Scholar] [CrossRef]
- Liu, X.; Song, J. The application of autopsy and explanted heart samples in scientific research. Cardiovasc. Pathol. 2022, 59, 107424. [Google Scholar] [CrossRef]
- Gloaguen, Y.; Morton, F.; Daly, R.; Gurden, R.; Rogers, S.; Wandy, J.; Wilson, D.; Barrett, M.; Burgess, K. PiMP my metabolome: An integrated, web-based tool for LC-MS metabolomics data. Bioinformatics 2017, 33, 4007–4009. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Ihnatova, I.; Popovici, V.; Budinska, E. A critical comparison of topology-based pathway analysis methods. PLoS ONE 2018, 13, e0191154. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. Wma Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. In Proceedings of the 64th WMA General Assembly, Fortaleza, Brazil, 16–19 October 2013. [Google Scholar]

| Metabolite Number | ID Peak | Identified Metabolites | Log2 Fold Change | m/Z | Retention Times (mins) | Chemical Class | p-Value | Adjusted p-Value * |
|---|---|---|---|---|---|---|---|---|
| 1 | 128 | N-Acetyl Methionine | −4.75 | 1.920.688 | 422.47 | C7H13NO3S | 0.0001 | 0.0004 |
| 2 | 559 | Aspartyl Methionine | −4.37 | 263.07 | 475.91 | C9H16N205S | <0.0001 | 0.0001 |
| 3 | 29 | PC (15:0/0:0) | −3.85 | 504.307 | 240.79 | C23H48NO7P | <0.0001 | 0.0001 |
| 4 | 436 | Lyso PE (16:0/0:0) | −3.68 | 4.522.792 | 250.58 | C21H44NO7P | <0.0001 | 0.0001 |
| 5 | 465 | L-Noradrenaline | −3.53 | 170.081 | 464.47 | C8H11NO3 | <0.0001 | 0.0001 |
| 6 | 12 | L-Isoleucyl l proline | −3.39 | 2.291.542 | 561.53 | C11H20N2O3 | <0.0001 | 0.0001 |
| 7 | 68 | O-malonyl L-carnitine | −3.08 | 2.481.122 | 695.43 | C10H17NO6 | <0.0001 | <0.0001 |
| 8 | 277 | Riboflavin | −3.0 | 3.771.445 | 486.78 | C17H20N4O6 | <0.0001 | <0.0001 |
| 9 | 34 | 5-Oxoproline | −2.88 | 1.300.498 | 785.71 | C5H7NO3 | <0.0001 | <0.0001 |
| 10 | 213 | N Formyl Methionine | −2.87 | 1.780.531 | 496.84 | C6H11NO3S | <0.0001 | <0.0001 |
| 11 | 45 | Adenine | −2.50 | 1.360.617 | 546.22 | C5H5N5 | 0.004 | 0.0128 |
| 12 | 92 | Pirbuterol | −2.36 | 2.411.541 | 590.07 | C12H20N2O3 | <0.0001 | <0.0001 |
| 13 | 192 | N-Acetyl-D glucosamine | −2.34 | 222.097 | 657.25 | C8H15NO6 | <0.0001 | 0.0003 |
| 14 | 550 | Fructose 6 phosphate | −2.12 | 2.580.383 | 795.46 | C6H14NO8P | <0.0001 | 0.0003 |
| 15 | 163 | Tiglylcarnitine | −2.09 | 2.441.538 | 464.78 | C12H21NO4 | 0.0001 | 0.0006 |
| 16 | 300 | Pyridoxine (B6) | −2.07 | 170.081 | 464.47 | C8H11NO3 | <0.0001 | 0.0003 |
| 17 | 23 | PC (16:0/0:0) | −2.03 | 496.339 | 249.4 | C24H50NO7P | <0.0001 | 0.0003 |
| 18 | 439 | Succinic acid | −2.00 | 1.170.193 | 797.5 | C4H6O4 | 0.002 | 0.007 |
| 19 | 455 | Lysine | −1.92 | 1.450.983 | 1160.9 | C6H14N2O2 | 0.0008 | 0.0033 |
| 20 | 69 | Hidroxyisovareoyl carnitine | −1.90 | 2.621.643 | 587.18 | C12H23NO5 | 0.002 | 0.0011 |
| 21 | 588 | D-Alanyl R Lactate | −1.81 | 1.600.616 | 505.38 | C6H11NO4 | 0.0004 | 0.0025 |
| 22 | 89 | 2 Methylcitrate | −1.80 | 2.070.505 | 1160.39 | C7H10O7 | 0.0005 | 0.0021 |
| 23 | 388 | Glycerol 3 phosphate | −1.70 | 1.710.066 | 744.30 | C3H9O6P | 0.0001 | 0.0005 |
| 24 | 75 | L-Citrulline | −1.61 | 1.360.617 | 546.22 | C6H13N3O3 | 0.0007 | 0.003 |
| 25 | 64 | L-Methionine | −1.57 | 1.500.583 | 644.53 | C5H11NO2S | 0.0001 | 0.0005 |
| 26 | 60 | PC (16:1(9Z)/18:1(11Z)) | −1.49 | 7.585.677 | 213.42 | C42H80NO8P | 0.0346 | 0.0052 |
| 27 | 367 | β-Alanine | −1.46 | 880.404 | 774.77 | C3H7NO2 | 0.0011 | 0.0043 |
| 28 | 633 | cis-Aconitate | −1.43 | 1.730.092 | 736.89 | C6H6O6 | 0.0114 | 0.0334 |
| 29 | 360 | L-Glutamine | −1.43 | 1.450.619 | 784.69 | C5H10N2O3 | 0.0154 | 0.0375 |
| 30 | 21 | D-Valine | −1.36 | 1.180.862 | 632.6 | C5H11NO2 | 0.0001 | 0.0008 |
| 31 | 18 | Betaine | −1.36 | 1.180.862 | 630.34 | C5H11NO2 | 0.0003 | 0.0015 |
| 32 | 96 | Asparagine | −1.31 | 1.330.605 | 797.42 | C4H8N2O3 | 0.0013 | 0.0048 |
| 33 | 52 | L-Arginine | −1.30 | 175.11 | 1249.8 | C6H14N4O2 | 0.0006 | 0.0025 |
| 34 | 35 | L-Phenylalanine | −1.19 | 1.660.861 | 581.32 | C9H11NO2 | 0.0022 | 0.0075 |
| 35 | 73 | 4 Aminobutanoate | 1.04 | 1.040.705 | 796.8 | C4H9NO2 | 0.0004 | 0.0019 |
| 36 | 471 | D-Ribose 5 phosphate | 1.13 | 2.290.119 | 799.22 | C5H11O8P | 0.00032 | 0.0022 |
| 37 | 395 | L-Aspartate | 1.83 | 1.320.303 | 783.88 | C4H7NO4 | 0.0031 | 0.0098 |
| 38 | 218 | 2 Phospho D glycerate | 1.36 | 187.000 | 847.47 | C3H707P | 0.0073 | 0.0201 |
| 39 | 496 | Methylmalonylcarnitine | 2.49 | 260.114 | 686.2 | C11H19NO6 | 0.0023 | 0.0078 |
| 40 | 39 | Tranexamic acid | 8.94 | 1.581.173 | 804.32 | C8H15NO2 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, M.L.; Burgess, K.; Burchmore, R.; Gómez, M.A.; Gómez-Ochoa, S.A.; Echeverría, L.E.; Morillo, C.; González, C.I. Metabolomic Profiling of End-Stage Heart Failure Secondary to Chronic Chagas Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 10456. https://doi.org/10.3390/ijms231810456
Díaz ML, Burgess K, Burchmore R, Gómez MA, Gómez-Ochoa SA, Echeverría LE, Morillo C, González CI. Metabolomic Profiling of End-Stage Heart Failure Secondary to Chronic Chagas Cardiomyopathy. International Journal of Molecular Sciences. 2022; 23(18):10456. https://doi.org/10.3390/ijms231810456
Chicago/Turabian StyleDíaz, Martha Lucía, Karl Burgess, Richard Burchmore, María Adelaida Gómez, Sergio Alejandro Gómez-Ochoa, Luis Eduardo Echeverría, Carlos Morillo, and Clara Isabel González. 2022. "Metabolomic Profiling of End-Stage Heart Failure Secondary to Chronic Chagas Cardiomyopathy" International Journal of Molecular Sciences 23, no. 18: 10456. https://doi.org/10.3390/ijms231810456
APA StyleDíaz, M. L., Burgess, K., Burchmore, R., Gómez, M. A., Gómez-Ochoa, S. A., Echeverría, L. E., Morillo, C., & González, C. I. (2022). Metabolomic Profiling of End-Stage Heart Failure Secondary to Chronic Chagas Cardiomyopathy. International Journal of Molecular Sciences, 23(18), 10456. https://doi.org/10.3390/ijms231810456







