Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings
Abstract
:1. Introduction
2. Methodology
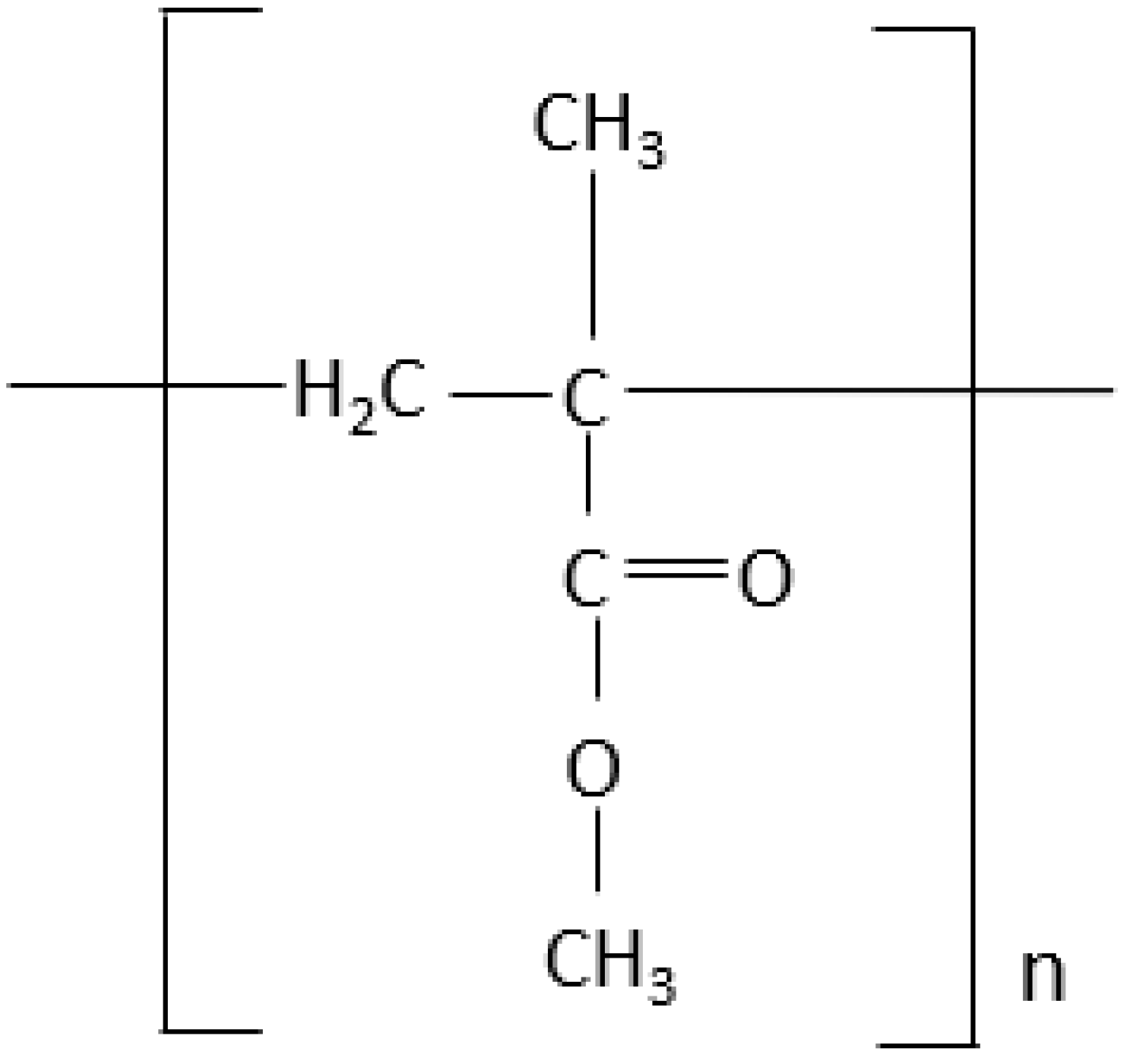
3. Polymethyl Methacrylate (PMMA)


4. Common Microbial Challenges Associated with PMMA in Conventional Denture
Denture Stomatitis


5. Pathogens in Denture Stomatitis
5.1. Candida sp. (Candida albicans)
5.2. Staphylococcus aureus
5.3. Streptococcus mutans
5.4. Fusobacterium nucleatum
6. Development of Digital Denture
7. Antimicrobial Properties on Digital Denture
8. Contemporary Modification on Denture Base Material
| Title | Material | Methodology | Effect | Recommendation |
|---|---|---|---|---|
| Poly (methyl methacrylate) with TiO2 nanoparticles inclusion for stereolithographic complete denture manufacturing—The future in dental care for elderly edentulous patients? [34] | Titanium Dioxide (TiO2) | The composite mixture has been obtained through subsequent additions of different amounts of TiO2 nanoparticles into PMMA the mixture by the weight of 0.2%, 0.4%, 0.6%, 1%, and 2.5%. Preparation used a combination of titanium tetrabutoxide (1.11 mmol) and dimedone (2.4 mmol) in 100 mL of considered alcohol (methanol, isopropanol) which has been allowed to react for 4 h in the 200 mL Teflon® shaft of a PM100 Retsch® (Retsch GmbH, Haan, Germany) colloid mill (with 25 g of quartz grinding balls of 1 mm diameter) at 250 rpm. The synthesization of TiO2 nanoparticles were evidenced by using scanning electron microscopy (SEM). Nanocomposites preparation procedure consisted of adding an appropriate amount of titania nanoparticles into PMMA solution under continuous stirring followed by ultrasound direct mixing in a sealed vial within an hour. | Have a large spectrum of activity against microorganisms including Gram-negative and positive bacteria and fungi. Intrinsically environmentally friendly. Effective antimicrobial activity. | Further studies are needed to document the evolution of thermal and rheological behaviour of the PMMA-TiO2 nanocomposites to have a complete image of the influence of the nanofiller content. Further mechanical and biocompatibility tests need to be investigated further. |
| Titanium Dioxide (TiO2) polymethylmethacrylate (PMMA) denture base nanocomposites: mechanical, viscoelastic and antibacterial behavior. [46] | Titanium Dioxide (TiO2) | A commercial heat curing PMMA denture acrylic was used (Lucitone 550, Dentsply International Inc., PA, USA). The resin was mixed according to the manufacturer’s instructions and packed into the mold space when the resin mix was in a doughy stage. Different ratios of TiO2 NPs were carefully weighed and mixed into the PMMA then tested (1 wt. %, 2 wt. %, and 3 wt. %). Characterization was assessed under the Fourier transform infrared (FTIR, Bruker, TENSOR Series FT-IR Spectrometer, Germany) and Scanning electron microscopy (SEM; FE-SEM-JEOL GSM-6610LV). Mechanical characteristics of the composite PMMA/TiO2 NP specimens were measured using a universal nanomechanical tester (Bruker, Campbell, CA, USA). The effects of TiO2 NP addition on the thermal behaviour of PMMA were examined using differential scanning calorimetry (DSC)/thermogravimetric analysis (TGA; Model SDT-Q600, TA-Instrument, USA). The PMMA/TiO2 nanocomposite specimens were cut into small parts weighing 7 mg, sealed in an aluminium pan, and then heated at 10 °C/min to 600 °C under nitrogen. The thermal behaviour data were obtained by software setup. | Improved in mechanical properties including the microhardness, creep related properties and modulus of elasticity. Improving antibacterial activity (E. faecalis & P. aeruginosa) by reducing bacterial adherence to cells. | - |
| TiO2 and PEEK reinforced 3D printing PMMA composite resin for dental denture base applications. [41] | Titanium Dioxide (TiO2) | The nano-TiO2 was prepared by the hydrothermal method as described by Souvereyns, B. et al. 2013. 34 mL of tetrabutyl titanate was slowly added to 100 mL of 4 mol/L HCl solution and stirred for 2 h. The lower layer of liquid processed using hydrothermal process at 180 °C for 12 h. The PMMA photosensitive resin were mixed according to resin base and nano filler weightage ratio. The mixture was then mechanically stirred at 500 rpm/min for 60 min, followed by ultrasonication at 40 W for 1 h. The mixture was placed in the vacuum oven to remove air bubbles and residue ethanol at 50 °C to remove unwanted materials. Digital Light Projection (DLP) Photocuring 3D printing system (Envision Tech, Gladbeck, Germany) were used to print the samples. | Excellent properties, such as stable chemical properties, good physical and mechanical properties, easy to polish, non-toxic, and antibacterial properties. TiO2 enhances the antimicrobial properties of the PMMA resin base. | Further research should focus on microwave light-heat conditions on the light-curing resins. Finite element analysis should be use as part of assessment to see the feasibility of stress of these samples intraorally. |
| A polymethyl methacrylate denture resin’s flexibility, biocompatibility, and antimicrobial activity are enhanced with graphene and silver nanoparticles. [35] | Graphene-Silver nanoparticles (G-AgNp) | The graphene silver nanoparticles (G-AgNp) composite was synthesized through the radio-frequency catalytic chemical vapor deposition (RF-CCVD) method. The mixture of G-AgNp with the acrylic material was done utilizing 95% ethyl alcoholic solution, at room temperature. Continuous mixing and stirring was performed within 30 min. The material was dried in an oven at 40 °C. Synthesis was performed using a methane. | The addition of graphene and silver nanoparticles to PMMA showed improvements in several physical and mechanical properties of the material, including flexural strength. Silver fillers were shown to exhibit an antimicrobial effect. | Further analysis is needed to determine the exact causative reduction of antimicrobial effect of the material. /-Adding other relevant bacterial / fungi strains particularly Candida Albican sp. |
| Characterization and evaluation of a novel silver nanoparticles-loaded polymethyl methacrylate denture base: In vitro and in vivo animal study. [36] | Silver nanoparticles (Nag) | Nag solution was synthetized and mixed with acrylic acid and methyl methyacrylate (MMA) monomer in order to prepare a new type of Nag solution (NS)/polymer methyl methacrylate denture base specimens (NS/PMMA). Dissolve 2.5 g of solid silver nitrate in 50 mL water followed by centrifugation at specified speed (rpm) and addition of alcohol. | The addition of NAg on PMMA has sufficient mechanical properties for clinical application. Biological properties showed that it possessed antibacterial effect with no cytotoxic effect. | - |
| Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. [47] | Nano graphene oxide (nGO) | nGO powder was washed with 70% alcohol for cleaning and sterilization purposes. nGO was added according to specific weight in relative to PMMA powder and further underwent sonication for 60 min. PMMA powder was mixed into the liquid at a powder (g) to liquid (ml) at a specific ratio (1.2:1.) and underwent low temperature polymerization afterwards | Addition of nGO increase the flexural strength and surface hardness (0.5 wt% onwards). It shows sustained, long-term antibacterial-adhesive effects. | Functionalization of nGO with PEG, salinization or carboxyl group are recommended to ensure homogenization of aggregation of the samples. |
| Denture Acrylic Resin Material with Antibacterial and Protein-Repelling Properties for the Prevention of Denture Stomatitis. [38] | 2-methacryloyloxyethyl phosphorylcholine (MPC) Dimethylaminohexadecyl methacrylate (DMAHDM). | Combination of 10 mmol of 2-(dimethylamino) ethyl methacrylate (DMAEMA, Sigma-Aldrich, St. Louis, MO, USA) and 10 mmol of 1-bromohexadecane with alcohol (3 g ethanol) in a 20 mL vial. Continuous stirring was done for 24 h at 70 °C. | The addition of MPC and DMAHDM causing decrease value in mechanical properties including flexural strength. The addition of those two biomaterials also promotes antibacterial effect, in this research to Candida albicans sp. | Multiple bacterial/ fungal strains should be included in future studies to see the effect on the relevant pathogen that can cause intraoral disease particularly to denture wearers. Long term effect of the addition of biomaterials should be evaluated in the context of biological and mechanical properties. |
| Heat-cured poly (methyl methacrylate) resin incorporated with different food preservatives as an anti-microbial denture base material [39] | Food preservatives, including zinc oxide, potassium sorbate and sodium metabisulfite | The food preservatives were mixed with the PMMA powder to specific concentrations (0.25% w/w of ZnO, 1.0% w/w of PS, and 0.5 % w/w of SM). The powder and liquid are mixed according to the manufacturer guidelines. | Incorporation of food preservative agent, such as ZnO, PS (Potassium Sulphate), and SM (Sodium Metabisulfite), into PMMA denture-based resin help in improving anti-microbial properties with no significant cytotoxicity exhibited. The addition of the food preservatives did not weaken the flexural strength and modulus in comparison to unmodified PMMA. | - |
| Surface silanization and grafting reaction of nano-silver loaded zirconium phosphate and properties strengthen 3D-printable dental base composites. [33] | Nano-silver loaded zirconium phosphate (6SNP3) | Surface alteration achieved by modification and salinization of γ-methacryloxypropyltrimethoxysilane (MPS) and grafting reaction of methyl methacrylate (MMA). Denture base resin composite materials were prepared with the ratio of P-6S-NP3 to E-Denture resin in a specified ratio (0, 1:100, 2:100 and 3:100, respectively). The procedure was briefed as follows: the 6S-NP3 or P-6S-NP3 nanoparticles were added to E-Denture resin and underwent mechanical stirring at 500 rpm/min for 60 min. The bubbles were removed by vacuum to obtain well-dispersed nanocomposite resin materials for additive manufacturing process. | Denture base composites displayed better mechanical properties in flexural strength, flexural modulus and impact strength. It also showed that the denture base composite possessed better antibacterial efficacy against E. coli sp. than S. aureus sp. | - |
| Novel dental poly (methyl methacrylate) containing phytoncide for antifungal effect and inhibition of oral multispecies biofilm. [40] | Phytoncide | The phytoncide was mixed with the denture base resin liquid monomer at various specific weight percentages; 0% (control), 1.25%, 2.5%, 3.75%, and 5%, respectively, and synthesizing further by sonication for 60 min and stirring for 4 h. Specimens were produced after polymerization into specific shapes were done. | The addition of phytoncide did not alter the general mechanical properties of the denture base, in the aspect of microhardness and flexural strength. Phytoncide exerted antimicrobial effects by reducing the amount of Candida albicans sp. colony forming units and biofilm thickness in this study. | Recommendation for more studies done to prove the effect of phytoncide incorporated in PMMA has antifungal properties when incorporated within denture base material. |
| Superhydrophobic coatings with self-cleaning and antibacterial adhesion properties for denture base. [42] | SiO2 nanoparticles | 0.2 g, 30 nm SiO2 NPs were dispersed into isopropyl alcohol (10 g), tetraethoxysilane (TEOS) (0.3 g) and 3-Glycidoxypropyltrimethoxysilane (KH560) (0.9 g) were added. Next, deionized water (30 µL) and acetic acid (30 µL) were added. The mixture was stirred vigorously for one day (24 h) at room temperature to obtain epoxy functionalized SiO2. S1, S2, and S3 coatings were made into 10% solution by mixing three solutions with different ratios and spraying on the substrate. | SiO2 micro nanoparticles produce an extra layer that prevents bacteria adhesion to the denture base surfaces and contains a self-cleaning effect. | - |
| Antibacterial activity, cytotoxicity, and mechanical behaviour of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. [41] | Titanium dioxide (TiO2) Silver-supported titanium dioxide (Ag/TiO2) Silver-supported zirconium phosphate (Novaron), Tetrapod-like zinc oxide whiskers (T-ZnOw) | Two weight percentage silanized nano-ZrO2 particles and 4 wt% silanized ABW (Aluminium Borate Whiskers) were mixed with PMMA powder by ball milling for 120 min at the speed of 180 rpm. Other materials, such as TiO2, Ag/TiO2, Novaron, and T-ZnOw, are also added to the composites with specific 3 wt%. The composites were further mixed with MMA monomer at specific ratio (2:1) powder-to-liquid ratio. | The addition of four antibacterial inorganic substrate reducing the colony forming units of S. mutans and C. albican. T-ZnOw and Novaron possessed the highest surface hardness and flexural strength in comparison to other groups, respectively. | - |
| Fabrication of denture base materials with antimicrobial properties. [44] | Quaternized N, N-dimethylaminoethyl methacrylate (DMAEMA) | The heat-polymerizing denture base resin was prepared according to the manufacturer’s guidelines. Next, DMAEMA-OB monomer was added to the monomer of the acrylic resin at 0%, 8%, 10%, and 12%, according to the polymer mass. Following the mixing of the powder and monomer, the dough was placed in a customised stainless-steel mold sandwiched between two steel plates and polymerized for 1 h at 70 °C and 2 h at 100 °C (dry heat), while the mould was crushed with a hydraulic press (20 MPa). | The antimicrobial activity was assessed using three different bacteria which are S. aureus (Gram-positive), E-coli (Gram-negative), and C. albicans. DMAEMA incorporated in denture base resin possessed antimicrobial effect towards all 3 different bacteria and fungi strains. Nonetheless, careful consideration on incorporating this material in the denture acrylic base as it may cause more plastic deformation, crack and reduce the mechanical properties of the denture base. | Recommendation for the need to improve the mechanical properties of a denture base composed of antibacterial properties. |
| Inhibitory effects of Lactobacillus rhamnosus and Lactobacillus casei on Candida biofilm of denture surface. [45] | Probiotics | Polymethylmethacrylate (PMMA) material specimens as denture base resin were prepared. Acrylic resin specimens were processed according to the manufacturer’s instructions. The flask was submerged in water at 74 °C for 90 min and at 99 °C for 30 min. After deflasking, PMMA disks were equivalent in size. Disks were placed in distilled water at room temperature until used. Next, denture base resin was immersed in the spent culture medium of L. rhamnosus and L. casei during the day and in tap water overnight, and this process was repeated for 30 days. | Both Lactobacillus rhamnosus sp. and Lactobacillus casei sp. possessed antifungal activity against Blastoconidia and C. albicans sp. and inhibit formation of biofilm on the denture base. They also did not affect the surface roughness of the denture base. | - |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, C.M. Suppl 1: A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Khindria, S.K.; Mittal, S.; Sukhija, U. Evolution of denture base materials. J. Indian Prosthodont. Soc. 2009, 9, 64–69. [Google Scholar]
- Wiesli, M.G.; Özcan, M. High-performance polymers and their potential application as medical and oral implant materials: A review. Implant. Dent. 2015, 24, 448–457. [Google Scholar] [CrossRef]
- Alla, R.; Raghavendra, K.N.; Vyas, R.; Konakanchi, A. Conventional and contemporary polymers for the fabrication of denture prosthesis: Part I—Overview, composition and properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Sun, D. Synthesis of nanoencapsulated Glauber’s salt using PMMA shell and its application on cotton for thermoregulating effect. Cellulose 2018, 25, 2103–2113. Available online: https://researchportal.hw.ac.uk/en/publications (accessed on 4 September 2022). [CrossRef]
- Arenas-Arrocena, M.C.; Argueta-Figueroa, L.; García-Contreras, R.; Martínez-Arenas, O.; Camacho-Flores, B.; del Pilar Rodriguez-Torres, M.; De la Fuente-Hernández, J.; Acosta-Torres, L.S. New Trends for the Processing of Poly(Methyl Methacrylate) Biomaterial for Dental Prosthodontics; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/55930 (accessed on 9 August 2022).
- Kopperud, H.M.; Kleven, I.S.; Wellendorf, H. Identification and quantification of leachable substances from polymer-based orthodontic base-plate materials. Eur. J. Orthod. 2011, 33, 26–31. [Google Scholar] [CrossRef]
- Al-Fouzan, A.F.; Al-Mejrad, L.A.; Albarrag, A.M. Adherence of Candida to complete denture surfaces in vitro: A comparison of conventional and CAD/CAM complete dentures. J. Adv. Prosthodont. 2017, 9, 402–408. [Google Scholar] [CrossRef]
- Hannah, V.E.; O’Donnell, L.; Robertson, D.; Ramage, G. Denture stomatitis: Causes, cures and prevention. Prim. Dent. J. 2017, 6, 46–51. [Google Scholar] [CrossRef]
- Shi, B.; Wu, T.; McLean, J.; Edlund, A.; Young, Y.; He, X.; Lv, H.; Zhou, X.; Shi, W.; Li, H.; et al. The denture-associated oral microbiome in health and stomatitis. mSphere 2016, 1, e00215–e00216. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Toledo, B.C.; Santos, C.T.; Costa, A.C.; Back-Brito, G.N.; Kaminagakura, E.; Jorge, A.O. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn. Microbiol. Infect. Dis. 2013, 76, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.; Bürgers, R.; Hahnel, S. Candida albicans adherence and proliferation on the surface of denture base materials. Gerodontology 2013, 30, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Emami, E.; de Souza, R.F.; Kabawat, M.; Feine, J.S. The impact of edentulism on oral and general health. Int. J. Dent. 2013, 2013, 498305. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, S.E.; Whincup, P.H.; Watt, R.G.; Tsakos, G.; Papacosta, A.O.; Lennon, L.T.; Wannamethee, S.G. Burden of poor oral health in older age: Findings from a population-based study of older British men. BMJ Open 2015, 5, e009476. [Google Scholar] [CrossRef]
- Wu, T.; Cen, L.; Kaplan, C.; Zhou, X.; Lux, R.; Shi, W.; He, X. Cellular components mediating coadherence of Candida albicans and Fusobacterium nucleatum. J. Dent. Res. 2015, 94, 1432–1438. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; Sampaio, F.C.; Sampaio, M.C.; Pereira, M.D.; Peixoto, M.H. Streptococcus mutans in denture stomatitis patients under antifungal therapy. Rev. Odonto Ciência 2010, 25, 120–125. [Google Scholar] [CrossRef]
- Jose, A.; Coco, B.J.; Milligan, S.; Young, B.; Lappin, D.F.; Bagg, J.; Murray, C.; Ramage, G. Reducing the incidence of denture stomatitis: Are denture cleansers sufficient? J. Prosthodont. 2010, 19, 252–257. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Zawrotniak, M.; Gogol, M.; Bartnicka, D.; Satala, D.; Smolarz, M.; Karkowska-Kuleta, J.; Kozik, A. Interactions of Candida albicans cells with aerobic and anaerobic bacteria during formation of mixed biofilms in the oral cavity. In Candida Albicans; IntechOpen: London, UK, 2019. [Google Scholar]
- Awad, A.K.; Jassim, R.K. The effect of plasma on transverse strength, surface roughness and Candida adhesion of two types of acrylic denture base materials (Heat cure and light cure). J. Baghdad Coll. Dent. 2012, 24, 10. [Google Scholar]
- Garbacz, K.; Kwapisz, E.; Wierzbowska, M. Denture stomatitis associated with small-colony variants of Staphylococcus aureus: A case report. BMC Oral Health 2019, 19, 219. [Google Scholar] [CrossRef]
- De Visschere, L.M.; Grooten, L.; Theuniers, G.; Vanobbergen, J.N. Oral hygiene of elderly people in long-term care institutions–a cross-sectional study. Gerodontology 2006, 23, 195–204. [Google Scholar] [CrossRef]
- Barbieri, D.S.V.; Vicente, V.A.; Fraiz, F.C.; Lavoranti, O.J.; Svidzinski, T.I.E.; Pinheiro, R.L. Analysis of the in vitro adherence of Streptococcus mutants and Candida albicans. Braz. J. Microbiol. 2007, 38, 624–631. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis—Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef]
- Delaney, C.; O’Donnell, L.E.; Kean, R.; Sherry, L.; Brown, J.L.; Calvert, G.; Nile, C.J.; Cross, L.; Bradshaw, D.J.; Brandt, B.W.; et al. Interkingdom interactions on the denture surface: Implications for oral hygiene. Biofilm 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The Modern and Digital Transformation of Oral Health Care: A Mini Review. Healthcare 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.S. A Review of Polymer Crown Materials: Biomechanical and Material Science. J. Clin. Diagn. Res. 2019, 13, ZE01–ZE05. [Google Scholar] [CrossRef]
- Alp, G.; Johnston, W.M.; Yilmaz, B. Optical properties and surface roughness of prepolymerized poly (methyl methacrylate) denture base materials. J. Prosthet. Dent. 2019, 121, 347–352. [Google Scholar] [CrossRef]
- Lee, S.; Hong, S.J.; Paek, J.; Pae, A.; Kwon, K.R.; Noh, K. Comparing accuracy of denture bases fabricated by injection molding, CAD/CAM milling, and rapid prototyping method. J. Adv. Prosthodont. 2019, 11, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Palaskar, J.N.; Mittal, S. Comparative evaluation of surface porosities in conventional heat polymerized acrylic resin cured by water bath and microwave energy with microwavable acrylic resin cured by microwave energy. Contemp. Clin. Dent. 2013, 4, 147–151. [Google Scholar]
- Aoun, G.; Cassia, A. Evaluation of denture-related factors predisposing to denture stomatitis in a Lebanese population. Mater. Socio-Med. 2016, 28, 392–396. [Google Scholar] [CrossRef]
- Liao, W.; Zheng, S.; Chen, S.; Zhao, L.; Huang, X.; Huang, L.; Kang, S. Surface silanization and grafting reaction of nano-silver loaded zirconium phosphate and properties strengthen in 3D-printable dental base composites. J. Mech. Behav. Biomed. Mater. 2020, 110, 103864. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly (methyl methacrylate) with TiO2 nanoparticles inclusion for stereolithographic complete denture manufacturing—The future in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Wang, J.; Li, Y.; Zhou, X.; Guo, X.; Zhang, T.; Guo, H. Characterization and evaluation of a novel silver nanoparticles-loaded polymethyl methacrylate denture base: In vitro and in vivo animal study. Dent. Mater. J. 2021, 40, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Shahin, S.Y.; Alsaqer, B.T.; Ali, A.A. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409–5419. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Weir, M.D.; Xu, H.H. Denture Acrylic Resin Material with Antibacterial and Protein-Repelling Properties for the Prevention of Denture Stomatitis. Polymers 2022, 14, 230. [Google Scholar] [CrossRef]
- Ratanajanchai, M.; Kanchanavasita, W.; Suputtamongkol, K.; Wonglamsam, A.; Thamapipol, S.; Sae-Khow, O. Heat-cured poly (methyl methacrylate) resin incorporated with different food preservatives as an anti-microbial denture base material. J. Dent. Sci. 2021, 16, 706–712. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, M.J.; Oh, S.H.; Kwon, J.S. Novel dental poly (methyl methacrylate) containing phytoncide for antifungal effect and inhibition of oral multispecies biofilm. Materials 2020, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef]
- Cheng, Q.; Cao, D.; Liu, X.; Zheng, Y.; Shi, Z.; Zhu, S.; Cui, Z. Superhydrophobic coatings with self-cleaning and antibacterial adhesion properties for denture base. J. Mech. Behav. Biomed. Mater. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Tsuji, M.; Ueda, T.; Sawaki, K.; Kawaguchi, M.; Sakurai, K. Biocompatibility of a titanium dioxide-coating method for denture base acrylic resin. Gerodontology 2016, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Mirizadeh, A.; Atai, M.; Ebrahimi, S. Fabrication of denture base materials with antimicrobial properties. J. Prosthet. Dent. 2018, 119, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.G.; Lee, S.H. Inhibitory effects of Lactobacillus rhamnosus and Lactobacillus casei on Candida biofilm of denture surface. Arch. Oral Biol. 2017, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Fouad, H.; Hashem, M.; Niazy, A.A.; AlBadah, A. Titanium oxide (TiO2)/polymethylmethacrylate (PMMA) denture base nanocomposites: Mechanical, viscoelastic and antibacterial behavior. Materials 2018, 11, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Farid, D.A.; Zahari, N.A.H.; Said, Z.; Ghazali, M.I.M.; Hao-Ern, L.; Mohamad Zol, S.; Aldhuwayhi, S.; Alauddin, M.S. Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings. Int. J. Mol. Sci. 2022, 23, 10426. https://doi.org/10.3390/ijms231810426
Mohd Farid DA, Zahari NAH, Said Z, Ghazali MIM, Hao-Ern L, Mohamad Zol S, Aldhuwayhi S, Alauddin MS. Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings. International Journal of Molecular Sciences. 2022; 23(18):10426. https://doi.org/10.3390/ijms231810426
Chicago/Turabian StyleMohd Farid, Durratul Aqwa, Nur A’fifah Husna Zahari, Zulfahmi Said, Mohd Ifwat Mohd Ghazali, Lee Hao-Ern, Syazwani Mohamad Zol, Sami Aldhuwayhi, and Muhammad Syafiq Alauddin. 2022. "Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings" International Journal of Molecular Sciences 23, no. 18: 10426. https://doi.org/10.3390/ijms231810426
APA StyleMohd Farid, D. A., Zahari, N. A. H., Said, Z., Ghazali, M. I. M., Hao-Ern, L., Mohamad Zol, S., Aldhuwayhi, S., & Alauddin, M. S. (2022). Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings. International Journal of Molecular Sciences, 23(18), 10426. https://doi.org/10.3390/ijms231810426






