Effects of Nitrogen Supply on Induced Defense in Maize (Zea mays) against Fall Armyworm (Spodoptera frugiperda)
Abstract
:1. Introduction
2. Results
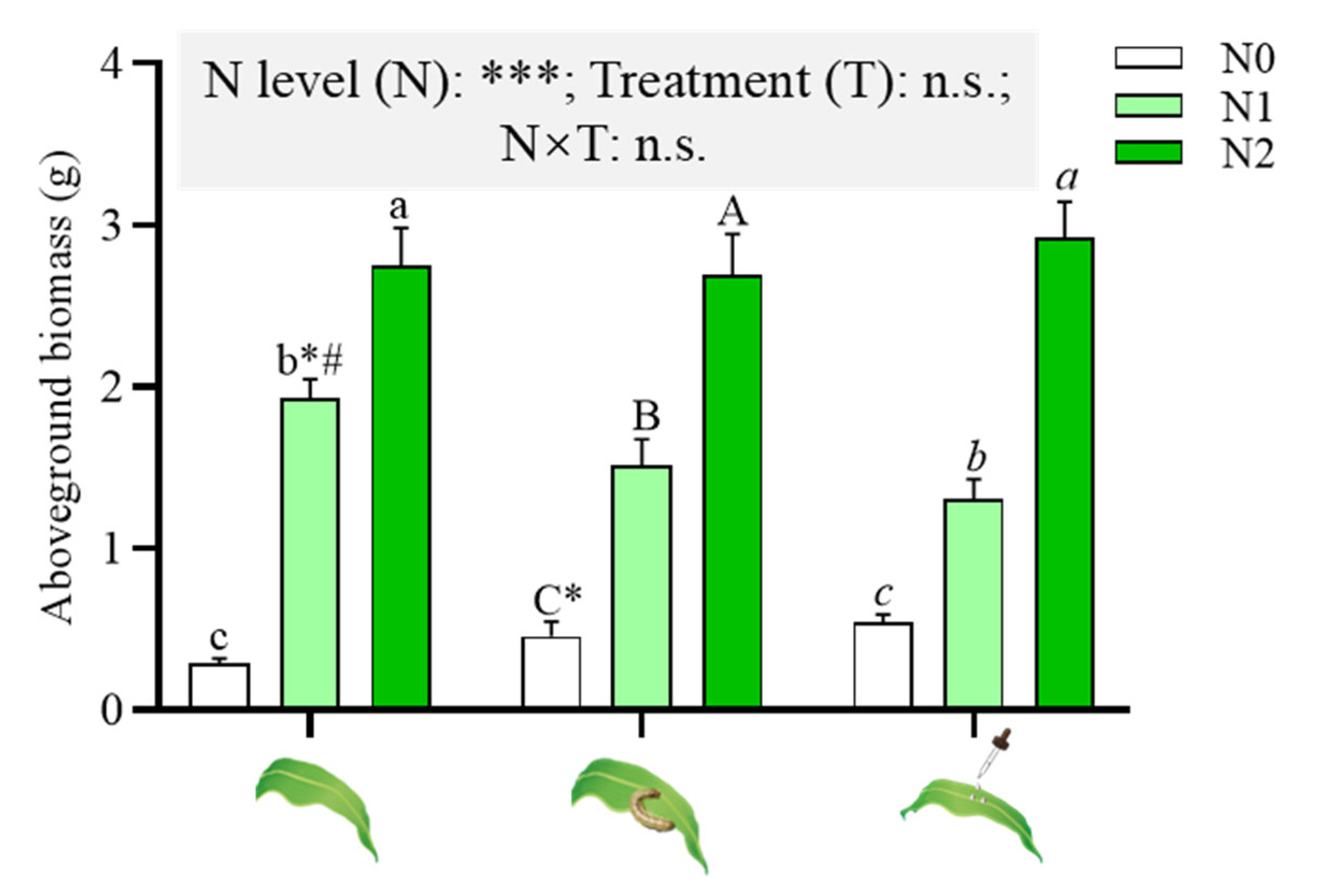
2.1. Effects of N Supply on Maize Aboveground Biomass
2.2. Effects of N Supply on Herbivore Performance in Maize
2.3. Effect of N Supply on Maize Nutrients and Secondary Metabolites
2.4. Effect of N Supply on Maize Phytohormones
2.5. Effect of N Supply on Maize VOCs
2.6. Effect of N Supply on Maize Defensive Genes
3. Discussion
4. Materials and Methods
4.1. Soils, Plants and Insects
4.2. Experimental Design and Conditions
4.3. Plant Aboveground Biomass
4.4. Herbivore Performance
4.4.1. Growth of S. frugiperda
4.4.2. Feeding Behavior of S. frugiperda
4.4.3. S. frugiperda Preference as Assessed by Olfactometer
4.5. Measurement of Total Protein, Soluble Sugar and Amino Acids
4.6. Secondary Metabolites Analyses
4.7. Measurement of Phytohormones
4.8. Volatile Organic Compound (VOC) Collection and Quantification
4.9. Quantitative Real-Time PCR Analysis of Maize Defensive Genes
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| N | nitrogen |
| P | phosphorus |
| K | potassium |
| VOCs | volatile organic compounds |
| MPI | maize proteinase inhibitor |
| PAL | phenylalanine ammonia-lyase |
| BX9 | glucosyltransferase |
| LOX | lipoxygenase |
| AOS | allene oxide synthase |
| UBQ | ubiquitin |
| ABA | abscisic acid |
| SA | salicylic acid |
| JA | jasmonic acid |
| MeJA | methyl jasmonate |
| MeSA | methyl salicylate |
| TCA | tricarboxylic acid |
| RGR | relative growth rate |
| DIMBOA | 2, 4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one |
| GC-MS | gas chromatography-mass spectrometer |
| PBS | phosphate buffered saline |
| GLM | generalized linear models |
| ANOVA | analysis of variance |
| PCA | principal components analysis |
References
- Chuang, W.; Ray, S.; Acevedo, F.E.; Peiffer, M.; Felton, G.W.; Luthe, D.S. Herbivore cues from the fall armyworm (Spodoptera frugiperda) larvae trigger direct defenses in maize. Mol. Plant-Microbe Interact. 2014, 27, 461–470. [Google Scholar] [CrossRef] [PubMed]
- ul Malook, S.; Xu, Y.; Qi, J.; Li, J.; Wang, L.; Wu, J. Mythimna separata herbivory primes maize resistance in systemic leaves. J. Exp. Bot. 2021, 72, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.M.; Persicke, M.; Mueller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef]
- Bozorov, T.A.; Son, T.D.; Baldwin, I.T. JA but not JA-Ile is the cell-nonautonomous signal activating JA mediated systemic defenses to herbivory in Nicotiana attenuate (FA). J. Integr. Plant Biol. 2017, 59, 552–571. [Google Scholar] [CrossRef]
- Guo, J.; Qi, J.; He, K.; Wu, J.; Bai, S.; Zhang, T.; Zhao, J.; Wang, Z. The Asian corn borer Ostrinia furnacalis feeding increases the direct and indirect defence of mid-whorl stage commercial maize in the field. Plant Biotechnol. J. 2018, 17, 88–102. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Chen, Y.; Olson, D.M.; Ruberson, J.R. Effects of nitrogen fertilization on tritrophic interactions. Arthropod Plant Interact. 2010, 4, 81–94. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Liu, X.; Qin, N.; Xu, K.; Zeng, R.; Liu, J.; Song, Y. Nitrogen supply alters rice defense against the striped stem borer Chilo suppressalis. Front. Plant Sci. 2021, 12, 691292. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Hardy, G.; Liu, Z.; Wei, W.; Dai, H. Corn defense responses to nitrogen availability and subsequent performance and feeding preferences of beet armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2013, 106, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xie, H.; Wu, S.; Wang, Z.; He, K. Effects of elevated CO2 and increased N fertilization on plant secondary metabolites and chewing insect fitness. Front. Plant Sci. 2019, 10, 739. [Google Scholar] [CrossRef]
- Gupta, S.; Roy, A. Deciphering the Role of Phytoanticipins, Phytoalexins, and Polyphenols in Plant-Insect Defense. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Springer: Singapore, 2021; pp. 305–335. [Google Scholar]
- Cousins, A.B.; Bloom, A.J. Influence of elevated CO2 and nitrogen nutrition on photosynthesis and nitrate photo-assimilation in maize (Zea mays L.). Plant Cell Evriron. 2003, 26, 1525–1530. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G.; Hodges, M. Respiration and nitrogen assimilation: Targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1467–1482. [Google Scholar] [CrossRef]
- Sun, X.; Yue, S.; Mangan, M.A. Decentralised neural model explaining optimal integration of navigational strategies in insects. eLife 2020, 9, e54026. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.K.; Pathania, V.S.; Thakur, S. Effect of plant nutrition in insect pest management: A review. J. Pharmacogn. Phytochem. 2018, 7, 2737–2742. [Google Scholar]
- Rashid, M.M.; Jahan, M.; Islam, K.S. Impact of nitrogen, phosphorus and potassium on brown planthopper and tolerance of its host rice plants. Rice Sci. 2016, 23, 119–131. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, X.; Heong, K.; Hu, C. Effect of nitrogen fertilizer on herbivores and its stimulation to major insect pests in rice. Rice Sci. 2007, 14, 56–66. [Google Scholar] [CrossRef]
- Lou, Y.; Baldwin, I.T. Nitrogen supply influences herbivore-induced direct and indirect defenses and transcriptional responses in Nicotiana attenuate. Plant Physiol. 2004, 135, 496–506. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2021, 167, 113516–113529. [Google Scholar] [CrossRef]
- Boateng, K.; Hawkins, B.J.; Yanchuk, A.; Fellenberg, C.; Constabel, C.P. Factors affecting foliar oregonin and condensed tannin in red alder (Alnus rubra Bong.): Phytochemicals implicated in defense against western tent caterpillar (Malacosoma californicum Packard). J. Chem. Ecol. 2021, 47, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Jiang, H.; Qin, Y.A.; Yan, H.Z.A.; Jiang, X.M.; Qin, Y. Nitrogen deficiency maintains the yield and improves the antioxidant activity of Coreopsis tinctoria Nutt. Biosci. Biotech. Bioch. 2021, 85, 1492–1505. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Y.; Cui, X.; Liu, Y.; Hu, G. Effects of nitrogen application on the distribution of photosynthates in roots, stems, leaves and alfalfa resistance to thrips. Plant Protect. 2021, 47, 109–115. [Google Scholar]
- Fallovo, C.; Schreiner, M.; Schwarz, D.; Colla, G.; Krumbein, A. Phytochemical changes induced by different nitrogen supply forms and radiation levels in two leafy Brassica species. J. Agric. Food Chem. 2011, 59, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Schmelz, E.A.; Waeckers, F.; Ruberson, J.R. Cotton plant, Gossypium hirsutum L., defense in response to nitrogen fertilization. J. Chem. Ecol. 2008, 34, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.T.; Shao, X.Q.; Li, J.X.; Ahammed, G.J.; Yao, Y.L.; Ding, J.; Shi, K. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef]
- Gouinguené, S.P.; Turlings, T. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Han, P.; Becker, C.; Le Bot, J.; Larbat, R.; Lavoir, A.V.; Desneux, N. Plant nutrient supply alters the magnitude of indirect interactions between insect herbivores: From foliar chemistry to community dynamics. J. Ecol. 2020, 108, 1497–1510. [Google Scholar] [CrossRef]
- Chen, Y. Analyzing blends of herbivore-induced volatile organic compounds with factor analysis: Revisiting “cotton plant, Gossypium hirsutum L., defense in response to nitrogen fertilization”. J. Econ. Entomol. 2013, 106, 1053–1057. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Alborn, H.T.; Engelberth, J.; Tumlinson, J.H. Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 2003, 133, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the stress: Breeding for climate resilience in maize for the tropical rainfed environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef] [PubMed]
- Wu, K. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Protect. 2020, 46, 1–5. [Google Scholar]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef]
- Qi, J.; ul Malook, S.; Shen, G.; Gao, L.; Zhang, C.; Li, J.; Zhang, J.; Wang, L.; Wu, J. Current understanding of maize and rice defense against insect herbivores. Plant Divers. 2018, 40, 189–195. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar]
- Endara, M.; Coley, P.D. The resource availability hypothesis revisited: A meta-analysis. Funct. Ecol. 2011, 25, 389–398. [Google Scholar] [CrossRef]
- Kim, J.; Felton, G.W. Priming of antiherbivore defensive responses in plants. Insect Sci. 2013, 20, 273–285. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [PubMed]
- Wang, J.; Song, Y.; Hu, L.; Yang, M.; Zeng, R. Plant anti-herbivore defense priming: Concept, mechanisms, and application. Chin. J. Appl. Ecol. 2018, 29, 2068–2078. [Google Scholar]
- Nykänen, H.; Koricheva, J. Damage-induced changes in woody plants and their effects on insect herbivore performance: A meta-analysis. Oikos 2004, 104, 247–268. [Google Scholar]
- Van Zandt, P.A. Plant defense, growth, and habitat: A comparative assessment of constitutive and induced resistance. Ecology 2007, 88, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Bodenhausen, N.; Reymond, P. Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol. Plant-Microbe Interact. 2007, 20, 1406–1420. [Google Scholar] [PubMed]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar]
- Stella De Freitas, T.F.; Stout, M.J.; Sant’Ana, J. Effects of exogenous methyl jasmonate and salicylic acid on rice resistance to Oebalus pugnax. Pest Manag. Sci. 2019, 75, 744–752. [Google Scholar]
- Abeed, A.H.A.; Eissa, M.A.; Abdel-Wahab, D.A. Effect of exogenously applied jasmonic acid and kinetin on drought tolerance of wheat cultivars based on morpho-physiological evaluation. J. Soil Sci. Plant Nut. 2021, 21, 131–144. [Google Scholar]
- Rasmann, S.; De Vos, M.; Casteel, C.L.; Tian, D.; Halitschke, R.; Sun, J.Y.; Agrawal, A.A.; Felton, G.W.; Jander, G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012, 158, 854–863. [Google Scholar]
- White, T.C.R. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 1984, 63, 90–105. [Google Scholar]
- Veyrat, N.; Robert, C.A.M.; Turlings, T.C.J.; Erb, M. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J. Ecol. 2016, 104, 591–600. [Google Scholar]
- Islam, M.N.; Hasanuzzaman, A.T.M.; Zhang, Z.; Zhang, Y.; Liu, T. High level of nitrogen makes tomato plants releasing less volatiles and attracting more Bemisia tabaci (Hemiptera: Aleyrodidae). Front. Plant Sci. 2017, 8, 466. [Google Scholar] [PubMed]
- Oluwafemi, S.; Bruce, T.J.A.; Pickett, J.A.; Ton, J.; Bikett, M.A. Behavioral responses of the leafhopper, cicadulina storeyi China, a major vector of maize streak virus, to volatile cues from intact and leafhopper-damaged maize. J. Chem. Ecol. 2011, 37, 40–48. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, L.; Feng, Y.; Guo, S.; Pang, X.; Zhang, D.; Geng, Z.; Du, S. Insecticidal and repellent efficacy against stored-product insects of oxygenated monoterpenes and 2-dodecanone of the essential oil from Zanthoxylum planispinum var. dintanensis. Environ. Sci. Pollut. Res. 2019, 26, 24988–24997. [Google Scholar]
- Annamma, A.A.; Abraghrer, V.; Muthangi, S. Role of colour and volatile in foraging behaviour of honeybee Apis cerana on Jacquemontia pentanthos. J. Asia-Pac. Entomol. 2018, 21, 1122–1128. [Google Scholar] [CrossRef]
- Sun, J.; Huang, L.; Wang, C. Electrophysiological and behavioral responses of Helicoverpa assulta (Lepidoptera: Noctuidae) to tobacco volatiles. Arthropod Plant Interact. 2012, 6, 375–384. [Google Scholar]
- Fang, Y.; Zeng, R.; Lu, S.; Dai, L.; Wan, X. The synergistic attractiveness effect of plant volatiles to sex pheromones in a moth. J. Asia-Pac. Entomol. 2018, 21, 380–387. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Lu, Y.; Cheng, D. Behavioral response of Bactrocera dorsalis female adults to the host volatile, β-caryophyllene. J. Environ. Entomol. 2021, 43, 253–259. [Google Scholar]
- Liu, Y.; Xie, D.; Hu, C.; Dong, W.; Zhang, F.; Zhang, J.; Xiao, C. Influence of β-caryophyllene on the behaviors of female Drosophila suzukii. J. Environ. Entomol. 2018, 40, 684–689. [Google Scholar]
- Capra, E.; Colombi, C.; De Poli, P.; Nocito, F.F.; Cocucci, M.; Vecchietti, A.; Marocco, A.; Stile, M.R.; Rossini, L. Protein profiling and tps23 induction in different maize lines in response to methyl jasmonate treatment and Diabrotica virgifera infestation. J. Plant Physiol. 2015, 175, 68–77. [Google Scholar]
- Li, S.; Wang, H.; Li, F.; Chen, Z.; Li, X.; Zhu, L.; Wang, G.; Yu, J.; Huang, D.; Lang, Z. The maize transcription factor EREB58 mediates the jasmonate-induced production of sesquiterpene volatiles. Plant J. 2015, 84, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Y.; Huang, Q.; Yin, G.; Pennerman, K.K.; Yu, J.; Liu, Z.; Li, D.; Guo, A. Analysis of key genes of jasmonic acid mediated signal pathway for defense against insect damages by comparative transcriptome sequencing. Sci. Rep. 2015, 5, 16500. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Teng, S.; Liang, H.; Xin, H.; Gao, H.; Huang, D.; Lang, Z. Transcriptome profiling revealed novel transcriptional regulators in maize responses to Ostrinia furnacalis and jasmonic acid. PLoS ONE 2017, 12, e0177739. [Google Scholar] [CrossRef] [PubMed]
- Quilis, J.; Lopez-Garcia, B.; Meynard, D.; Guiderdoni, E.; San Segundo, B. Inducible expression of a fusion gene encoding two proteinase inhibitors leads to insect and pathogen resistance in transgenic rice. Plant Biotechnol. J. 2014, 12, 367–377. [Google Scholar] [CrossRef]
- Tamayo, M.C.; Rufat, M.; Bravo, J.M.; San Segundo, B. Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity toward digestive proteinases of Spodoptera littoralis larvae. Planta 2000, 211, 62–71. [Google Scholar] [CrossRef]
- Paulillo, L.C.; Lopes, A.R.; Cristofoletti, P.T.; Parra, J.R.; Terra, W.R.; Silva-Filho, M.C. Changes in midgut endopeptidase activity of Spodoptera frugiperda (Lepidoptera: Noctuidae) are responsible for adaptation to soybean proteinase inhibitors. J. Econ. Entomol. 2000, 93, 892–896. [Google Scholar] [CrossRef]
- Ritter, H.; Schulz, G.E. Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell 2004, 16, 3426–3436. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, Y.; Wang, N.; Wang, J.; Zhou, Y.; Zhang, Z. Activities of lipoxygenase and phenylalanine ammonia lyase in poplar leaves induced by insect herbivory and volatiles. J. Forestry Res. 2009, 20, 372–376. [Google Scholar] [CrossRef]
- Jin, P.; Chen, J.; Zhan, H.; Huang, S.; Wang, J.; Shu, Y. Accumulation and excretion of zinc and their effects on growth and food utilization of Spodoptera litura (Lepidoptera: Noctuidae). Ecotox. Environ. Saf. 2020, 202, 110883. [Google Scholar] [CrossRef]
- Farrar, R.R.; Barbour, J.J.; Kennedy, G. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 1989, 82, 593–598. [Google Scholar] [CrossRef]
- Damerval, C.; de Vienne, D.; Zivy, M.; Thiellement, H. The technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 1986, 7, 52–54. [Google Scholar] [CrossRef]
- Jellouli, N.; Ben Salem, A.; Ghorbel, A.; Ben Jouira, H. Evaluation of protein extraction methods for vitis vinifera leaf and root proteome analysis by two-dimensional electrophoresis. J. Integr. Plant Biol. 2010, 52, 933–940. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principal of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Gao, L.; Shen, G.; Zhang, L.; Qi, J.; Zhang, C.; Ma, C.; Li, J.; Wang, L.; ul Malook, S.; Wu, J. An efficient system composed of maize protoplast transfection and HPLC–MS for studying the biosynthesis and regulation of maize benzoxazinoids. Plant Methods 2019, 15, 144. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, X.; Liao, H.; Feng, Y.; Guo, Y.; Shu, Y.; Wang, J. Effects of Nitrogen Supply on Induced Defense in Maize (Zea mays) against Fall Armyworm (Spodoptera frugiperda). Int. J. Mol. Sci. 2022, 23, 10457. https://doi.org/10.3390/ijms231810457
Wang W, Wang X, Liao H, Feng Y, Guo Y, Shu Y, Wang J. Effects of Nitrogen Supply on Induced Defense in Maize (Zea mays) against Fall Armyworm (Spodoptera frugiperda). International Journal of Molecular Sciences. 2022; 23(18):10457. https://doi.org/10.3390/ijms231810457
Chicago/Turabian StyleWang, Wenxin, Xiaoyi Wang, Huimin Liao, Yuanjiao Feng, Yeshan Guo, Yinghua Shu, and Jianwu Wang. 2022. "Effects of Nitrogen Supply on Induced Defense in Maize (Zea mays) against Fall Armyworm (Spodoptera frugiperda)" International Journal of Molecular Sciences 23, no. 18: 10457. https://doi.org/10.3390/ijms231810457




