Early Rehabilitation Exercise after Stroke Improves Neurological Recovery through Enhancing Angiogenesis in Patients and Cerebral Ischemia Rat Model
Abstract
:1. Introduction
2. Results
2.1. The Effects of Different Rehabilitation Training Timings on the Neurological Function and Motor Ability of the Patients before and after Rehabilitation
2.2. Effects of Different Rehabilitation Training Timings on ADL Ability and Quality of Life of the Patients before and after Rehabilitation
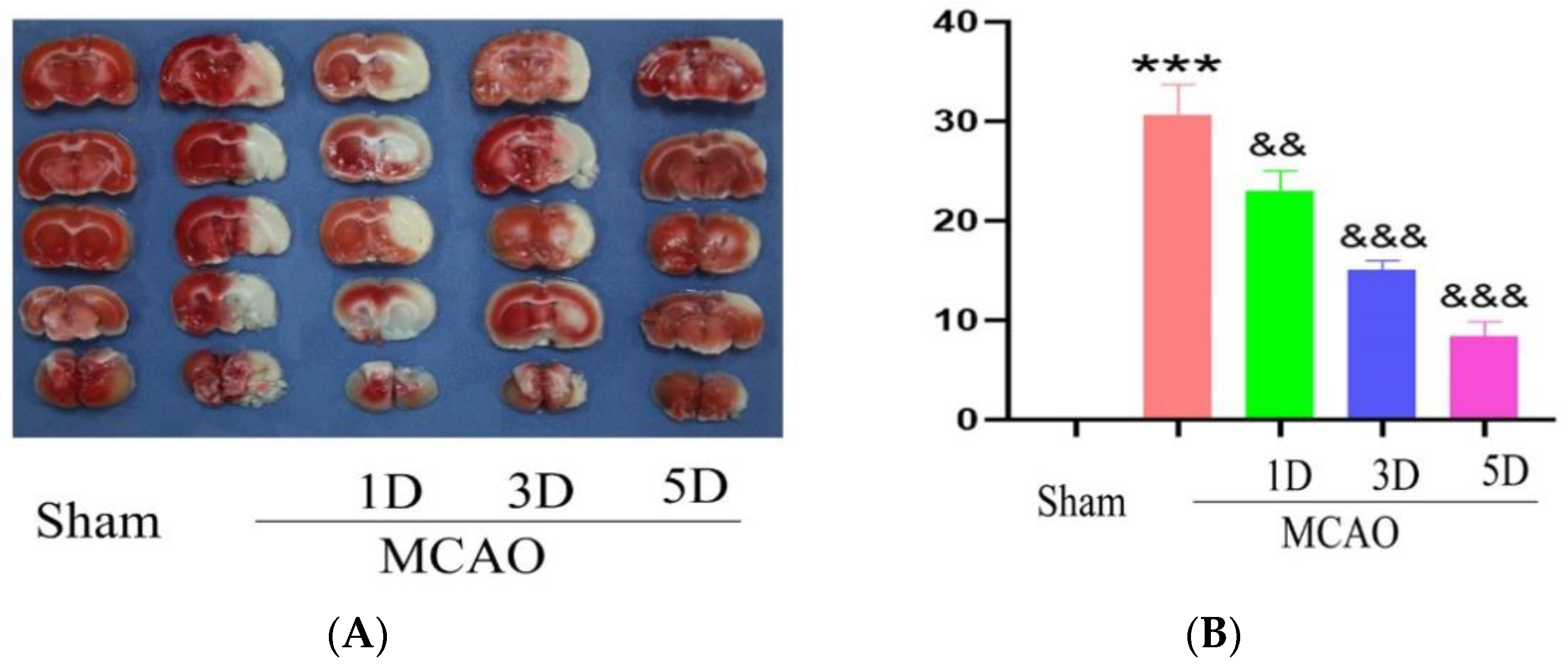
2.3. The Effect of Treadmill Exercise on Cerebral Infarction Volume and Neurological Function
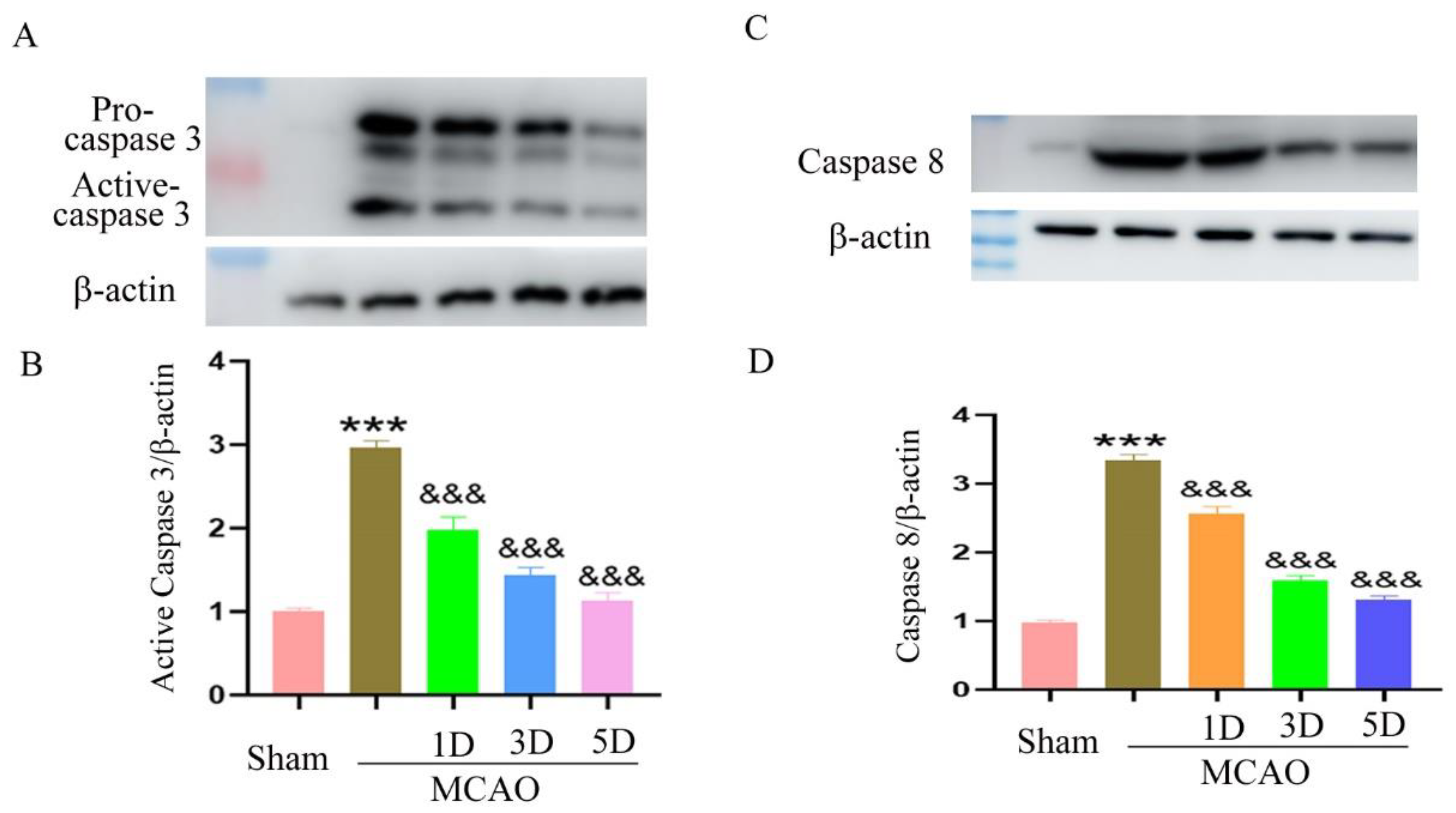
2.4. The Effect of Treadmill Exercise on Neuronal Apoptosis after MCAO and Treadmill Exercise
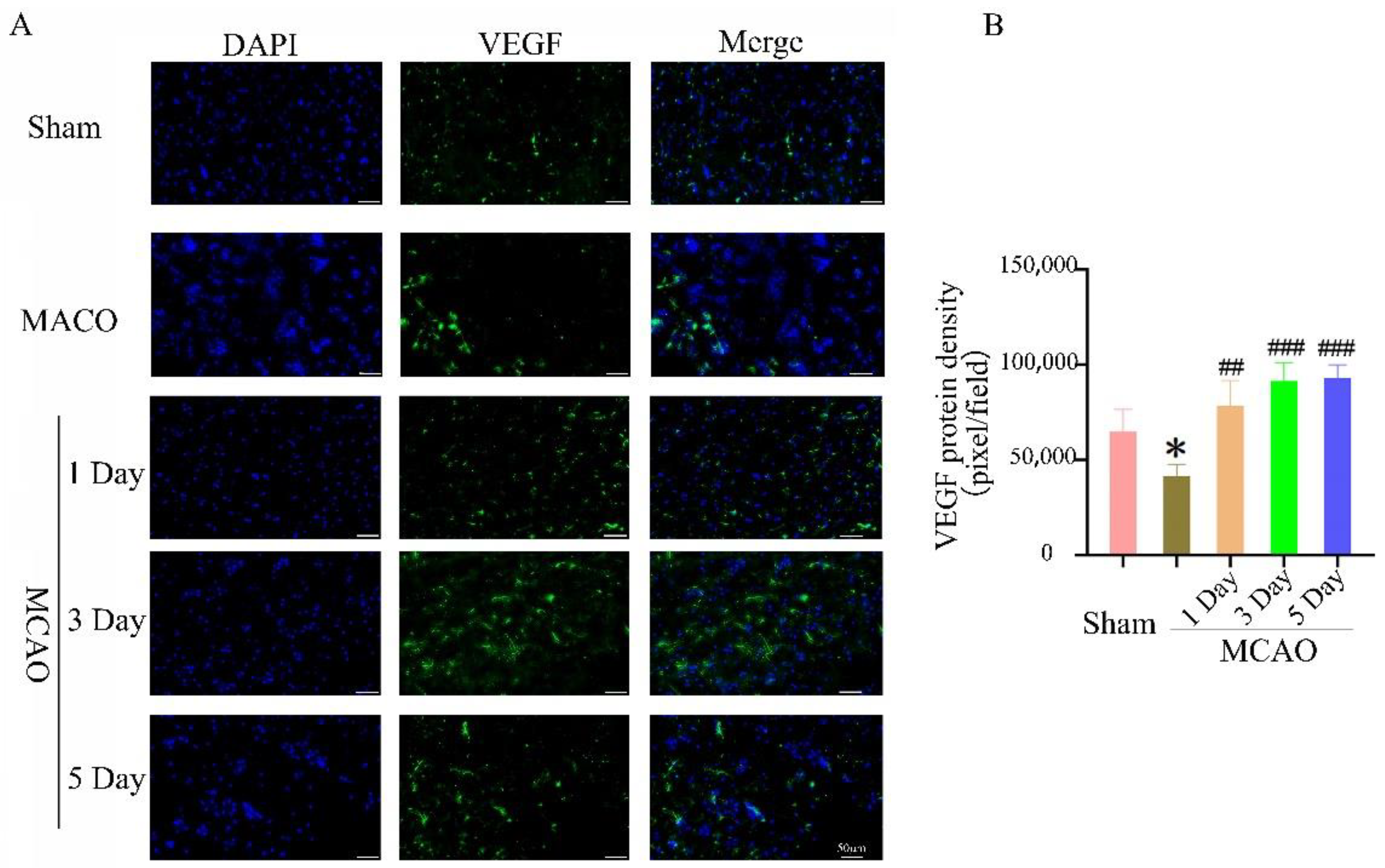
2.5. The Effect of Treadmill Exercise on Angiogenesis in the Ischemic Penumbra
3. Discussion
4. Materials and Methods
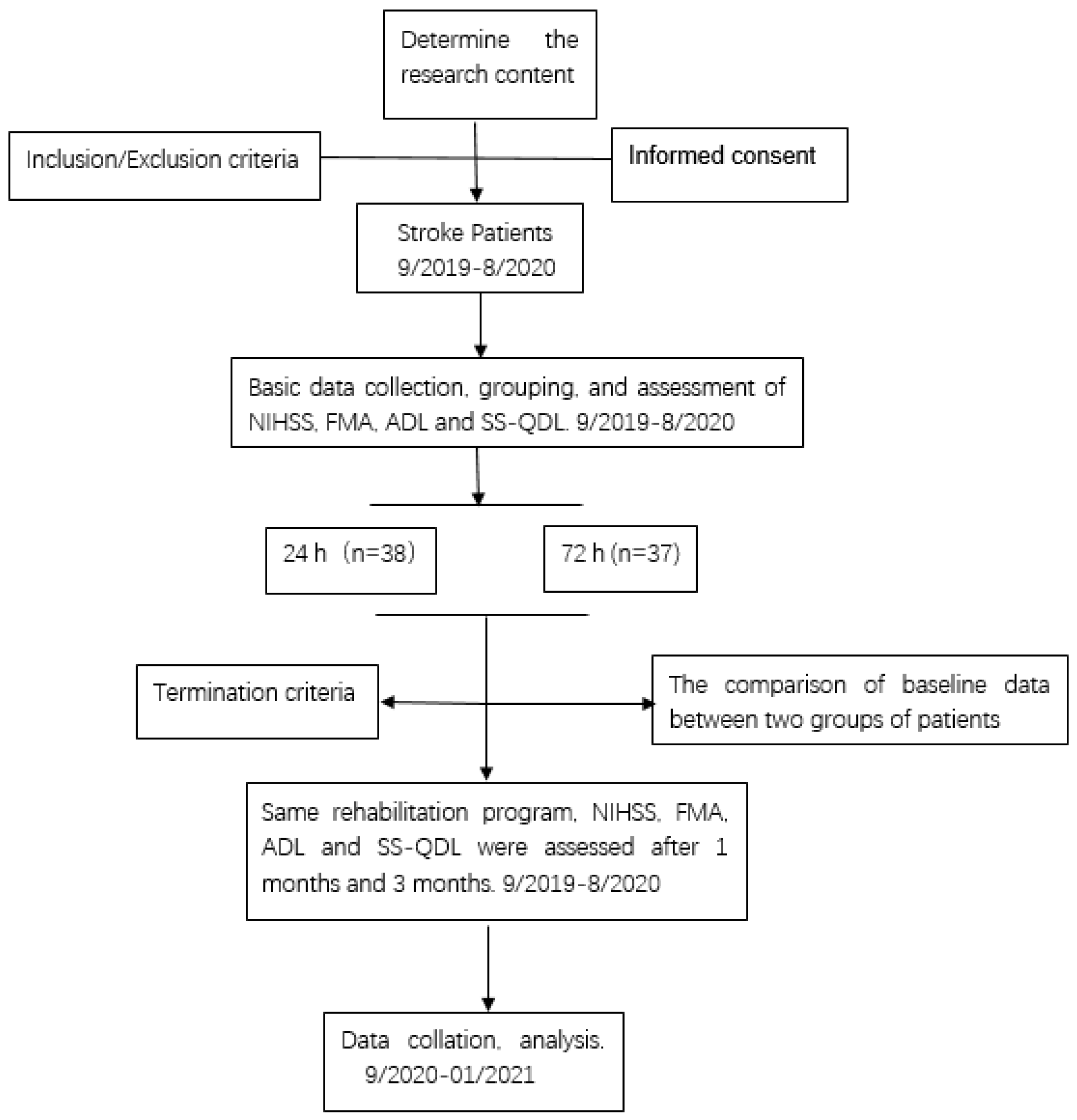
4.1. Patients
4.2. Patient Rehabilitation
4.3. Patient Limb Function Testing
4.4. Animals
4.5. Middle Cerebral Artery Occlusion (MCAO)
4.6. Treadmill Exercise
4.7. Infarct Volume Analyses
4.8. Modified Neurological Severity Scores (mNSS)
4.9. Western Blot
4.10. Immunofluorescence
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, P.C.; Yu, I.C.; Scofield, B.A.; Brown, D.A.; Curfman, E.T.; Paraiso, H.C.; Chang, F.L.; Yen, J.H. 3H-1,2-Dithiole-3-thione as a novel therapeutic agent for the treatment of ischemic stroke through Nrf2 defense pathway. Brain Behav. Immun. 2017, 62, 180–192. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, J.; Li, X.; Bai, Y.; Wang, B.; Qu, Y.; Li, B.; Zhao, S. Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of stroke rehabilitation. Stroke Vasc. Interv. Neurol. 2020, 5, 250–259. [Google Scholar] [CrossRef]
- Nesin, S.M.; Sabitha, K.R.; Gupta, A.; Laxmi, T.R. Constraint Induced Movement Therapy as a Rehabilitative Strategy for Ischemic Stroke-Linking Neural Plasticity with Restoration of Skilled Movements. J. Stroke Cerebrovasc. Dis. 2019, 28, 1640–1653. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, J.; He, Y.; Yuan, M.; Dong, L.; Ye, Z.; Mao, W. The Mechanism and Clinical Application of Constraint-Induced Movement Therapy in Stroke Rehabilitation. Front. Behav. Neurosci. 2022, 16, 828599. [Google Scholar] [CrossRef]
- Sandel, M.E. Dr. Herman Kabat: Neuroscience in translation … from bench to bedside. PM R 2013, 5, 453–461. [Google Scholar] [CrossRef]
- Gray, L.A. Living the Full Catastrophe: A Mindfulness-Based Program to Support Recovery from Stroke. Healthcare 2020, 8, 498. [Google Scholar] [CrossRef]
- Telles, S.; Kozasa, E.; Bernardi, L.; Cohen, M. Yoga and rehabilitation: Physical, psychological, and social. Evid. Based Complementary Altern. Med. 2013, 2013, 624758. [Google Scholar] [CrossRef]
- Wathugala, M.; Saldana, D.; Juliano, J.M.; Chan, J.; Liew, S.L. Mindfulness Meditation Effects on Poststroke Spasticity: A Feasibility Study. J. Evid. Based Integr. Med. 2019, 24, 2515690X19855941. [Google Scholar] [CrossRef]
- Johansson, B.; Bjuhr, H.; Rönnbäck, L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj. 2012, 26, 1621–1628. [Google Scholar] [CrossRef]
- Scrivener, K.; Dorsch, S.; McCluskey, A.; Schurr, K.; Graham, P.L.; Cao, Z.; Shepherd, R.; Tyson, S. Bobath therapy is inferior to task-specific training and not superior to other interventions in improving lower limb activities after stroke: A systematic review. J. Physiother. 2020, 66, 225–235. [Google Scholar] [CrossRef]
- Pathak, A.; Gyanpuri, V.; Dev, P.; Dhiman, N.R. The Bobath Concept (NDT) as rehabilitation in stroke patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 3983–3990. [Google Scholar]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Sheppard, L.; Dewey, H.; Bernhardt, J.; Collier, J.M.; Ellery, F.; Churilov, L.; Tay-Teo, K.; Wu, O.; Moodie, M. AVERT Trial Collaboration Group. Economic Evaluation Plan (EEP) for A Very Early Rehabilitation Trial (AVERT): An international trial to compare the costs and cost-effectiveness of commencing out of bed standing and walking training (very early mobilization) within 24 h of stroke onset with usual stroke unit care. Int. J. Stroke 2016, 11, 492–494. [Google Scholar] [PubMed]
- Lynch, E.A.; Cumming, T.; Janssen, H.; Bernhardt, J. Early Mobilization after Stroke: Changes in Clinical Opinion Despite an Unchanging Evidence Base. J. Stroke Cerebrovasc. Dis. 2017, 26, 1–6. [Google Scholar] [CrossRef]
- Wu, D.; Ma, J.; Zhang, L.; Wang, S.; Tan, B.; Jia, G. Effect and Safety of Transcutaneous Auricular Vagus Nerve Stimulation on Recovery of Upper Limb Motor Function in Subacute Ischemic Stroke Patients: A Randomized Pilot Study. Neural Plast. 2020, 2020, 8841752. [Google Scholar] [CrossRef]
- Bernhardt, J.; English, C.; Johnson, L.; Cumming, T.B. Early mobilization after stroke: Early adoption but limited evidence. Stroke 2015, 46, 1141–1146. [Google Scholar] [CrossRef]
- Cumming, T.B.; Churilov, L.; Collier, J.; Donnan, G.; Ellery, F.; Dewey, H.; Langhorne, P.; Lindley, R.I.; Moodie, M.; Thrift, A.G.; et al. AVERT Trial Collaboration group. Early mobilization and quality of life after stroke: Findings from AVERT. Neurology 2019, 93, e717–e728. [Google Scholar] [CrossRef]
- AVERT Trial Collaboration Group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet 2015, 386, 46–55. [Google Scholar] [CrossRef]
- Gittler, M.; Davis, A.M. Guidelines for Adult Stroke Rehabilitation and Recovery. JAMA 2018, 319, 820–821. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.; Fullerton, H.J.; et al. Executive Summary:Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. Adv. Exp. Med. Biol. 2017, 1000, 131–151. [Google Scholar] [PubMed]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Tollár, J.; Nagy, F.; Csutorás, B.; Prontvai, N.; Nagy, Z.; Török, K.; Blényesi, E.; Vajda, Z.; Farkas, D.; Tóth, B.E.; et al. High Frequency and Intensity Rehabilitation in 641 Subacute Ischemic Stroke Patients. Arch. Phys. Med. Rehabil. 2021, 102, 9–18. [Google Scholar] [CrossRef]
- Haruyama, K.; Kawakami, M.; Otsuka, T. Effect of Core Stability Training on Trunk Function, Standing Balance, and Mobility in Stroke Patients. Neurorehabilit. Neural Repair 2017, 31, 240–249. [Google Scholar] [CrossRef]
- Zhang, P.; Xianglei, J.; Hongbo, Y.; Zhang, J.; Xu, C. Neuroprotection of Early Locomotor Exercise Poststroke: Evidence From Animal Studies. Can. J. Neurol. Sci. 2015, 42, 213–220. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Zhang, L.Y.; Luo, J.; Li, L.L.; Li, M.; Zhang, Q.; Hu, X.Q. Physical exercise promotes recovery of neurological function after ischemic stroke in rats. Int. J. Mol. Sci. 2014, 15, 10974–10988. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.; Kim, S.S.; Lee, T.H.; Lim, B.V.; Chang, H.K.; Jang, M.H.; Shin, M.C.; Shin, M.S.; Kim, C.J. Treadmill exercise suppresses ischemia-induced increment in apoptosis and cell proliferation in hippocampal dentate gyrus of gerbils. Life Sci. 2003, 3, 2455–2465. [Google Scholar] [CrossRef]
- Luo, C.X.; Jiang, J.; Zhou, Q.G.; Zhu, X.J.; Wang, W.; Zhang, Z.J.; Han, X.; Zhu, D.Y. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J. Neurosci. Res. 2007, 85, 1637–1646. [Google Scholar] [CrossRef]
- Risedal, A.; Zeng, J.; Johansson, B.B. Early training may exacerbate brain damage after focal brain ischemia in the rat. J. Cereb. Blood Flow Metab. 1999, 19, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Komitova, M.; Zhao, L.R.; Gidö, G.; Johansson, B.B.; Eriksson, P. Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur. J. Neurosci. 2005, 21, 2397–2405. [Google Scholar] [CrossRef]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early Rehabilitation After Stroke: A Narrative Review. Curr. Atheroscler. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Kennedy, C.; Bernhardt, J.; Churilov, L.; Collier, J.M.; Ellery, F.; Rethnam, V.; Carvalho, L.B.; Donnan, G.A.; Hayward, K.S. Factors associated with time to independent walking recovery post-stroke. J. Neurol. Neurosurg. Psychiatry 2021, 92, 702–708. [Google Scholar] [CrossRef]
- Bernhardt, J.; Dewey, H.; Thrift, A.; Collier, J.; Donnan, G. A very early rehabilitation trial for stroke (AVERT): Phase II safety and feasibility. Stroke 2008, 39, 390–396. [Google Scholar] [CrossRef]
- Bernhardt, J.; Godecke, E.; Johnson, L.; Langhorne, P. Early rehabilitation after stroke. Curr. Opin. Neurol. 2017, 30, 48–54. [Google Scholar] [CrossRef]
- Gor-García-Fogeda, M.D.; Molina-Rueda, F.; Cuesta-Gómez, A.; Carratalá-Tejada, M.; Alguacil-Diego, I.M.; Miangolarra-Page, J.C. Scales to assess gross motor function in stroke patients: A systematic review. Arch. Phys. Med. Rehabil. 2014, 95, 1174–1183. [Google Scholar] [CrossRef]
- van Wijk, R.; Cumming, T.; Churilov, L.; Donnan, G.; Bernhardt, J. An early mobilization protocol successfully delivers more and earlier therapy to acute stroke patients: Further results from phase II of AVERT. Neurorehabilit. Neural Repair 2012, 26, 20–26. [Google Scholar] [CrossRef]
- Reijneveld, S.A.; Spijker, J.; Dijkshoorn, H. Katz’ ADL index assessed functional performance of Turkish, Moroccan, and Dutch elderly. J. Clin. Epidemiol. 2007, 60, 382–388. [Google Scholar] [CrossRef]
- Ren, C.L.; Fu, J.J.; Wang, H.X. Effect of early rehabilitation clinical pathway on ischemic stroke patients: A randomized controlled trial. Chin. J. Rehabil. Med. 2017, 32, 275–282. [Google Scholar]
- Cumming, T.B.; Thrift, A.G.; Collier, J.M.; Churilov, L.; Dewey, H.M.; Donnan, G.A.; Bernhardt, J. Very early mobilization after stroke fast-tracks return to walking: Further results from the phase II AVERT randomized controlled trial. Stroke 2011, 42, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Cunningham, D.; Carl, D.; Jansen, C.; Khoury, J.C.; et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. J. Appl. Physiol. 2019, 126, 431–443. [Google Scholar] [CrossRef]
- Oshikawa, M.; Okada, K.; Kaneko, N.; Sawamoto, K.; Ajioka, I. Affinity-Immobilization of VEGF on Laminin Porous Sponge Enhances Angiogenesis in the Ischemic Brain. Adv. Healthc. Mater. 2017, 6, 1700183. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar] [PubMed]
- Li, F.; Geng, X.; Yip, J.; Ding, Y. Therapeutic Target and Cell-signal Communication of Chlorpromazine and Promethazine in Attenuating Blood-Brain Barrier Disruption after Ischemic Stroke. Cell Transplant. 2019, 28, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, K.; Riddick, S.; Awa, F.; Chen, X.; Virgintino, D.; Stonestreet, B.S. Time Course of Changes in the Neurovascular Unit after Hypoxic-Ischemic Injury in Neonatal Rats. Int. J. Mol. Sci. 2022, 23, 4180. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.Y.; Ko, A.R.; Kang, T.C. Over-expression of laminin correlates to recovery of vasogenic edema following status epilepticus. Neuroscience 2014, 275, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Tsirka, S.E. Inflammation modulates expression of laminin in the central nervous system following ischemic injury. J. Neuroinflamm. 2012, 9, 159. [Google Scholar] [CrossRef]
- Ishrat, T.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Kozak, A.; Johnson, M.H.; Ergul, A.; Fagan, S.C. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol. Neurobiol. 2015, 51, 1542–1553. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Leng, X.; Zheng, D.; Chen, F.; Wong, L.K.S.; Shi, L.; Leung, T.W.H. State-of-the-Art Computational Models of Circle of Willis With Physiological Applications: A Review. IEEE Access 2020, 8, 156261–156273. [Google Scholar] [CrossRef]
- Lan, L.; Leng, X.; Ip, V.; Soo, Y.; Abrigo, J.; Liu, H.; Fan, F.; Ma, S.H.; Ma, K.; Ip, B.Y.; et al. Sustaining cerebral perfusion in intracranial atherosclerotic stenosis: The roles of antegrade residual flow and leptomeningeal collateral flow. J. Cereb. Blood Flow Metab. 2020, 40, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Arnold, M.; Nedeltchev, K.; Brekenfeld, C.; Ballinari, P.; Remonda, L.; Schroth, G.; Mattle, H.P. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005, 36, 2121–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Chen, S.; Jia, J.; Shull, P.B. Cellphone-Based Automated Fugl-Meyer Assessment to Evaluate Upper Extremity Motor Function After Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tsang, R.C.; Zhou, J.; Zhou, M.; Zha, F.; Long, J.; Wang, Y. Relationship of Barthel Index and its Short Form with the Modified Rankin Scale in acute stroke patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 105033. [Google Scholar] [CrossRef]
- Williams, L.S.; Weinberger, M.; Harris, L.E.; Clark, D.O.; Biller, J. Development of a stroke-specific quality of life scale. Stroke 1999, 30, 1362–1369. [Google Scholar] [CrossRef]
- Geng, H.X.; Li, R.P.; Li, Y.G.; Wang, X.Q.; Zhang, L.; Deng, J.B.; Wang, L.; Deng, J.X. 14,15-EET Suppresses Neuronal Apoptosis in Ischemia-Reperfusion Through the Mitochondrial Pathway. Neurochem. Res. 2017, 42, 2841–2849. [Google Scholar] [CrossRef]
- Wen, Z.; Xie, Y.; Li, J.; Li, R.; Lv, Q.; Liu, Q.; Dai, Q.; Liu, X.; Xu, G. Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. J. Neuroinflamm. 2019, 16, 168. [Google Scholar]

| Group | Before Rehabilitation | 1 Month after Rehabilitation | 3 Months after Rehabilitation | F | p |
|---|---|---|---|---|---|
| 24 h rehabilitation | 6.47 ± 1.589 | 3.21 ± 1.069 | 0.76 ± 0.852 | 564.881 | <0.001 |
| 72 h rehabilitation | 6.76 ± 1.739 | 5.05 ± 1.246 | 2.51 ± 0.989 | 380.673 | <0.001 |
| t | −0.736 | −6.882 | −8.216 | ||
| p | 0.464 | <0.001 | <0.001 |
| Group | Before Rehabilitation | 1 Month after Rehabilitation | 3 Months after Rehabilitation | F | p |
|---|---|---|---|---|---|
| 24 h rehabilitation | 15.68 ± 5.951 | 62.32 ± 9.286 | 91.18 ± 7.377 | 776.513 | <0.001 |
| 72 h rehabilitation | 16.27 ± 6.475 | 41.43 ± 7.065 | 73.22 ± 7.811 | 592.256 | <0.001 |
| t | −0.408 | 10.939 | 10.244 | ||
| p | 0.684 | <0.001 | <0.001 |
| Group | Before Rehabilitation | 1 Month after Rehabilitation | 3 Months after Rehabilitation | F | p |
|---|---|---|---|---|---|
| 24 h rehabilitation | 23.37 ± 5.196 | 66.95 ± 4.707 | 96.55 ± 2.873 | 543.936 | <0.001 |
| 72 h rehabilitation | 22.84 ± 5.036 | 52.95 ± 6.725 | 84.46 ± 6.971 | 316.315 | <0.001 |
| t | 0.449 | 10.469 | 9.870 | ||
| p | 0.655 | <0.001 | <0.001 |
| Group | Before Rehabilitation | 1 Month after Rehabilitation | 3 Months after Rehabilitation | F | p |
|---|---|---|---|---|---|
| 24 h rehabilitation | 114.39 ± 7.772 | 168.61 ± 6.323 | 215.95 ± 10.977 | 414.795 | <0.001 |
| 72 h rehabilitation | 113.70 ± 8.323 | 149.92 ± 6.264 | 195.78 ± 8.145 | 271.184 | <0.001 |
| t | 0.372 | 12.855 | 9.015 | ||
| p | 0.711 | <0.001 | <0.001 |
| Days | MCAO Group | Treadmill Exercise Group in 24 h | t | p |
|---|---|---|---|---|
| 1 d | 10.22 ± 1.263 | 10.17 ± 1.505 | 0.120 | 0.905 |
| 3 d | 8.28 ± 1.018 | 7.22 ± 1.478 | 2.496 | 0.018 |
| 5 d | 6.00 ± 0.767 | 4.44 ± 0.784 | 6.018 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Li, M.; Tang, J.; Lv, Q.; Li, R.; Wang, L. Early Rehabilitation Exercise after Stroke Improves Neurological Recovery through Enhancing Angiogenesis in Patients and Cerebral Ischemia Rat Model. Int. J. Mol. Sci. 2022, 23, 10508. https://doi.org/10.3390/ijms231810508
Geng H, Li M, Tang J, Lv Q, Li R, Wang L. Early Rehabilitation Exercise after Stroke Improves Neurological Recovery through Enhancing Angiogenesis in Patients and Cerebral Ischemia Rat Model. International Journal of Molecular Sciences. 2022; 23(18):10508. https://doi.org/10.3390/ijms231810508
Chicago/Turabian StyleGeng, Huixia, Min Li, Jing Tang, Qing Lv, Ruiling Li, and Lai Wang. 2022. "Early Rehabilitation Exercise after Stroke Improves Neurological Recovery through Enhancing Angiogenesis in Patients and Cerebral Ischemia Rat Model" International Journal of Molecular Sciences 23, no. 18: 10508. https://doi.org/10.3390/ijms231810508
APA StyleGeng, H., Li, M., Tang, J., Lv, Q., Li, R., & Wang, L. (2022). Early Rehabilitation Exercise after Stroke Improves Neurological Recovery through Enhancing Angiogenesis in Patients and Cerebral Ischemia Rat Model. International Journal of Molecular Sciences, 23(18), 10508. https://doi.org/10.3390/ijms231810508






