Tissue Characteristics in Endodontic Regeneration: A Systematic Review
Abstract
:1. Introduction
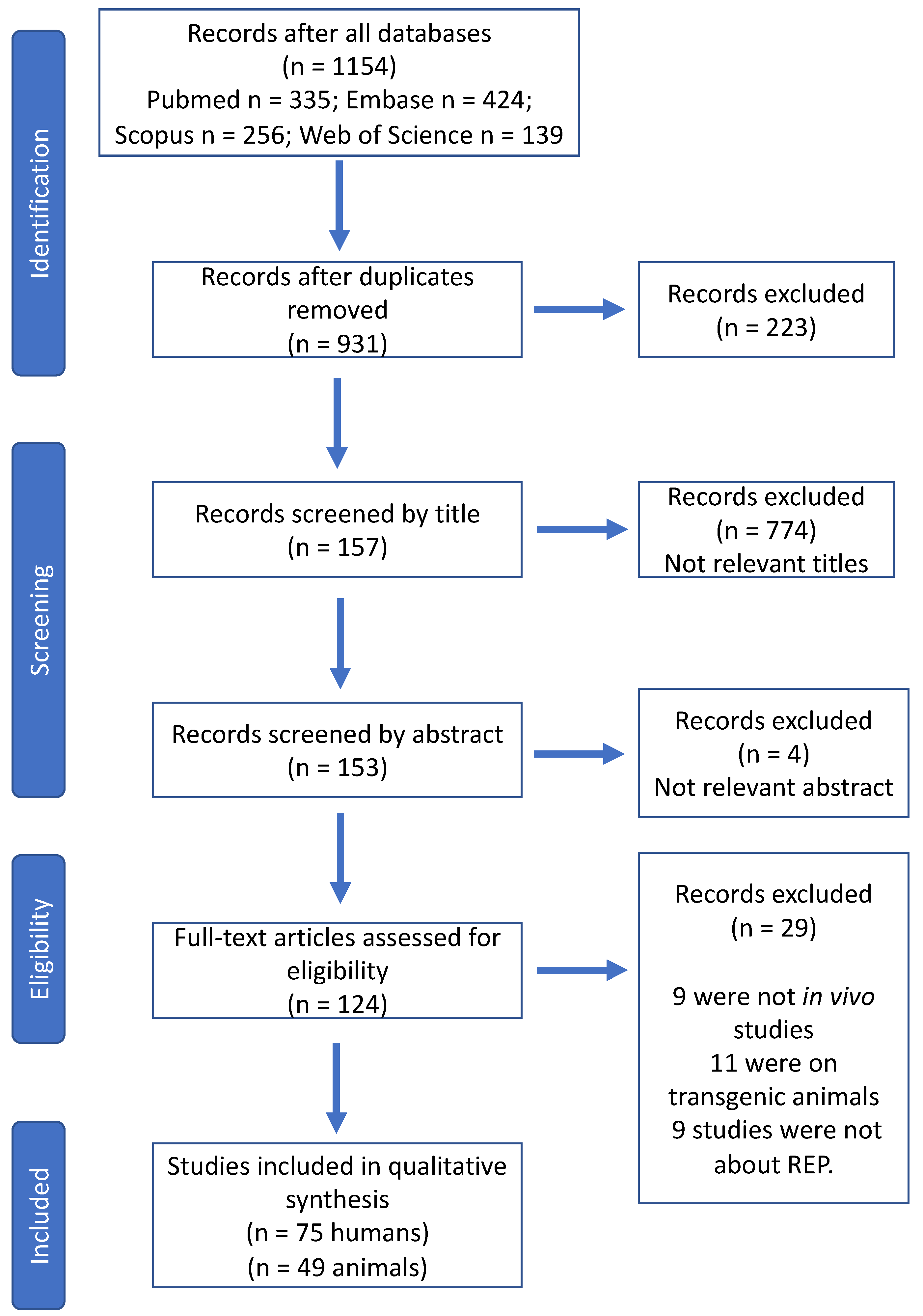
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Analysis
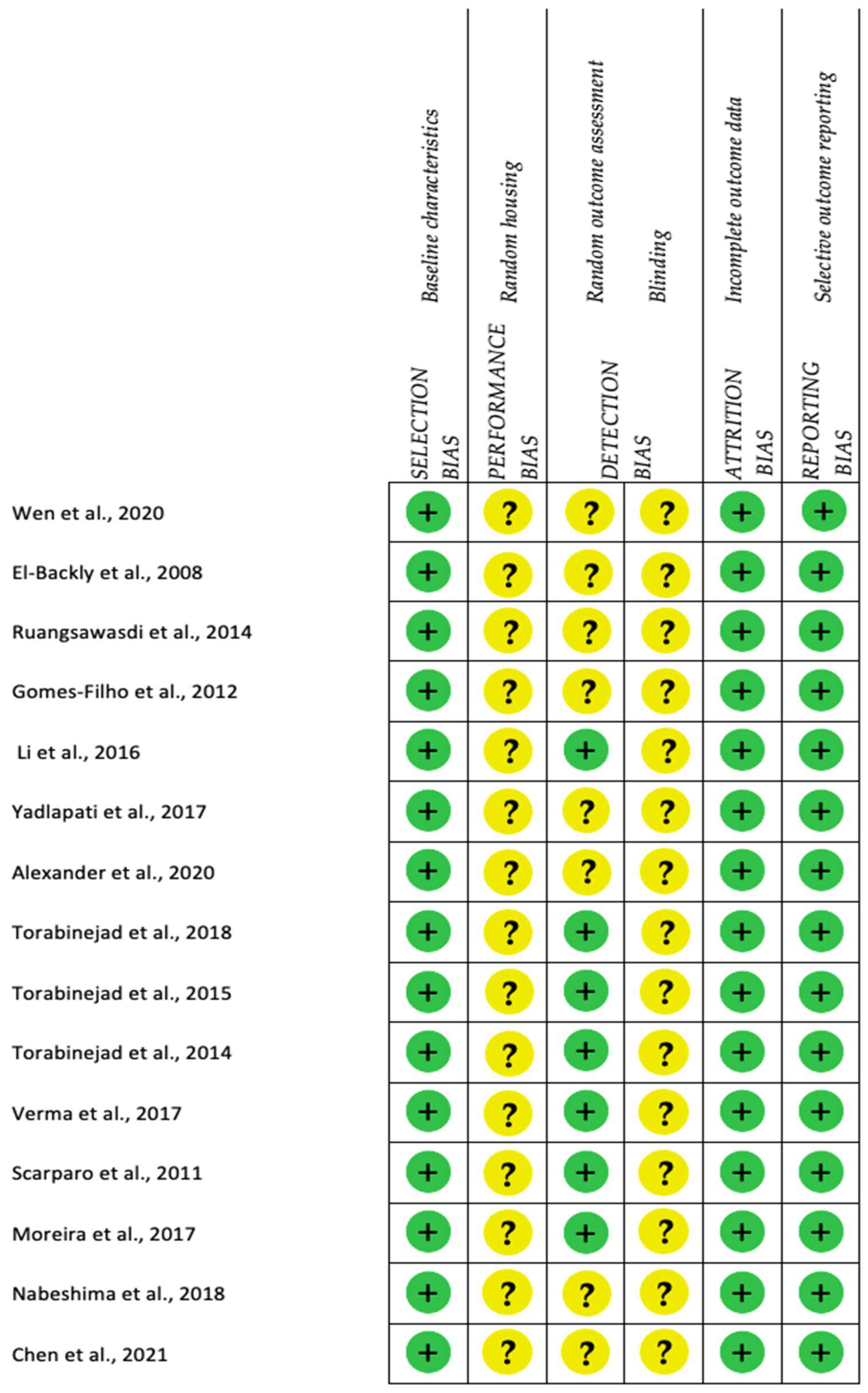
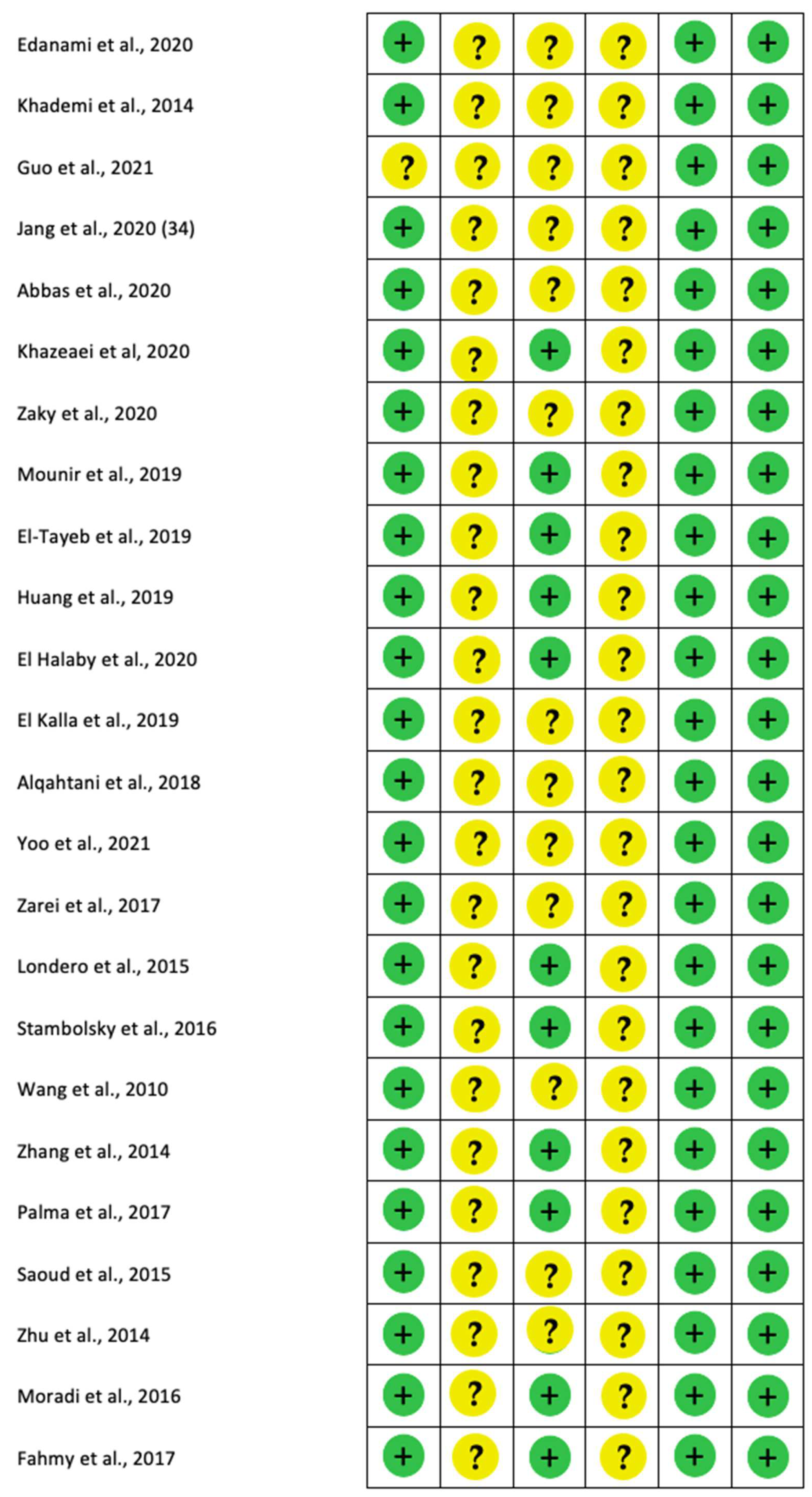
2.4. Quality Analysis and Level of Evidence
3. Results
3.1. Study Design and Characteristics of Included Studies
3.2. Animals Studies
3.2.1. Ectopic REP
Procedure
- -
- Dentine slices or entire tooth roots:
- -
- Polymers such as poly(lactic-co-glycolic acid) and rabbit DPSCs.
Follow-up
Evaluation Criteria
3.2.2. Orthotopic REP
Procedure
Follow-up
Evaluation Criteria
- -
- Dentin: Presence/absence of dentinal tubules.
- -
- Cementum: Absence of dentinal tubules and adherence onto dentin, and the presence of cementocyte-like cells.
- -
- Bone: Presence of Haversian canals with uniformly distributed osteocyte-like cells.
- -
- PDL: Presence of Sharpey’s fibers and fibers bridging cementum and bone.
Others
| Animal Models: Orthotopic REP Procedure | ||||
|---|---|---|---|---|
| Assessment | Main Results | Procedure | Follow-Up | Model |
| Histology | Presence of pulp-like / vital tissue | Gelatin and fibrin- based matrix BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. Nanosphere w/o BMSCs | 3 months 3–7 months 3 months 1–2 months | Mini pig [34] Dogs [32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,61,62,63] Ferrets [23,24,25,26] Rats [28,29,30,31] |
| New formation of mineralized tissue | Gelatin and fibrine-based matrix BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. | 3 months 3–7 months 3 months 3 months | Mini pig [34] Dogs [32,34,36,37,38,39,41,42,43,44,45,47,48,49,50,51,52,53,54,56,57,59,61,62,63,64,66] Sheep [64] Ferrets [23,24,25,26] | |
| Presence of odontoblastic palisade | Gelatin and fibrine-based matrix Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. | 3–6 months 3–6 months | Mini pig [33,67] Dogs [33,35,36,42,45,46,54] | |
| Inflammatory cell infiltration | Gelatin and fibrine-based matrix Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. TAP + silver amalgam | 3 months 3–6 months 3 months 1.5 months | Mini pig [34] Dogs [32,35,37,38,39,43,48,56,61,66] Ferrets [22,25] Rats [27] | |
| Presence of blood vessels | Gelatin and fibrine-based matrix Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. | 3–6 months 3–7 months 3 months 1–1.5 months | Mini pig [33,34] Dogs [35,37,42,43,47,52,55,56,64] Ferrets [24] Rats [28,29] | |
| Presence of nerve fibers | SLan angiogenictarget peptide vs. SLed dentinogenic control peptide Autologous pulp + BC + MTA | 3 months | Dogs [35,42] | |
| Presence of resorption | Gelatin and fibrine-based matrix Collagen sponge vs. PRF vs. MTA | 3 months | Mini pig [34] Dogs [39] | |
| No intraradicular mineralized tissue deposition | Gelatin and fibrine-based matrix | 3 months | Mini pig [34] | |
| Root maturation | BC + MTA | 3 months | Sheep [64] | |
| Apex maturation | Gelatin and fibrine-based matrix Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. | 3 months | Mini pig [34] Dogs [34,36,38,41,42,44,47,48,49,51,52,57,62,66] Sheep [64] Ferrets [22,24,25] | |
| Cementum cells/ tissue | Gelatin and fibrine-based matrix BC + Gelfoam BC + PRP BC + MTA | 3–7 months | Mini pig [34] Dogs [41,48,50,52,56] Ferrets [24] Rats [28] | |
| Dentin tissue | BC + Gelfoam BC + PRP Propolis vs. MTA Autologous stem cells | 1–3 months | Dogs [41,42,52] Rats [31] | |
| Osteodentin | (Buccal fat) vs. (BC + Buccal fat) + MTA BC + PRP BC + MTA | 3–6 months | Dogs [38] Ferrets [26] Rats [31] | |
| Bone tissue | Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. | 6 months | Dogs [39,48,49,50,52,53,59,60,63] | |
| Mineralized tissue deposition | Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. | 3–6 months | Dogs [32,41,42,43,45,49,57,58] Ferrets [23,26] | |
| Radiology | Presence of pulp-like / vital tissue | Gelatin and fibrine-based matrix BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. Nanosphere w/o BMSCs | 3 months 3–7 months 3 months 1–2 months | Mini pig [34] Dogs [32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,61,62,63] Ferrets [23,24,25,26] Rats [28,29,30,31] |
| Apex closure | Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. TAP + silver amalgam | 3–6 months | Dogs [32,41,42,43,46,49,51,57,58,59,66] Sheep [64] Ferrets [23,26] Rats [27] | |
| Increase root length | Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. TAP + silver amalgam | 3–6 months | Dogs [32,41,42,46,49,51,57,58,59,66] Sheep [64] Ferrets [23,26] Rats [27] | |
| Increase dentin thickness | Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. TAP + silver amalgam | 3–6 months | Dogs [32,41,42,46,49,51,57,58,59,66] Sheep [64] Ferrets [23,26] Rats [27] | |
| Periapical healing | Autologous stem cells BC alone + MTA PRP or PRF with cement and BC DPSCs and Buccal fat with BC and MTA. TAP + silver amalgam | 3–6 months | Dogs [32,42,46,57,58,59] Sheep [64] Ferrets [23,26] Rats [27] | |
| qPCR | DSPP, COL1A1, ALP, DMP1 expression | (Buccal fat) vs. (BC + Buccal fat) + MTA | 3 months | Dogs [38] |
3.3. Human Studies
3.3.1. REP protocol
3.3.2. Follow-Up
3.3.3. Clinical and Radiographic Evaluation of REP
3.3.4. Others
| Human Model: Regenerative Endodontic Procedure | ||||
|---|---|---|---|---|
| Assessment | Main Results | Procedure | Follow-Up | Articles |
| Clinical tests | Asymptomatic teeth | BC + Biodentine or MTA BC +PRF + MTA or Biodentine or GIC BC + PRP + MTA or Biodentine or GIC BC + Collagen + MTA or BC+ UC-MSCs + collagen + MTA BC + PRF + Collagen + Biodentine or Portland mDPSCs + G-CSF + Collagen + MTA Medication on different Appointment TAP vs. CaOH2 vs. formocresol Bi antibiotic + GIC | 21 days–79 months | [61,66,68,69,70,71,72,86,87,90,91,94,95,96,97,101,102,103,104,106,107,110,111,113,116,117,118,119,120,121,122,123,124,125,126,128,130,131,132] |
| PAI | BC + MTA Sealbio vs. obturation | 12–24 months | [80,98,134] | |
| Dyschromia | BC + collaplug MTA vs. Biodentine vs. GIC BC + MTA vs. Biodentine BC + PRF vs. PRP + MTA Bi-antibiotic paste + BC + GIC | 12–96 months | [75,79,85,86,98,126,130] | |
| Mobility | BC + Synoss Putty | 72 months | [120] | |
| Radiographic observation | Apical lesion | BC + hydrogel with FGF+ MTA BC + DPSC In hydrogel + MTA or GIC BC + MTA BC + PRF + Biodentine BC + PRP + MTA BC + PRF vs. PRP + Collagen + GIC BC + Synoss putty + MTA BC + Collagen + Portland + MTA BC + LPRF + Portland cement | 21 days– 72 months | [8,66,70,71,79,80,81,82,83,90,92,93,95,97,99,100,103,105,107,112,113,116,118,120,122,124,125,128,133] |
| Root length | BC + hydrogel with FGF+ MTA BC + DPSC In hydrogel + MTA or GIC BC + MTA BC + PRF + Biodentine BC + PRP + MTA BC + PRF vs. PRP + Collagen + GIC BC + Synoss putty + MTA BC + Collagen + Portland + MTA BC + LPRF + Portland cement | 21 days– 78 months | [66,67,69,73,80,82,83,87,90,91,94,96,97,99,100,101,102,104,105,108,110,112,119,122,124,127,128,130,132] | |
| Root thickness | BC + hydrogel with FGF+ MTA BC + DPSC In hydrogel + MTA or GIC BC + MTA BC + PRF + Biodentine BC + PRP + MTA BC + PRF vs. PRP + Collagen + GIC BC + Synoss putty + MTA BC + Collagen + Portland + MTA BC + LPRF + Portland cement | 21 days– 60 months | [65,66,67,71,80,83,87,90,91,96,97,99,100,101,102,104,105,108,111,112,118,119,122,124,127,128,130,132] | |
| Apical closure | BC + hydrogel with FGF+ MTA BC + DPSC In hydrogel + MTA or GIC BC + Collagen + coltosol BC + MTA BC + PRF + Biodentine BC + PRP + MTA BC + PRF vs. PRP + Collagen + GIC BC + Synoss putty + MTA BC + Collagen + Portland + MTA BC + LPRF + Portland cement | 21 days– 78 months | [8,67,69,70,71,72,73,74,76,77,78,84,88,92,97,98,99,100,102,104,105,110,111,112,118,119,120,122,124,128,132] | |
| Radiolucy | BC + PRF + MTA BC + MTA or CEM | 6 months– 78 months | [8,73,97,102,103,107,108,109,110,119] | |
| Bone density | BC + hydrogel with FGF+ MTA BC + DPSC in hydrogel + MTA or GIC BC + MTA BC + PRP + MTA BC + Synoss putty + MTA | 24–70 months | [70,96,97,109,111,112,120] | |
| Resorption | BC + PRF + Collagen + Biodentine Medication on different appointment | 21 days– 30 months | [117,128,133] | |
| Calcification in the pulp | BC + MTA or CEM BC + Collagen + MTA BC + PRP + MTA BC + iPRF + Biodentine BC + Amelogen Plus | 12–60 months | [66,73,78,85,88,93,96,99,110,118,119,127,133] | |
| Ligament repair | BC + PRF or PRP + MTA Vs BC + MTA BC + PRP + MTA BC + PRF + Collagen + Biodentine | 50 months | [91,95,117] | |
| qPCR | Quantify bacteria | Different appointment medication TAP vs. calcium hydroxide medication | 21 days– 19 months | [128,129] |
| Cells identification in the canal | Intracanal blood sample after BC | 1 month | [135] | |
| Histology | Regenerate tissue observation | BC + MTA BC + Synoss Putty BC + Amelogen Plus BC + Collagen / MTA | 7.5–36 months | [106,107,121,127,136] |
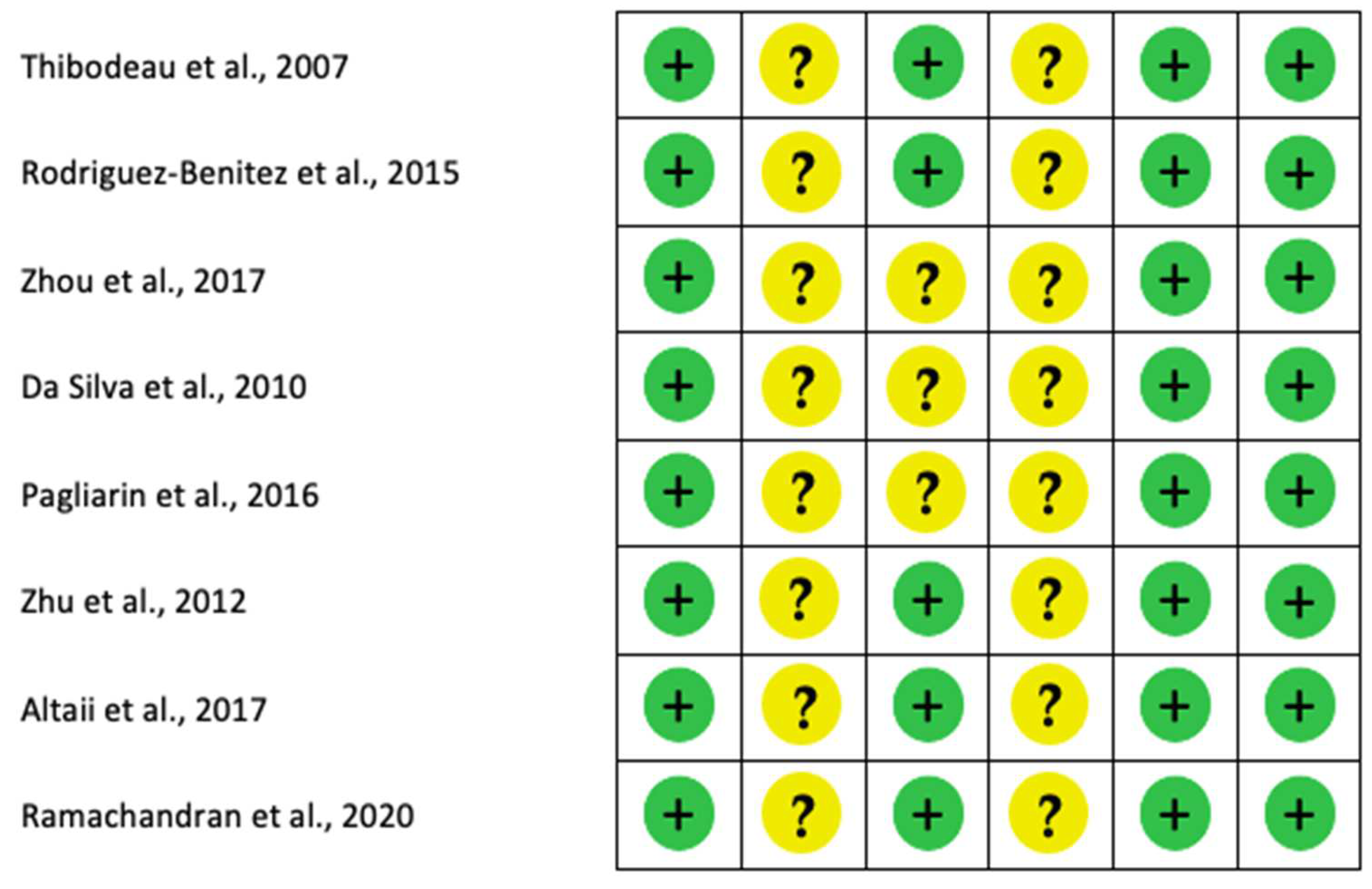
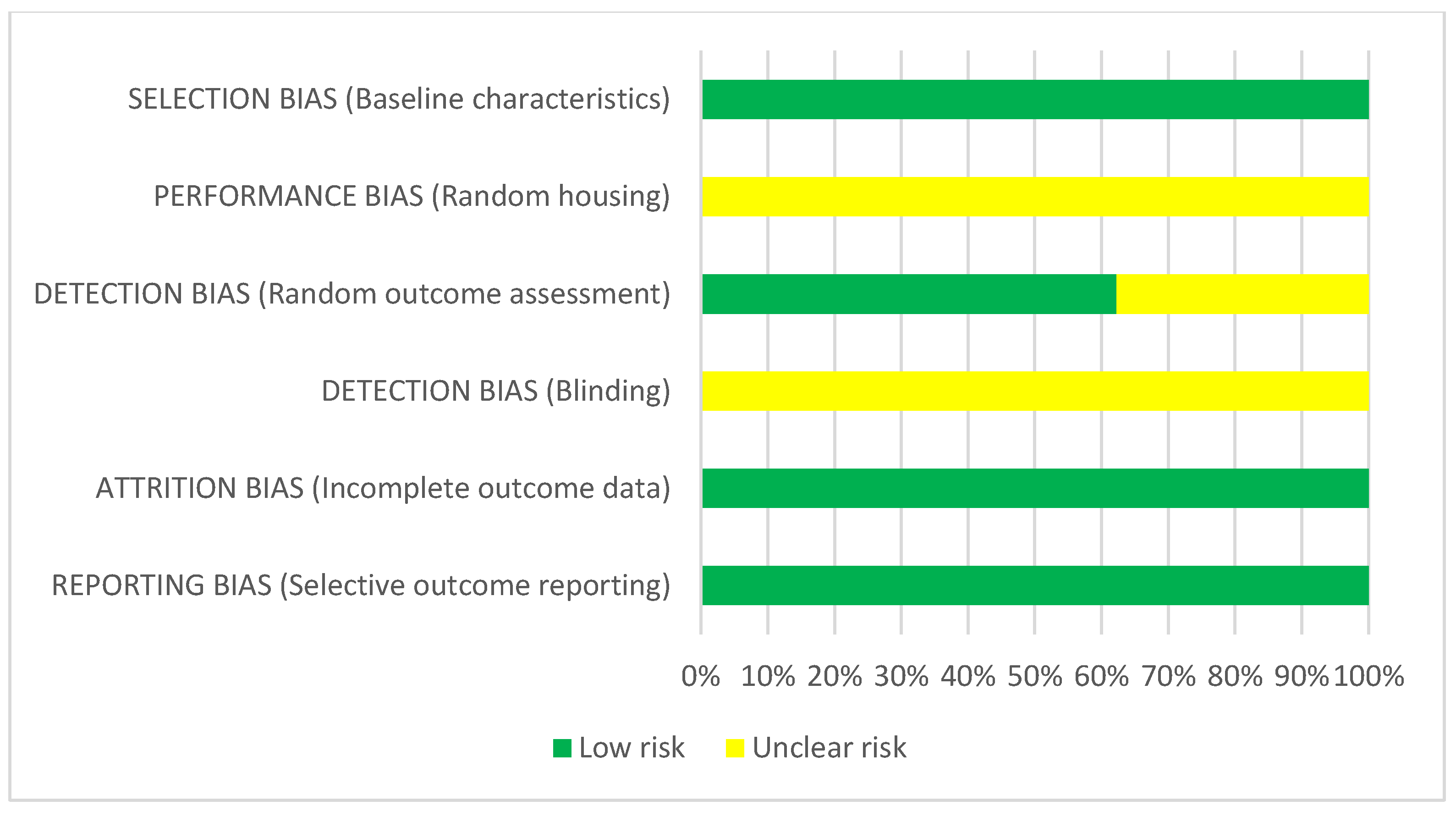
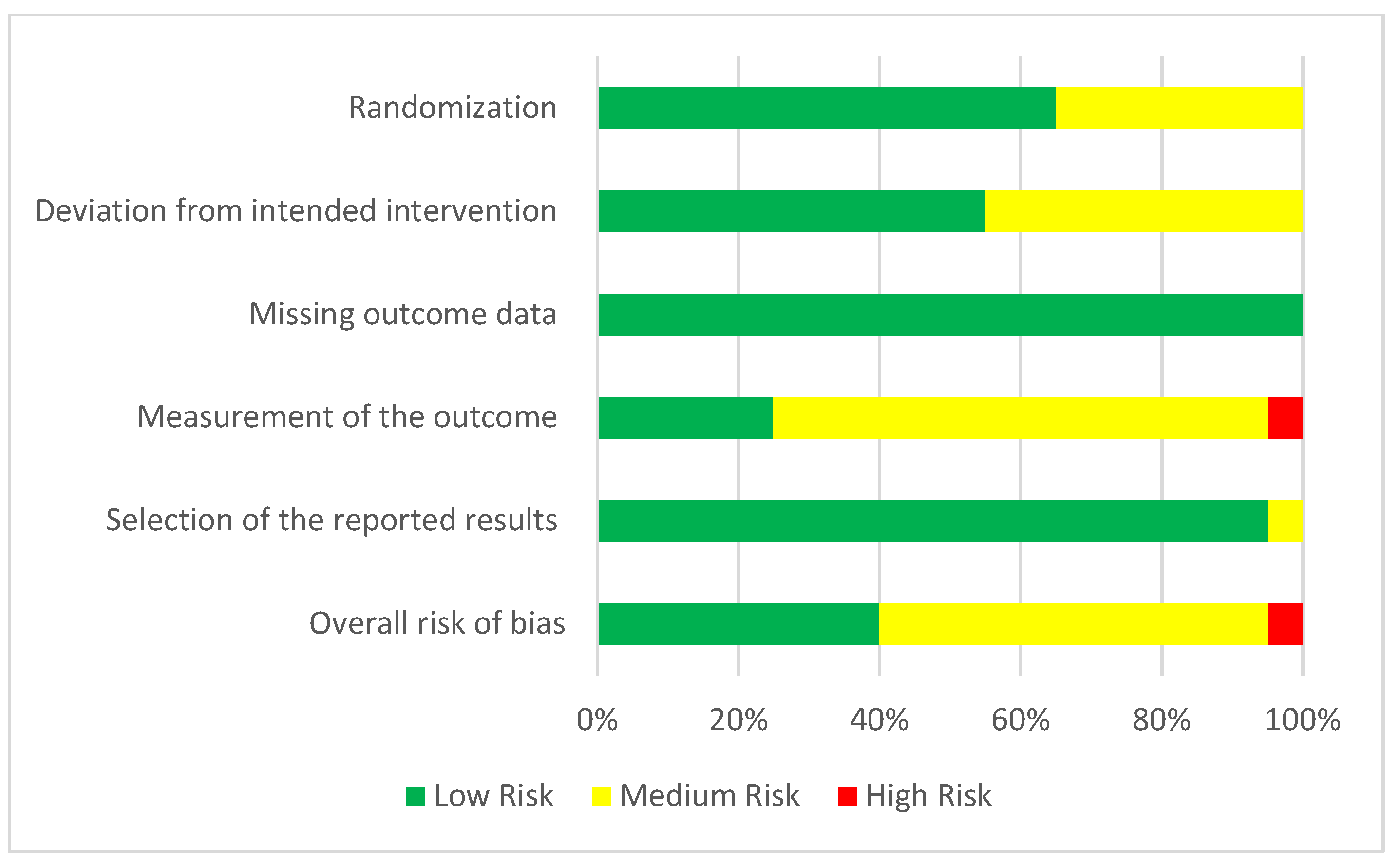
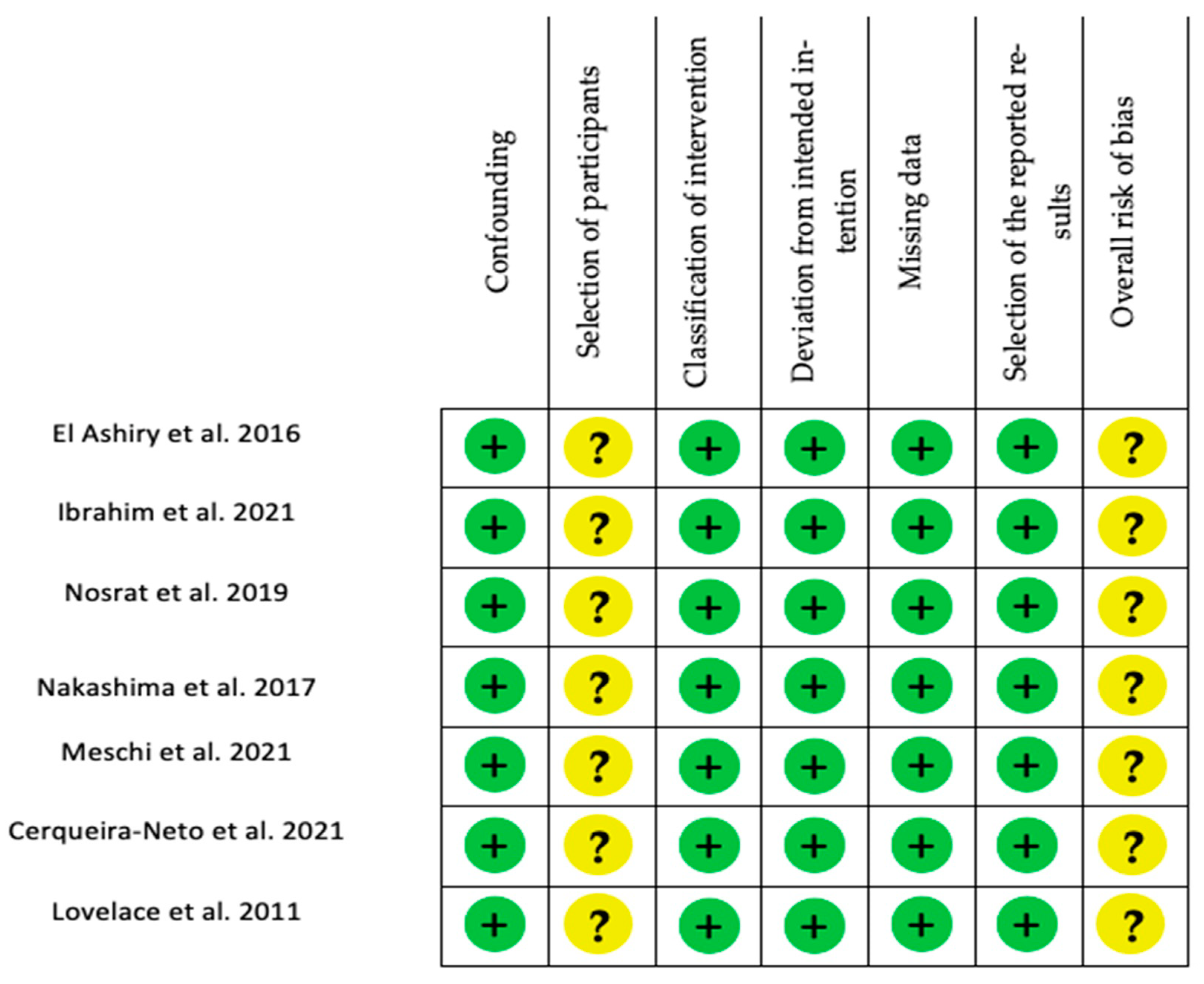
3.4. Risk of Bias
4. Discussion
4.1. Success Criteria Assessment of REP
4.2. Ectopic Model
4.3. Animal Models
4.4. Clinical Studies
4.5. Risk of Bias
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ALP | Alkaline Phosphatase |
| BC | Blood Clot |
| BMSCs | Bone Marrow Stem Cells |
| CaOH2 | Calcium Hydroxide |
| CAP | Catabolite Activator Protein |
| CD31 | Platelet endothelial cell adhesion molecule |
| CEM | Calcium Enriched Mixture |
| CEMP | Cathelicidin Antimicrobial Peptide |
| CHP | Calcium Hydroxide Paste |
| CGRP | Calcitonin Gene-Related Peptide |
| GIC | Glass Ionomer Cement |
| Col1A1 | Collagen type I alpha 1 |
| DMP1 | Dentin Matrix acidic Phosphoprotein 1 |
| DMP4 | Dentin matrix protein 4 |
| (m) DPSCs | (mobilized) Dental Pulp Stem Cells |
| DSPP | Dentin Sialophosphoprotein |
| DXL1 | Distal-Less Homeobox 1 |
| FGF | Fibroblast growth factor |
| G-CSF | Granulocyte colony-stimulating factor |
| GLI2 | GLI Family Zinc Finger 2 |
| LPS | Lipopolysaccharide |
| MTA | Mineral Trioxide Aggregate |
| MRI | Magnetic Resonance Imaging |
| NF | Neurofilament |
| PAI | Periapical Index |
| PDLs | Periodontal ligament cells |
| PGP 9,5 | Neuronal marker |
| (i)- (L)- PRF | (Injection-) (Leucocyte-) Platelet Rich Fibrin |
| PRP | Platelet Rich Plasma |
| qPCR | quantitative Polymerase Chain Reaction |
| rBMSC | rabbit Bone Marrow Stem Cells |
| REP | Regenerative Endodontic Procedure |
| SOX2 | Sex determining region Y)-box 2 |
| TAP | Tri-Antibiotic Paste |
| UC-MSCs | Umbilical Cord Mesenchymental Stem Cells |
| VEGF | Vascular Endothelial Growth Factor |
| vVW | Von Willebrand |
| 2D radiography | X-ray / Panoramic radiography |
| 3D radiography | CBCT (Cone Beam Computed Tomography) |
References
- Diogenes, A.; Ruparel, N.B. Regenerative Endodontic Procedures: Clinical Outcomes. Dent. Clin. N. Am. 2017, 61, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists. Endodontic Diagnosis. 2013. Available online: https://www.aae.org/specialty/newsletter/endodontic-diagnosis/ (accessed on 28 July 2022).
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Duggal, M.; Tong, H.J.; Al-Ansary, M.; Twati, W.; Day, P.F.; Nazzal, H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: A systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur. Arch. Paediatr. Dent. 2017, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.M.; El Kalla, I.H.; Wahba, A.H.; Salama, N.M. Clinical and radiographic evaluation of platelet-rich fibrin for revascularization of necrotic immature permanent teeth: A controlled clinical trial. Pediatr. Dent. J. 2020, 30, 182–190. [Google Scholar] [CrossRef]
- ElSheshtawy, A.S.; Nazzal, H.; El Shahawy, O.I.; El Baz, A.A.; Ismail, S.M.; Kang, J.; Ezzat, K.M. The effect of platelet-rich plasma as a scaffold in regeneration/revitalization endodontics of immature permanent teeth assessed using 2-dimensional radiographs and cone beam computed tomography: A randomized controlled trial. Int. Endod. J. 2020, 53, 905–921. [Google Scholar] [CrossRef]
- Yoshpe, M.; Kaufman, A.Y.; Lin, S.; Ashkenazi, M. Regenerative endodontics: A promising tool to promote periapical healing and root maturation of necrotic immature permanent molars with apical periodontitis using platelet-rich fibrin (PRF). Eur. Arch. Paediatr. Dent. 2021, 22, 527–534. [Google Scholar] [CrossRef]
- He, L.; Zhong, J.; Gong, Q.; Cheng, B.; Kim, S.G.; Ling, J.; Mao, J.J. Regenerative Endodontics by Cell Homing. Dent. Clin. 2017, 61, 143–159. [Google Scholar] [CrossRef]
- Kim, S.G.; Solomon, C.; Zheng, Y.; Suzuki, T.; Mo, C.; Song, S.; Jiang, N.; Cho, S.; Zhou, J.; Mao, J.J. Effects of Growth Factors on Dental Stem/ProgenitorCells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef]
- Saoud, T.M.A.; Ricucci, D.; Lin, L.M.; Gaengler, P. Regeneration and Repair in Endodontics—A Special Issue of the Regenerative Endodontics—A New Era in Clinical Endodontics. Dent. J. 2016, 4, 3. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ EBM 2018, 23, 60–63. [Google Scholar] [CrossRef]
- Wen, R.; Wang, X.; Lu, Y.; Du, Y.; Yu, X. The combined application of rat bone marrow mesenchymal stem cells and bioceramic materials in the regeneration of dental pulp-like tissues. Int. J. Clin. Exp. Pathol. 2020, 13, 1492–1499. [Google Scholar] [PubMed]
- El-Backly, R.M.; Massoud, A.G.; El-Badry, A.M.; Sherif, R.A.; Marei, M.K. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust. Endod. J. 2008, 34, 52–67. [Google Scholar] [CrossRef]
- Ruangsawasdi, N.; Zehnder, M.; Weber, F.E. Fibrin Gel Improves Tissue Ingrowth and Cell Differentiation in Human Immature Premolars Implanted in Rats. J. Endod. 2014, 40, 246–250. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; Duarte, P.C.T.; de Oliveira, C.B.; Watanabe, S.; Lodi, C.S.; Cintra, L.T.Â.; Bernabé, P.F.E. Tissue Reaction to a Triantibiotic Paste Used for Endodontic Tissue Self-regeneration of Nonvital Immature Permanent Teeth. J. Endod. 2012, 38, 91–94. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Xie, X.; Sun, H.; Liu, X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016, 35, 57–67. [Google Scholar] [CrossRef]
- Yadlapati, M.; Biguetti, C.; Cavalla, F.; Nieves, F.; Bessey, C.; Bohluli, P.; Garlet, G.P.; Letra, A.; Fakhouri, W.D.; Silva, R.M. Characterization of a Vascular Endothelial Growth Factor–loaded Bioresorbable Delivery System for Pulp Regeneration. J. Endod. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Alexander, A.; Torabinejad, M.; Vahdati, S.A.; Nosrat, A.; Verma, P.; Grandhi, A.; Shabahang, S. Regenerative Endodontic Treatment in Immature Noninfected Ferret Teeth Using Blood Clot or SynOss Putty as Scaffolds. J. Endod. 2020, 46, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Alexander, A.; Vahdati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-pulp Complex: An In Vivo Investigation. J. Endod. 2018, 44, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Milan, M.; Shabahang, S.; Wright, K.R.; Faras, H. Histologic Examination of Teeth with Necrotic Pulps and Periapical Lesions Treated with 2 Scaffolds: An Animal Investigation. J. Endod. 2015, 41, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Faras, H.; Corr, R.; Wright, K.R.; Shabahang, S. Histologic Examinations of Teeth Treated with 2 Scaffolds: A Pilot Animal Investigation. J. Endod. 2014, 40, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Scarparo, R.K.; Dondoni, L.; Böttcher, D.E.; Grecca, F.S.; Rockenbach, M.I.B.; Batista, E.L. Response to intracanal medication in immature teeth with pulp necrosis: An experimental model in rat molars. J. Endod. 2011, 37, 1069–1073. [Google Scholar] [CrossRef]
- Moreira, M.S.; Diniz, I.M.; Rodrigues, M.F.S.D.; de Carvalho, R.A.; de Almeida Carrer, F.C.; Neves, I.I.; Gavini, G.; Marques, M.M. In vivo experimental model of orthotopic dental pulp regeneration under the influence of photobiomodulation therapy. J. Photochem. Photobiol. B Biol. 2017, 166, 180–186. [Google Scholar] [CrossRef]
- Nabeshima, C.K.; Valdivia, J.E.; Caballero-Flores, H.; Arana-Chavez, V.E.; de Lima Machado Machado, M.E. Immunohistological study of the effect of vascular Endothelial Growth Factor on the angiogenesis of mature root canals in rat molars. J. Appl. Oral Sci. 2018, 26, e20170437. [Google Scholar] [CrossRef]
- Chen, W.-J.; Xie, J.; Lin, X.; Ou, M.-H.; Zhou, J.; Wei, X.-L.; Chen, W.-X. The Role of Small Extracellular Vesicles Derived from Lipopolysaccharide-preconditioned Human Dental Pulp Stem Cells in Dental Pulp Regeneration. J. Endod. 2021, 47, 961–969. [Google Scholar] [CrossRef]
- Edanami, N.; Yoshiba, K.; Shirakashi, M.; Ibn Belal, R.S.; Yoshiba, N.; Ohkura, N.; Tohma, A.; Takeuchi, R.; Okiji, T.; Noiri, Y. Impact of remnant healthy pulp and apical tissue on outcomes after simulated regenerative endodontic procedure in rat molars. Sci. Rep. 2020, 10, 20967. [Google Scholar] [CrossRef]
- Khademi, A.A.; Dianat, O.; Mahjour, F.; Razavi, S.M.; Younessian, F. Outcomes of revascularization treatment in immature dog’s teeth. Dent. Traumatol. 2014, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, B.; Wu, M.; Zhao, W.; He, X.; Sui, B.; Dong, Z.; Wang, L.; Shi, S.; Huang, X.; et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials 2021, 279, 121223. [Google Scholar] [CrossRef]
- Jang, J.-H.; Moon, J.-H.; Kim, S.G.; Kim, S.-Y. Pulp regeneration with hemostatic matrices as a scaffold in an immature tooth minipig model. Sci. Rep. 2020, 10, 12536. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.; Sarkar, B.; Kim, K.-K.; Kadincesme, N.; Paul, R.; Kumar, A.; Kobayashi, Y.; Roy, A.; Choudhury, M.; Yang, J.; et al. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021, 126, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Solomon, C.S. Regenerative Endodontic Therapy in Mature Teeth Using Human-Derived Composite Amnion-Chorion Membrane as a Bioactive Scaffold: A Pilot Animal Investigation. J. Endod. 2021, 47, 1101–1109. [Google Scholar] [CrossRef]
- Abbas, K.F.; Tawfik, H.; Hashem, A.A.R.; Ahmed, H.M.A.; Abu-Seida, A.M.; Refai, H.M. Histopathological evaluation of different regenerative protocols using Chitosan-based formulations for management of immature non-vital teeth with apical periodontitis: In vivo study. Aust. Endod. J. 2020, 46, 405–414. [Google Scholar] [CrossRef]
- Khazaei, S.; Khademi, A.; Torabinejad, M.; Nasr Esfahani, M.H.; Khazaei, M.; Razavi, S.M. Improving pulp revascularization outcomes with buccal fat autotransplantation. J. Tissue Eng. Regen. Med. 2020, 14, 1227–1235. [Google Scholar] [CrossRef]
- Zaky, S.H.; AlQahtani, Q.; Chen, J.; Patil, A.; Taboas, J.; Beniash, E.; Ray, H.; Sfeir, C. Effect of the Periapical “Inflammatory Plug” on Dental Pulp Regeneration: A Histologic In Vivo Study. J. Endod. 2020, 46, 51–56. [Google Scholar] [CrossRef]
- Mounir, M.M.F.; Rashed, F.M.; Bukhary, S.M. Regeneration of Neural Networks in Immature Teeth with Non-Vital Pulp Following a Novel Regenerative Procedure. Int. J. Stem Cells 2019, 12, 410–418. [Google Scholar] [CrossRef]
- El-Tayeb, M.M.; Abu-Seida, A.M.; El Ashry, S.H.; El-Hady, S.A. Evaluation of antibacterial activity of propolis on regenerative potential of necrotic immature permanent teeth in dogs. BMC Oral Health 2019, 19, 174. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Tang, X.; Cehreli, Z.C.; Dai, X.; Xu, J.; Zhu, H. Autologous transplantation of deciduous tooth pulp into necrotic young permanent teeth for pulp regeneration in a dog model. J. Int. Med. Res. 2019, 47, 5094–5105. [Google Scholar] [CrossRef] [PubMed]
- El Halaby, H.M.; Abu-Seida, A.M.; Fawzy, M.I.; Farid, M.H.; Bastawy, H.A. Evaluation of the regenerative potential of dentin conditioning and naturally derived scaffold for necrotic immature permanent teeth in a dog model. Int. J. Exp. Pathol. 2020, 101, 264–276. [Google Scholar] [CrossRef] [PubMed]
- El Kalla, I.H.; Salama, N.M.; Wahba, A.H.; Sallam, N.M. Histological evaluation of platelet-rich fibrin for revascularization of immature permanent teeth in dogs. Pediatr. Dent. J. 2019, 29, 72–77. [Google Scholar] [CrossRef]
- Alqahtani, Q.; Zaky, S.H.; Patil, A.; Beniash, E.; Ray, H.; Sfeir, C. Decellularized Swine Dental Pulp Tissue for Regenerative Root Canal Therapy. J. Dent. Res. 2018, 97, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.-J.; Perinpanayagam, H.; Choi, Y.; Gu, Y.; Chang, S.-W.; Baek, S.-H.; Zhu, Q.; Fouad, A.F.; Kum, K.-Y. Characterization of Histopathology and Microbiota in Contemporary Regenerative Endodontic Procedures: Still Coming up Short. J. Endod. 2021, 47, 1285–1293.e1. [Google Scholar] [CrossRef]
- Zarei, M.; Jafarian, A.H.; Harandi, A.; Javidi, M.; Gharechahi, M. Evaluation of the expression of VIII factor and VEGF in the regeneration of non-vital teeth in dogs using propolis. Iran. J. Basic Med. Sci. 2017, 20, 172–177. [Google Scholar] [CrossRef]
- De Londero, C.L.D.; Pagliarin, C.M.L.; Felippe, M.C.S.; Felippe, W.T.; Danesi, C.C.; Barletta, F.B. Histologic Analysis of the Influence of a Gelatin-based Scaffold in the Repair of Immature Dog Teeth Subjected to Regenerative Endodontic Treatment. J. Endod. 2015, 41, 1619–1625. [Google Scholar] [CrossRef]
- Stambolsky, C.; Rodríguez-Benítez, S.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D.; Martín-González, J.; Segura-Egea, J.J. Histologic characterization of regenerated tissues after pulp revascularization of immature dog teeth with apical periodontitis using tri-antibiotic paste and platelet-rich plasma. Arch. Oral Biol. 2016, 71, 122–128. [Google Scholar] [CrossRef]
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.-J. Histologic Characterization of Regenerated Tissues in Canal Space after the Revitalization/Revascularization Procedure of Immature Dog Teeth with Apical Periodontitis. J. Endod. 2010, 36, 56–63. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Chen, X.; Bao, Z.-F.; Chen, M.; Ding, Z.-J.; Zhong, M. Histologic Comparison between Platelet-rich Plasma and Blood Clot in Regenerative Endodontic Treatment: An Animal Study. J. Endod. 2014, 40, 1388–1393. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Saoud, T.M.A.; Zaazou, A.; Nabil, A.; Moussa, S.; Aly, H.M.; Okazaki, K.; Rosenberg, P.A.; Lin, L.M. Histological observations of pulpal replacement tissue in immature dog teeth after revascularization of infected pulps. Dent. Traumatol. 2015, 31, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Liu, Y.; Huang, G.T.-J.; Zhang, C. Immunohistochemical and Histochemical Analysis of Newly Formed Tissues in Root Canal Space Transplanted with Dental Pulp Stem Cells Plus Platelet-rich Plasma. J. Endod. 2014, 40, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Talati, A.; Forghani, M.; Jafarian, A.H.; Naseri, M.; Shojaeian, S. Immunohistological Evaluation of Revascularized Immature Permanent Necrotic Teeth Treated by Platelet-Rich Plasma: An Animal Investigation. Cell J. 2016, 18, 389–396. [Google Scholar]
- Fahmy, S.H.; Hassanien, E.E.S.; Nagy, M.M.; El Batouty, K.M.; Mekhemar, M.; Fawzy El Sayed, K.; Hassanein, E.H.; Wiltfang, J.; Dörfer, C. Investigation of the regenerative potential of necrotic mature teeth following different revascularisation protocols. Aust. Endod. J. 2017, 43, 73–82. [Google Scholar] [CrossRef]
- Thibodeau, B.; Teixeira, F.; Yamauchi, M.; Caplan, D.J.; Trope, M. Pulp Revascularization of Immature Dog Teeth With Apical Periodontitis. J. Endod. 2007, 33, 680–689. [Google Scholar] [CrossRef]
- Rodríguez-Benítez, S.; Stambolsky, C.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D.; Segura-Egea, J.J. Pulp Revascularization of Immature Dog Teeth with Apical Periodontitis Using Triantibiotic Paste and Platelet-rich Plasma: A Radiographic Study. J. Endod. 2015, 41, 1299–1304. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Chen, Y.; Chen, S.; Lyu, H.; Cai, Z.; Huang, X. Radiographic, Histologic, and Biomechanical Evaluation of Combined Application of Platelet-rich Fibrin with Blood Clot in Regenerative Endodontics. J. Endod. 2017, 43, 2034–2040. [Google Scholar] [CrossRef]
- El Ashiry, E.A.; Farsi, N.M.; Abuzeid, S.T.; El Ashiry, M.M.; Bahammam, H.A. Dental Pulp Revascularization of Necrotic Permanent Teeth with Immature Apices. J. Clin. Pediatr. Dent. 2016, 40, 361–366. [Google Scholar] [CrossRef]
- Da Silva, L.A.B.; Nelson-Filho, P.; da Silva, R.A.B.; Flores, D.S.H.; Heilborn, C.; Johnson, J.D.; Cohenca, N. Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs’ teeth with apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, 779–787. [Google Scholar] [CrossRef]
- Pagliarin, C.M.L.; de Londero, C.L.D.; Felippe, M.C.S.; Felippe, W.T.; Danesi, C.C.; Barletta, F.B. Tissue characterization following revascularization of immature dog teeth using different disinfection pastes. Braz. Oral Res. 2016, 30, S1806-83242016000100273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, C.; Huang, G.T.-J.; Cheung, G.S.P.; Dissanayaka, W.L.; Zhu, W. Transplantation of Dental Pulp Stem Cells and Platelet-rich Plasma for Pulp Regeneration. J. Endod. 2012, 38, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Altaii, M.; Cathro, P.; Broberg, M.; Richards, L. Endodontic regeneration and tooth revitalization in immature infected sheep teeth. Int. Endod. J. 2017, 50, 480–491. [Google Scholar] [CrossRef]
- Ramachandran, N.; Singh, S.; Podar, R.; Kulkarni, G.; Shetty, R.; Chandrasekhar, P. A comparison of two pulp revascularization techniques using platelet-rich plasma and whole blood clot. J. Conserv. Dent. 2020, 23, 637–643. [Google Scholar] [CrossRef]
- El Ashry, S.H.; Abu-Seida, A.M.; Bayoumi, A.A.; Hashem, A.A. Regenerative potential of immature permanent non-vital teeth following different dentin surface treatments. Exp. Toxicol. Pathol. 2016, 68, 181–190. [Google Scholar] [CrossRef] [PubMed]
- McTigue, D.J.; Subramanian, K.; Kumar, A. Case Series: Management of Immature Permanent Teeth With Pulpal Necrosis: A Case Series. Pediatric Dent. 2013, 35, 55–60. [Google Scholar]
- Meschi, N.; Castro, A.B.; Vandamme, K.; Quirynen, M.; Lambrechts, P. The impact of autologous platelet concentrates on endodontic healing: A systematic review. Platelets 2016, 27, 613–633. [Google Scholar] [CrossRef]
- Bezgin, T.; Yilmaz, A.D.; Celik, B.N.; Kolsuz, M.E.; Sonmez, H. Efficacy of Platelet-rich Plasma as a Scaffold in Regenerative Endodontic Treatment. J. Endod. 2015, 41, 36–44. [Google Scholar] [CrossRef]
- Nagy, M.M.; Tawfik, H.E.; Hashem, A.A.R.; Abu-Seida, A.M. Regenerative Potential of Immature Permanent Teeth with Necrotic Pulps after Different Regenerative Protocols. J. Endod. 2014, 40, 192–198. [Google Scholar] [CrossRef]
- Alagl, A.; Bedi, S.; Hassan, K.; AlHumaid, J. Use of platelet-rich plasma for regeneration in non-vital immature permanent teeth: Clinical and cone-beam computed tomography evaluation. J. Int. Med. Res. 2017, 45, 583–593. [Google Scholar] [CrossRef]
- Meschi, N.; EzEldeen, M.; Torres Garcia, A.E.; Jacobs, R.; Lambrechts, P. A Retrospective Case Series in Regenerative Endodontics: Trend Analysis Based on Clinical Evaluation and 2- and 3-dimensional Radiology. J. Endod. 2018, 44, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Elfrink, M.E.C.; Heijdra, J.S.C.; Krikken, J.B.; Kouwenberg-Bruring, W.H.; Kouwenberg, H.; Weerheijm, K.L.; Veerkamp, J.S.J. Regenerative endodontic therapy: A follow-up of 47 anterior traumatised teeth. Eur. Arch. Paediatr. Dent. 2021, 22, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; de Oliveira, M.L.; Cerqueira-neto, A.C.C.L.; Gomes, B.P.F.A.; Ferraz, C.C.R.; de Almeida, J.F.A.; Marciano, M.A.; de-Jesus-Soares, A. Treatment outcomes of pulp revascularization in traumatized immature teeth using calcium hydroxide and 2% chlorhexidine gel as intracanal medication. J. Appl. Oral Sci. 2020, 28, e20200217. [Google Scholar] [CrossRef] [PubMed]
- Chrepa, V.; Joon, R.; Austah, O.; Diogenes, A.; Hargreaves, K.M.; Ezeldeen, M.; Ruparel, N.B. Clinical Outcomes of Immature Teeth Treated with Regenerative Endodontic Procedures—A San Antonio Study. J. Endod. 2020, 46, 1074–1084. [Google Scholar] [CrossRef]
- Mittmann, C.W.; Kostka, E.; Ballout, H.; Preus, M.; Preissner, R.; Karaman, M.; Preissner, S. Outcome of revascularization therapy in traumatized immature incisors. BMC Oral Health 2020, 20, 207. [Google Scholar] [CrossRef]
- Linsuwanont, P.; Sinpitaksakul, P.; Lertsakchai, T. Evaluation of root maturation after revitalization in immature permanent teeth with nonvital pulps by cone beam computed tomography and conventional radiographs. Int. Endod. J. 2017, 50, 836–846. [Google Scholar] [CrossRef]
- Estefan, B.S.; Batouty, K.M.E.; Nagy, M.M.; Diogenes, A. Influence of Age and Apical Diameter on the Success of Endodontic Regeneration Procedures. J. Endod. 2016, 42, 1620–1625. [Google Scholar] [CrossRef]
- Peng, C.; Yang, Y.; Zhao, Y.; Liu, H.; Xu, Z.; Zhao, D.; Qin, M. Long-term treatment outcomes in immature permanent teeth by revascularisation using MTA and GIC as canal-sealing materials: A retrospective study. Int. J. Paediatr. Dent. 2017, 27, 454–462. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, L.-P. Radiographic outcome of necrotic immature teeth treated with two endodontic techniques: A retrospective analysis. Biomed. J. 2016, 39, 366–371. [Google Scholar] [CrossRef]
- Jeeruphan, T.; Jantarat, J.; Yanpiset, K.; Suwannapan, L.; Khewsawai, P.; Hargreaves, K.M. Mahidol Study 1: Comparison of Radiographic and Survival Outcomes of Immature Teeth Treated with Either Regenerative Endodontic or Apexification Methods: A Retrospective Study. J. Endod. 2012, 38, 1330–1336. [Google Scholar] [CrossRef]
- Bukhari, S.; Kohli, M.R.; Setzer, F.; Karabucak, B. Outcome of Revascularization Procedure: A Retrospective Case Series. J. Endod. 2016, 42, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.M.; Desmeules, M.; Cielecki, M.; Dabbagh, B.; Ferraz dos Santos, B. Longitudinal Cohort Study of Regenerative Endodontic Treatment for Immature Necrotic Permanent Teeth. J. Endod. 2017, 43, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cao, Y.; Shin, S.-J.; Shon, W.-J.; Chugal, N.; Kim, R.H.; Kim, E.; Kang, M.K. Revascularization-associated Intracanal Calcification: Assessment of Prevalence and Contributing Factors. J. Endod. 2017, 43, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.M.; Taha, S.E.E.-D.; El Sayed, M.A.; Youssef, R.; Omar, H.M. Clinical and radiographic evaluation of Biodentine and Mineral Trioxide Aggregate in revascularization of non-vital immature permanent anterior teeth (randomized clinical study). Int. J. Paediatr. Dent. 2019, 29, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.; Ahmed, H.M.A.; Şahin, Y.; Doğanay Yıldız, E.; Gündoğdu, E.C.; Güven, Y.; Khalilov, R. Regenerative Endodontic Procedures in Necrotic Mature Teeth with Periapical Radiolucencies: A Preliminary Randomized Clinical Study. J. Endod. 2019, 45, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Ragab, R.A.; Lattif, A.E.A.E.; Dokky, N.A.E.W.E. Comparative Study between Revitalization of Necrotic Immature Permanent Anterior Teeth with and without Platelet Rich Fibrin: A Randomized Controlled Trial. J. Clin. Pediatr. Dent. 2019, 43, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, H.; Peng, C. Clinical and Radiographic Assessment of the Efficacy of a Collagen Membrane in Regenerative Endodontics: A Randomized, Controlled Clinical Trial. J. Endod. 2017, 43, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.M.; AL-Deen, M.S.S.; Emam, A.A. Regenerative Endodontic Treatment of Bilateral Necrotic Immature Permanent Maxillary Central Incisors with Platelet-rich Plasma versus Blood Clot: A Split Mouth Double-blinded Randomized Controlled Trial. Int. J. Clin. Pediatr. Dent. 2019, 12, 332–339. [Google Scholar] [CrossRef]
- Botero, T.M.; Tang, X.; Gardner, R.; Hu, J.C.C.; Boynton, J.R.; Holland, G.R. Clinical Evidence for Regenerative Endodontic Procedures: Immediate versus Delayed Induction? J. Endod. 2017, 43, S75–S81. [Google Scholar] [CrossRef]
- Shivashankar, V.Y.; Johns, D.A.; Maroli, R.K.; Sekar, M.; Chandrasekaran, R.; Karthikeyan, S.; Renganathan, S.K. Comparison of the Effect of PRP, PRF and Induced Bleeding in the Revascularization of Teeth with Necrotic Pulp and Open Apex: A Triple Blind Randomized Clinical Trial. J. Clin. Diagn. Res. 2017, 11, ZC34–ZC39. [Google Scholar] [CrossRef]
- Chen, M.Y.-H.; Chen, K.-L.; Chen, C.-A.; Tayebaty, F.; Rosenberg, P.A.; Lin, L.M. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int. Endod. J. 2012, 45, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, H. An uncommon type of segmental root development after revitalization. Int. Endod. J. 2020, 53, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, M.; Golchert, K.; Torabinejad, M. Regeneration of Pulp-Dentin Complex in a Tooth with Symptomatic Irreversible Pulpitis and Open Apex Using Regenerative Endodontic Procedures. J. Endod. 2021, 47, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Gaviño Orduña, J.F.; Caviedes-Bucheli, J.; Manzanares Céspedes, M.C.; Berástegui Jimeno, E.; Martín Biedma, B.; Segura-Egea, J.J.; López-López, J. Use of Platelet-rich Plasma in Endodontic Procedures in Adults: Regeneration or Repair? A Report of 3 Cases with 5 Years of Follow-up. J. Endod. 2017, 43, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, Y.; Bakland, L.K.; Bogen, G. Combined Root Canal Therapies in Multirooted Teeth with Pulpal Disease. J. Endod. 2021, 47, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-Y.; Lee, S.-J.; Hargreaves, K.M. Biologically Based Treatment of Immature Permanent Teeth with Pulpal Necrosis: A Case Series. J. Endod. 2008, 34, 876–887. [Google Scholar] [CrossRef]
- Žižka, R.; Belák, Š.; Šedý, J.; Fačevicová, K.; Voborná, I.; Marinčák, D. Clinical and Radiographic Outcomes of Immature Teeth Treated with Different Treatment Protocols of Regenerative Endodontic Procedures: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 1600. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.; Mei, L.; Li, J. Clinical and Radiographic Outcomes in Immature Permanent Necrotic Evaginated Teeth Treated with Regenerative Endodontic Procedures. J. Endod. 2017, 43, 246–251. [Google Scholar] [CrossRef]
- Saoud, T.M.A.; Zaazou, A.; Nabil, A.; Moussa, S.; Lin, L.M.; Gibbs, J.L. Clinical and Radiographic Outcomes of Traumatized Immature Permanent Necrotic Teeth after Revascularization/Revitalization Therapy. J. Endod. 2014, 40, 1946–1952. [Google Scholar] [CrossRef]
- Dabbagh, B.; Alvaro, E.; Vu, D.-D.; Rizkallah, J.; Schwartz, S. Clinical Complications in the Revascularization of Immature Necrotic Permanent Teeth. Pediatr. Dent. 2012, 34, 4. [Google Scholar]
- Dudeja, P.G.; Grover, S.; Srivastava, D.; Dudeja, K.K.; Sharma, V. Pulp Revascularization- It’s your Future Whether you Know it or Not? J. Clin. Diagn. Res. 2015, 9, ZR01–ZR04. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, A.T.; Cehreli, Z.C. Regenerative Endodontic Treatment of Necrotic Primary Molars with Missing Premolars: A Case Series. Pediatr. Dent. 2017, 39, 131–134. [Google Scholar] [PubMed]
- Cehreli, Z.C.; Isbitiren, B.; Sara, S.; Erbas, G. Regenerative Endodontic Treatment (Revascularization) of Immature Necrotic Molars Medicated with Calcium Hydroxide: A Case Series. J. Endod. 2011, 37, 1327–1330. [Google Scholar] [CrossRef]
- Sachdeva, G.S.; Sachdeva, L.T.; Goel, M.; Bala, S. Regenerative endodontic treatment of an immature tooth with a necrotic pulp and apical periodontitis using platelet-rich plasma (PRP) and mineral trioxide aggregate (MTA): A case report. Int. Endod. J. 2015, 48, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Shimizu, E.; Gibbs, J.L.; Loghin, S.; Ricucci, D. Histologic and Histobacteriologic Observations of Failed Revascularization/Revitalization Therapy: A Case Report. J. Endod. 2014, 40, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Becerra, P.; Ricucci, D.; Loghin, S.; Gibbs, J.L.; Lin, L.M. Histologic Study of a Human Immature Permanent Premolar with Chronic Apical Abscess after Revascularization/Revitalization. J. Endod. 2014, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bao, Z.-F.; Liu, Y.; Liu, M.; Jin, X.-Q.; Xu, X.-B. Regenerative Endodontic Treatment of an Immature Permanent Tooth at an Early Stage of Root Development: A Case Report. J. Endod. 2013, 39, 719–722. [Google Scholar] [CrossRef]
- Chang, S.-W.; Oh, T.-S.; Lee, W.; Shun-Pan Cheung, G.; Kim, H.-C. Long-term observation of the mineral trioxide aggregate extrusion into the periapical lesion: A case series. Int. J. Oral Sci. 2013, 5, 54–57. [Google Scholar] [CrossRef]
- Lenzi, R.; Trope, M. Revitalization Procedures in Two Traumatized Incisors with Different Biological Outcomes. J. Endod. 2012, 38, 411–414. [Google Scholar] [CrossRef]
- Shin, S.Y.; Albert, J.S.; Mortman, R.E. One step pulp revascularization treatment of an immature permanent tooth with chronic apical abscess: A case report. Int. Endod. J. 2009, 42, 1118–1126. [Google Scholar] [CrossRef]
- Shiehzadeh, V.; Aghmasheh, F.; Shiehzadeh, F.; Joulae, M.; Kosarieh, E.; Shiehzadeh, F. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: New method and three case reports. Indian J. Dent. Res. 2014, 25, 248. [Google Scholar] [CrossRef] [PubMed]
- Plascencia, H.; Cruz, Á.; Díaz, M.; Jiménez, A.L.; Solís, R.; Bernal, C. Root Canal Filling after Revascularization/Revitalization. J. Clin. Pediatr. Dent. 2016, 40, 445–449. [Google Scholar] [CrossRef] [PubMed]
- El-Kateb, N.M.; El-Backly, R.N.; Amin, W.M.; Abdalla, A.M. Quantitative Assessment of Intracanal Regenerated Tissues after Regenerative Endodontic Procedures in Mature Teeth Using Magnetic Resonance Imaging: A Randomized Controlled Clinical Trial. J. Endod. 2020, 46, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.; Tawfik, M.; Abu Naeem, F. Evaluation of The Periapical Healing Following Pulp Revascularization Using Injectable PRF VS nonsurgical Root Canal Treatment in Mature Permanent Teeth with periapical periodontitis. A Clinical Study. Egypt. Dent. J. 2021, 67, 2663–2672. [Google Scholar] [CrossRef]
- Yoshpe, M.; Einy, S.; Ruparel, N.; Lin, S.; Kaufman, A.Y. Regenerative Endodontics: A Potential Solution for External Root Resorption (Case Series). J. Endod. 2020, 46, 192–199. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Esmaeili, S.; Fakhr Tabatabayi, S.; Ellini, M.R.; Nekoofar, M.H.; Dummer, P.M.H. Second-generation Platelet Concentrate (Platelet-rich Fibrin) as a Scaffold in Regenerative Endodontics: A Case Series. J. Endod. 2017, 43, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mehrvarzfar, P.; Abbott, P.V.; Akhavan, H.; Savadkouhi, S.T. Modified Revascularization in Human Teeth Using an Intracanal Formation of Treated Dentin Matrix: A Report of Two Cases. J. Int. Soc. Prev. Community Dent. 2017, 7, 218–221. [Google Scholar] [CrossRef]
- Cymerman, J.J.; Nosrat, A. Regenerative Endodontic Treatment as a Biologically Based Approach for Non-Surgical Retreatment of Immature Teeth. J. Endod. 2020, 46, 44–50. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Khatibi, A.H.; Verma, P.; Jamshidi, D.; Nevins, A.J.; Torabinejad, M. Clinical, Radiographic, and Histologic Outcome of Regenerative Endodontic Treatment in Human Teeth Using a Novel Collagen-hydroxyapatite Scaffold. J. Endod. 2019, 45, 136–143. [Google Scholar] [CrossRef]
- Narang, I.; Mittal, N.; Mishra, N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: A clinical study. Contemp. Clin. Dent. 2015, 6, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Meschi, N.; EzEldeen, M.; Garcia, A.E.T.; Lahoud, P.; Van Gorp, G.; Coucke, W.; Jacobs, R.; Vandamme, K.; Teughels, W.; Lambrechts, P. Regenerative Endodontic Procedure of Immature Permanent Teeth with Leukocyte and Platelet-rich Fibrin: A Multicenter Controlled Clinical Trial. J. Endod. 2021, 47, 1729–1750. [Google Scholar] [CrossRef] [PubMed]
- Meschi, N.; Hilkens, P.; Van Gorp, G.; Strijbos, O.; Mavridou, A.; Cadenas de Llano Perula, M.; Lambrichts, I.; Verbeken, E.; Lambrechts, P. Regenerative Endodontic Procedures Posttrauma: Immunohistologic Analysis of a Retrospective Series of Failed Cases. J. Endod. 2019, 45, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.M.; Salah Al-Deen, M.S.M.; Emam, A.A. Comparative evaluation of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) scaffolds in regenerative endodontic treatment of immature necrotic permanent maxillary central incisors: A double blinded randomized controlled trial. Saudi Dent. J. 2020, 32, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Ricucci, D.; Albert, J.; Alobaid, A.S.; Gibbs, J.L.; Huang, G.T.-J.; Lin, L.M. Clinical, Radiographic, and Histological Observation of a Human Immature Permanent Tooth with Chronic Apical Abscess after Revitalization Treatment. J. Endod. 2013, 39, 1078–1083. [Google Scholar] [CrossRef]
- De-Jesus-Soares, A.; Prado, M.C.; Nardello, L.C.L.; Pereira, A.C.; Cerqueira-Neto, A.C.C.L.; Nagata, J.Y.; Martinez, E.F.; Frozoni, M.; Gomes, B.P.F.A.; Pinheiro, E.T. Clinical and Molecular Microbiological Evaluation of Regenerative Endodontic Procedures in Immature Permanent Teeth. J. Endod. 2020, 46, 1448–1454. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Soares, A.J.; Souza-Filho, F.J.; Zaia, A.A.; Ferraz, C.C.R.; Almeida, J.F.A.; Gomes, B.P.F.A. Microbial Evaluation of Traumatized Teeth Treated with Triple Antibiotic Paste or Calcium Hydroxide with 2% Chlorhexidine Gel in Pulp Revascularization. J. Endod. 2014, 40, 778–783. [Google Scholar] [CrossRef]
- Nazzal, H.; Kenny, K.; Altimimi, A.; Kang, J.; Duggal, M.S. A prospective clinical study of regenerative endodontic treatment of traumatized immature teeth with necrotic pulps using bi-antibiotic paste. Int. Endod. J. 2018, 51, e204–e215. [Google Scholar] [CrossRef]
- Bose, R.; Nummikoski, P.; Hargreaves, K. A Retrospective Evaluation of Radiographic Outcomes in Immature Teeth With Necrotic Root Canal Systems Treated With Regenerative Endodontic Procedures. J. Endod. 2009, 35, 1343–1349. [Google Scholar] [CrossRef]
- Cerqueira-Neto, A.C.C.L.; Prado, M.C.; Pereira, A.C.; Oliveira, M.L.; Vargas-Neto, J.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Almeida, J.F.A.; de-Jesus-Soares, A. Clinical and Radiographic Outcomes of Regenerative Endodontic Procedures in Traumatized Immature Permanent Teeth: Interappointment Dressing or Single-Visit? J. Endod. 2021, 47, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Sutam, N.; Jantarat, J.; Ongchavalit, L.; Sutimuntanakul, S.; Hargreaves, K.M. A Comparison of 3 Quantitative Radiographic Measurement Methods for Root Development Measurement in Regenerative Endodontic Procedures. J. Endod. 2018, 44, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Virdi, M.S.; Nain, S. A Regenerative Approach for Root Canal Treatment of Mature Permanent Teeth: Comparative Evaluation with 18 Months Follow-up. Int. J. Clin. Pediatr. Dent. 2019, 12, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the Delivery of Mesenchymal Stem Cells into the Root Canal Space of Necrotic Immature Teeth after Clinical Regenerative Endodontic Procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef]
- Meschi, N.; Hilkens, P.; Lambrichts, I.; Van den Eynde, K.; Mavridou, A.; Strijbos, O.; De Ketelaere, M.; Van Gorp, G.; Lambrechts, P. Regenerative endodontic procedure of an infected immature permanent human tooth: An immunohistological study. Clin. Oral Investig. 2016, 20, 807–814. [Google Scholar] [CrossRef]
- Shah, N.; Logani, A. SealBio: A novel, non-obturation endodontic treatment based on concept of regeneration. J. Conserv. Dent. 2012, 15, 328–332. [Google Scholar] [CrossRef]
- Adnan, S.; Ullah, R. Top-cited Articles in Regenerative Endodontics: A Bibliometric Analysis. J. Endod. 2018, 44, 1650–1664. [Google Scholar] [CrossRef]
- Kahler, B.; Rossi-Fedele, G.; Chugal, N.; Lin, L.M. An Evidence-based Review of the Efficacy of Treatment Approaches for Immature Permanent Teeth with Pulp Necrosis. J. Endod. 2017, 43, 1052–1057. [Google Scholar] [CrossRef]
- Wigler, R.; Kaufman, A.Y.; Lin, S.; Steinbock, N.; Hazan-Molina, H.; Torneck, C.D. Revascularization: A Treatment for Permanent Teeth with Necrotic Pulp and Incomplete Root Development. J. Endod. 2013, 39, 319–326. [Google Scholar] [CrossRef]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Tziafas, D. Dynamics for Pulp-Dentin Tissue Engineering in Operative Dentistry. In Regenerative Dentistry; Marei, M.K., Ed.; Synthesis Lectures on Tissue Engineering; Springer International Publishing: Cham, Switzerland, 2010; pp. 111–158. ISBN 978-3-031-02581-5. [Google Scholar] [CrossRef]
- Yoshiba, K.; Yoshiba, N.; Nakamura, H.; Iwaku, M.; Ozawa, H. Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J. Dent. Res. 1996, 75, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T. Pulp and dentin tissue engineering and regeneration: Current progress. Regen. Med. 2009, 4, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laureys, W.G.M.; Cuvelier, C.A.; Dermaut, L.R.; De Pauw, G.A.M. The Critical Apical Diameter to Obtain Regeneration of the Pulp Tissue after Tooth Transplantation, Replantation, or Regenerative Endodontic Treatment. J. Endod. 2013, 39, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of Dental-Pulp-like Tissue by Chemotaxis-Induced Cell Homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Mangione, F.; Salmon, B.; EzEldeen, M.; Jacobs, R.; Chaussain, C.; Vital, S. Characteristics of Large Animal Models for Current Cell-Based Oral Tissue Regeneration. Tissue Eng. Part B Rev. 2022, 28, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, G.; Chen, Z. Pulp Regeneration: Current Approaches and Future Challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef]
- Cooke, J.P. Inflammation and Its Role in Regeneration and Repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef]
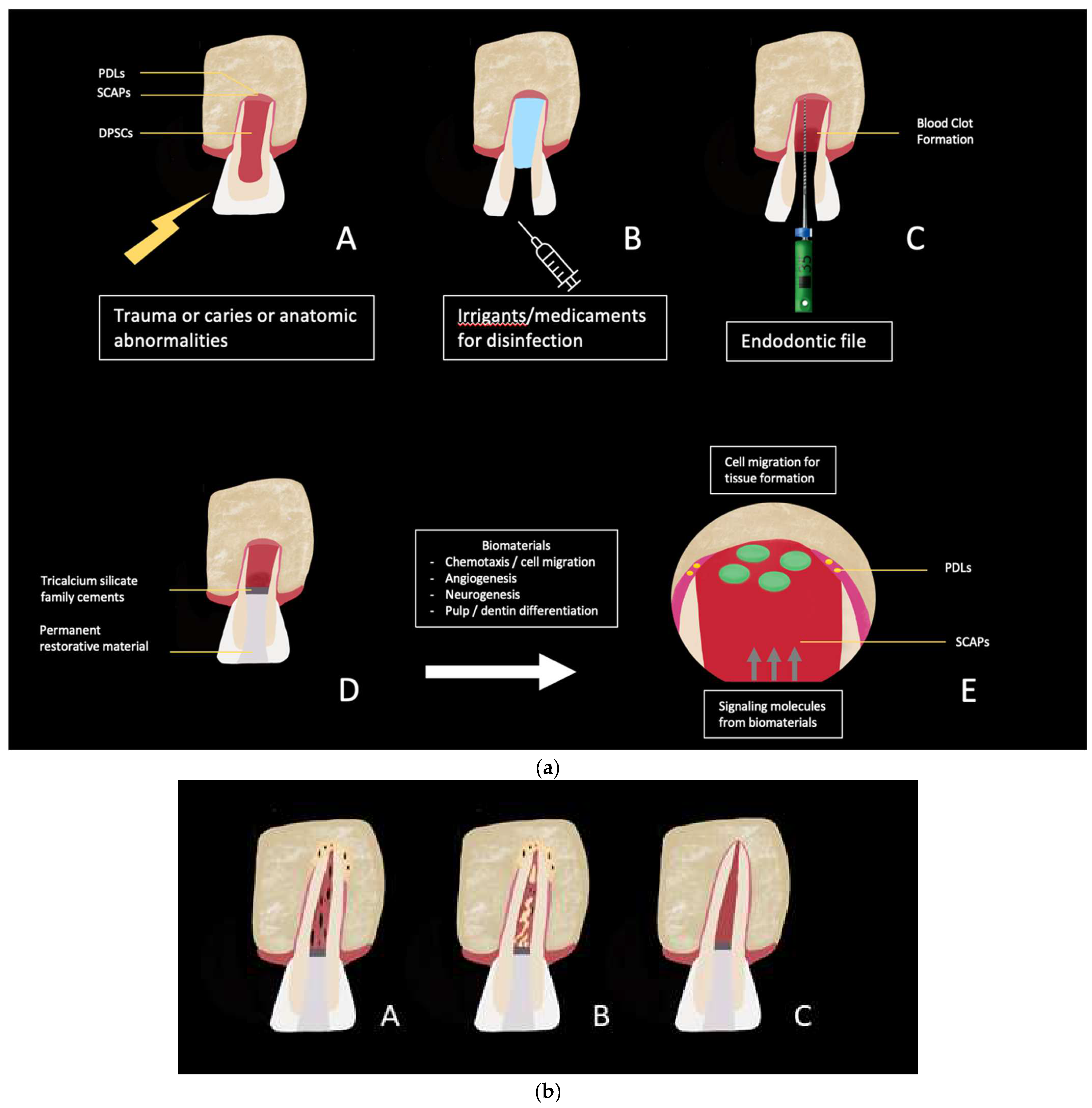


| Animal Models: Ectopic Procedure | ||||
|---|---|---|---|---|
| Assessment | Main Results | Procedure | Follow-Up | Model |
| Histology | Soft tissue formation | Root Tooth: VEGF + DPSCs + MTA Dentin slice: rBMSC + collagen scaffold + iRoot BP Human teeth roots + fibrin gel DPSCs + polymers scaffold | 12 days–3 months | Mice [20] Rats [16,18] Rabbit [17] |
| Presence of odontoblasts cells | Root Tooth: VEGF + DPSCs + MTA Dentin slice: rBMSC + collagen scaffold + iRoot BP Human teeth roots + fibrin gel DPSCs + polymers scaffold | 12 days–3 months | Mice [20] Rats [16,18] Rabbit [17] | |
| Presence of Inflammation | Polyethylene tubes: TAP vs. CHP calcium VEGF-loaded fiber + root fragment + MTA | 1.5–3 months | Mice [21] Rats [19] | |
| Presence of Vessels | Polyethylene tubes: TAP vs. CHP calcium Dentin slice: rBMSC + collagen scaffold + iRoot BP Human teeth roots + fibrin gel DPSCs + polymers scaffold | 12 days–3 months | Mice [20,21] Rats [16,18] Rabbit [17] | |
| Presence of Nerves | Dentin slice: rBMSC + collagen scaffold + iRoot BP Root Tooth: VEGF + DPSCs + MTA | 2–3 months | Mice [20] Rats [16] | |
| Presence of mineralization | Polyethylene tubes: TAP vs. CHP calcium | 3 months | Rats [19] | |
| Author/Year | Selection | Ascertainment | Causality | Reporting | Results | Finality | |
|---|---|---|---|---|---|---|---|
| Yoshpe et al., 2021 [8] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Jiang et al., 2020 [93] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Sabeti et al., 2021 [94] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Gaviño et al., 2017 [95] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Terauchi et al., 2021 [96] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Jung et al., 2008 [97] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| McTigue et al., 2013 [67] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Li et al., 2017 [99] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Saoud et al., 2014 [100] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Dabbagh et al., 2012 [101] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Dudeja et al., 2015 [102] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Ulusoy et al., 2017 [103] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Cehreli et al., 2011 [104] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Sachdeva et al., 2015 [105] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Lin et al., 2014 [106] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Becerra et al., 2014 [107] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Chen et al., 2013 [108] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Chang et al., 2013 [109] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Lenzi et al., 2012 [110] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Shin et al., 2009 [111] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Shiehzadeh et al., 2014 [112] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Plascencia et al., 2016 [113] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Yoshpe et al., 2020 [117] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Bakhtian et al., 2017 [118] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Mehrvarzfar et al., 2017 [119] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Cymerman et al., 2020 [120] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Shimizu et al., 2013 [127] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Nazzal et al., 2018 [130] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Meschi et al., 2016 [136] | 1 | 1 | 1 | 1 | 1 | 5 | Low |
| Author/Year | Selection | Outcome | Results | Finality | ||||
|---|---|---|---|---|---|---|---|---|
| Meschi et al., 2018 [72] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Elfrink et al., 2021 [73] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Pereira et al., 2020 [74] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Chrepa et al., 2020 [75] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Mittman et al., 2020 [76] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Linsuwanont et al., 2017 [77] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Estefan et al., 2016 [78] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Peng et al., 2017 [79] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Chen et al., 2016 [80] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Jeeruphan et al., 2012 [81] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Bukhari et al., 2016 [82] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Chan et al., 2017 [83] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Song et al., 2017 [84] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Zizka et al., 2021 [98] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Meschi et al., 2019 [125] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Bose et al., 2009 [131] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Shah et al., 2012 [137] | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Sutam et al., 2018 [133] | 1 | 1 | 1 | 1 | 0 | 0 | 4 | Mild |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minic, S.; Vital, S.; Chaussain, C.; Boukpessi, T.; Mangione, F. Tissue Characteristics in Endodontic Regeneration: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10534. https://doi.org/10.3390/ijms231810534
Minic S, Vital S, Chaussain C, Boukpessi T, Mangione F. Tissue Characteristics in Endodontic Regeneration: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(18):10534. https://doi.org/10.3390/ijms231810534
Chicago/Turabian StyleMinic, Sandra, Sibylle Vital, Catherine Chaussain, Tchilalo Boukpessi, and Francesca Mangione. 2022. "Tissue Characteristics in Endodontic Regeneration: A Systematic Review" International Journal of Molecular Sciences 23, no. 18: 10534. https://doi.org/10.3390/ijms231810534
APA StyleMinic, S., Vital, S., Chaussain, C., Boukpessi, T., & Mangione, F. (2022). Tissue Characteristics in Endodontic Regeneration: A Systematic Review. International Journal of Molecular Sciences, 23(18), 10534. https://doi.org/10.3390/ijms231810534







