Selective Fluorescent Probes for High-Throughput Functional Diagnostics of the Human Multidrug Transporter P-Glycoprotein (ABCB1)
Abstract
:1. Introduction
2. Results
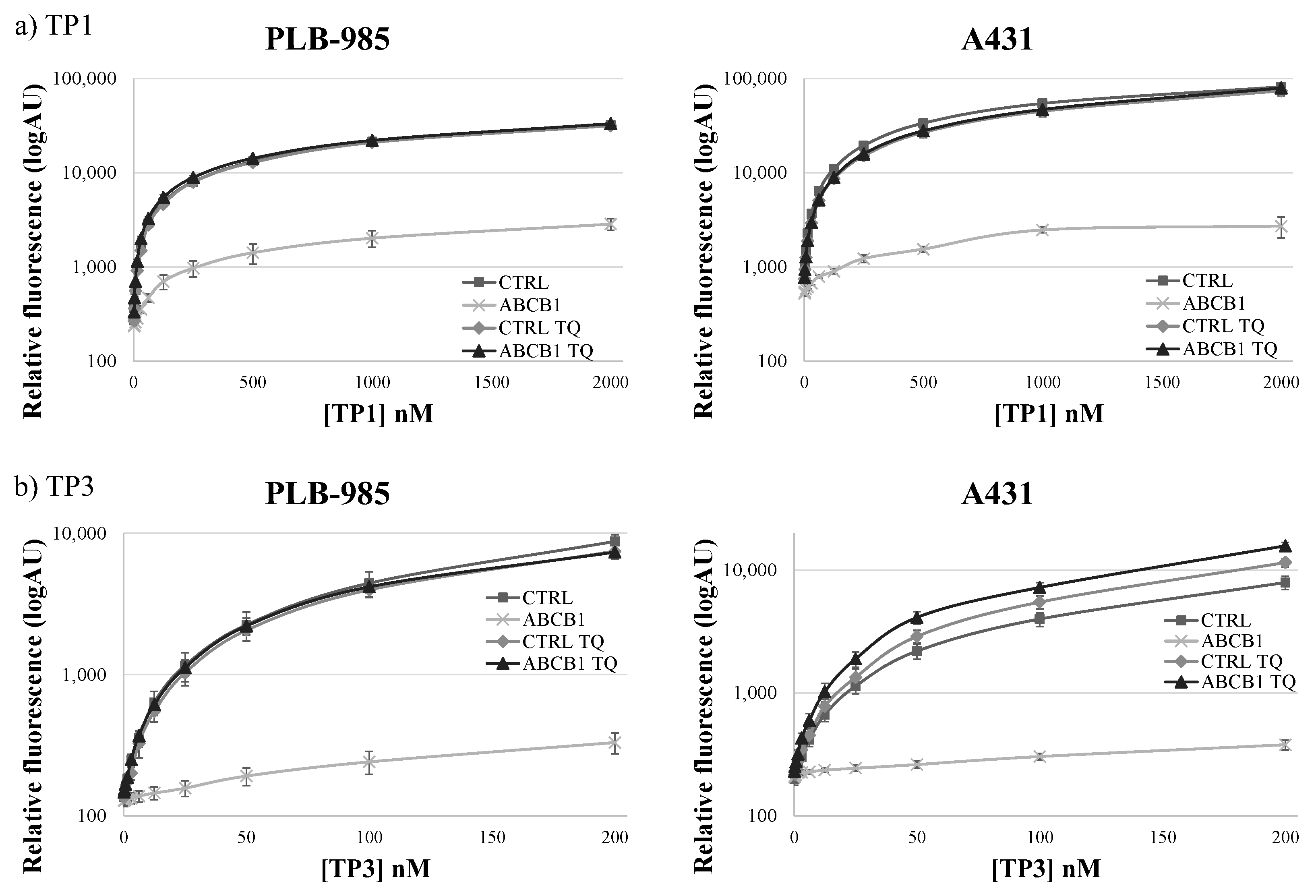
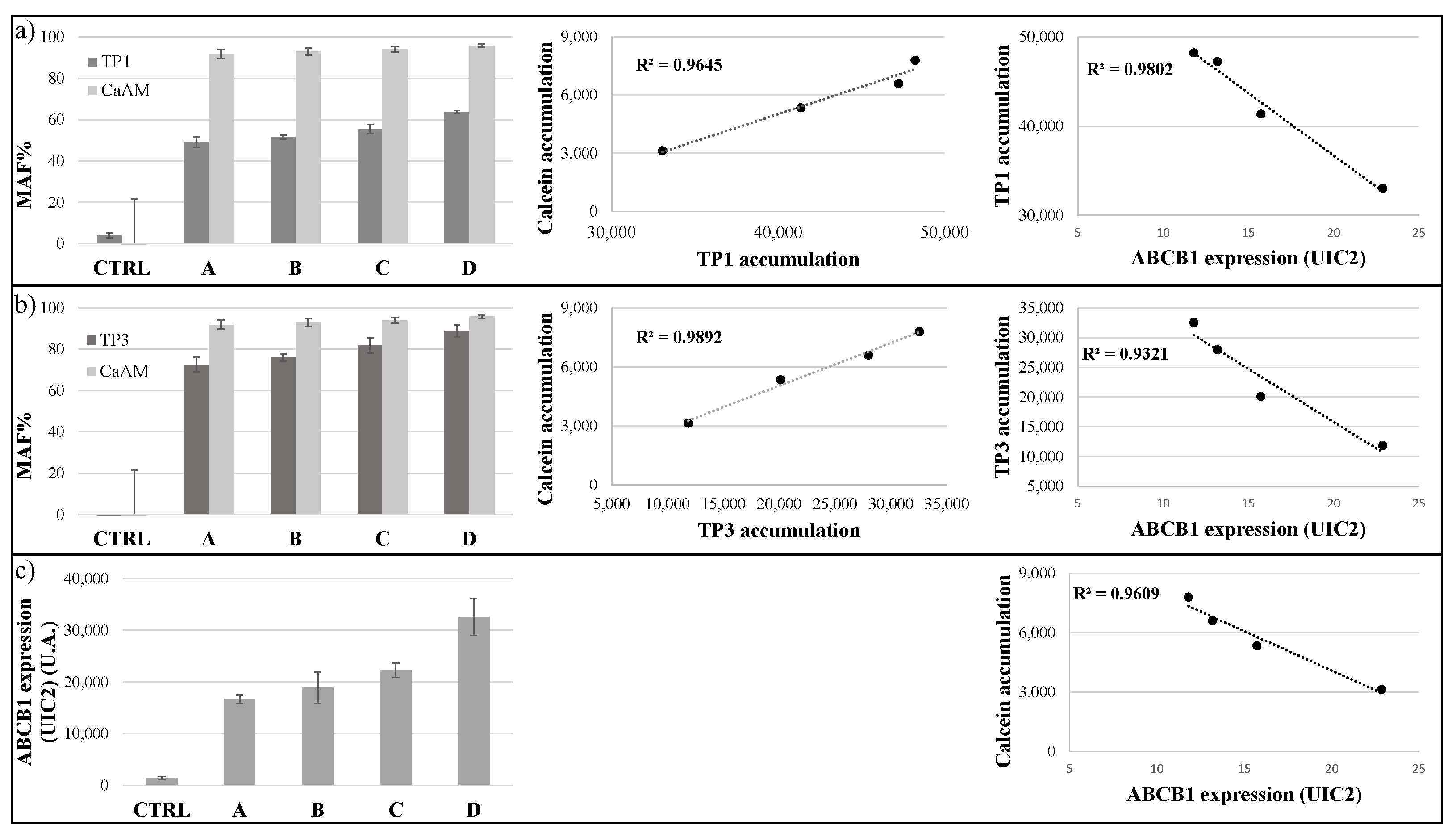
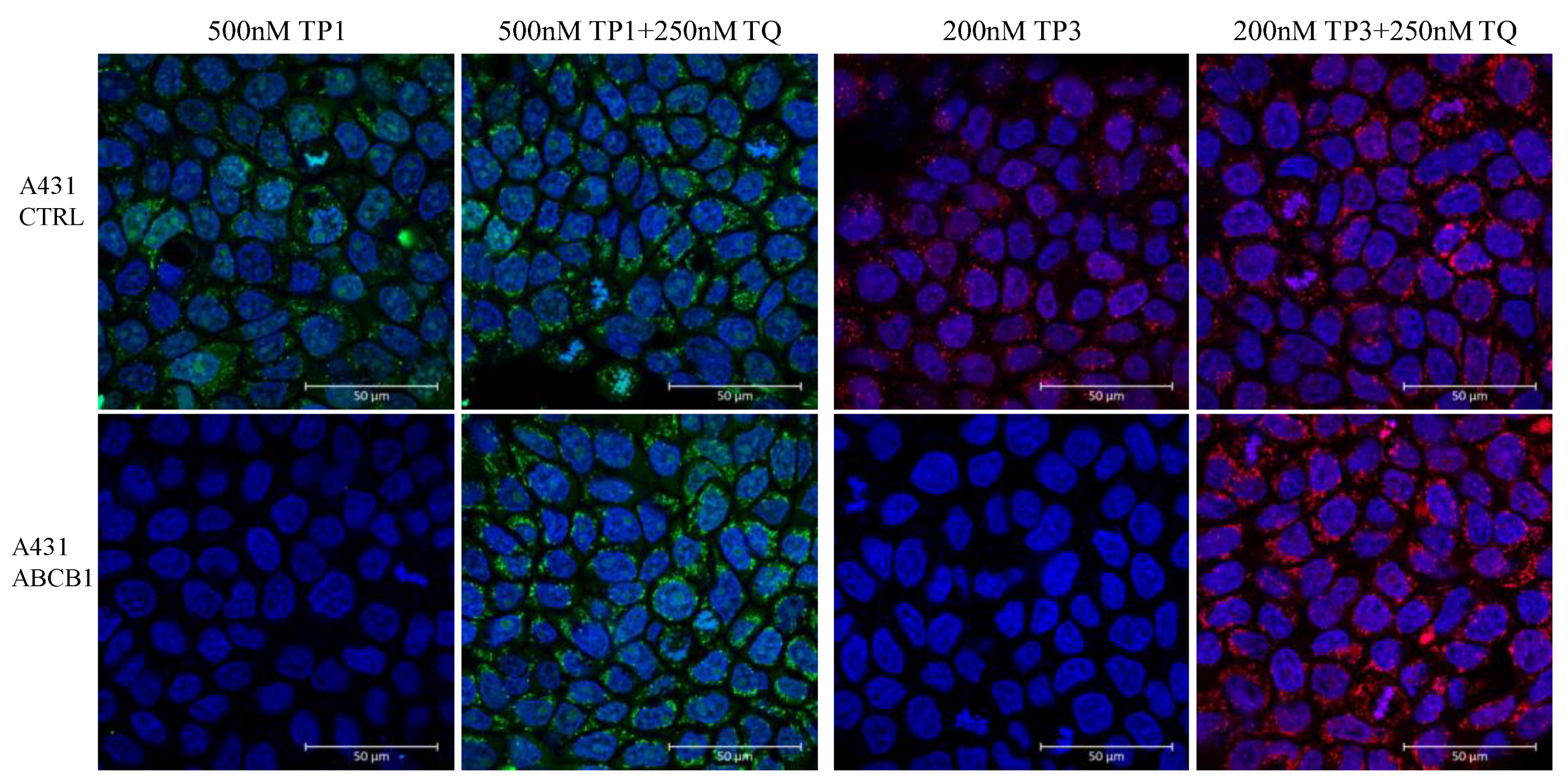
TP1 or TP3 Accumulation in Live Cells—Effects of ABC Transporters
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Flow Cytometry
4.4. Microplate-Based TO-PRO-1 or TO-PRO-3 Accumulation Measurements by Flow Cytometry
4.5. Calcein Assay
4.6. DCV Assay
4.7. Membrane Expression Studies
4.8. Flow Cytometry Data Analysis
4.9. Confocal Microscopy Images
5. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juan-Carlos, P.-D.M.; Perla-Lidia, P.-P.; Stephanie-Talia, M.-M.; Mónica-Griselda, A.-M.; Luz-María, T.-E. ABC Transporter Superfamily. An Updated Overview, Relevance in Cancer Multidrug Resistance and Perspectives with Personalized Medicine. Mol. Biol. Rep. 2021, 48, 1883–1901. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Türk, D.; Telbisz, Á.; Kucsma, N.; Horváth, T.; Szakács, G.; Homolya, L.; Sarkadi, B.; Várady, G. A New Fluorescent Dye Accumulation Assay for Parallel Measurements of the ABCG2, ABCB1 and ABCC1 Multidrug Transporter Functions. PLoS ONE 2018, 13, e0190629. [Google Scholar] [CrossRef] [PubMed]
- Zámbó, B.; Bartos, Z.; Mózner, O.; Szabó, E.; Várady, G.; Poór, G.; Pálinkás, M.; Andrikovics, H.; Hegedűs, T.; Homolya, L.; et al. Clinically Relevant Mutations in the ABCG2 Transporter Uncovered by Genetic Analysis Linked to Erythrocyte Membrane Protein Expression. Sci. Rep. 2018, 8, 7487. [Google Scholar] [CrossRef] [PubMed]
- Nerada, Z.; Hegyi, Z.; Szepesi, Á.; Tóth, S.; Hegedűs, C.; Várady, G.; Matula, Z.; Homolya, L.; Sarkadi, B.; Telbisz, Á. Application of Fluorescent Dye Substrates for Functional Characterization of ABC Multidrug Transporters at a Single Cell Level. Cytom. Part A 2016, 89, 826–834. [Google Scholar] [CrossRef]
- Szakács, G.; Váradi, A.; Özvegy-Laczka, C.; Sarkadi, B. The Role of ABC Transporters in Drug Absorption, Distribution, Metabolism, Excretion and Toxicity (ADME–Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human Multidrug Resistance ABCB and ABCG Transporters: Participation in a Chemoimmunity Defense System. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour Stem Cells and Drug Resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A Surface Glycoprotein Modulating Drug Permeability in Chinese Hamster Ovary Cell Mutants. Biochim. Biophys. Acta Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. P-Glycoprotein (ABCB1) and Oxidative Stress: Focus on Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2017, 2017, 7905486. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.B.; Kuo, C.L.; Lien, L.L.; Lien, E.J. Structure-Activity Relationship: Analyses of p-Glycoprotein Substrates and Inhibitors. J. Clin. Pharm. Ther. 2003, 28, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC Transporters as Mediators of Drug Resistance and Contributors to Cancer Cell Biology. Drug Resist. Updates 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Sehgal, A.; Kumar, A.; Uddin, M.S.; Bungau, S. The Interplay of ABC Transporters in Aβ Translocation and Cholesterol Metabolism: Implicating Their Roles in Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- Tziastoudi, M.; Pissas, G.; Raptis, G.; Cholevas, C.; Eleftheriadis, T.; Dounousi, E.; Stefanidis, I.; Theoharides, T.C. A Systematic Review and Meta-Analysis of Pharmacogenetic Studies in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 4480. [Google Scholar] [CrossRef]
- Tan, E.-K.; Chan, D.K.-Y.; Ng, P.-W.; Woo, J.; Teo, Y.Y.; Tang, K.; Wong, L.-P.; Chong, S.S.; Tan, C.; Shen, H.; et al. Effect of MDR1 Haplotype on Risk of Parkinson Disease. Arch. Neurol. 2005, 62, 460–464. [Google Scholar] [CrossRef]
- Johnson, I.D. Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies; Life Technologies Corporation: Carlsbad, CA, USA, 2010; ISBN 9780982927915. [Google Scholar]
- Sato, Y.; Yajima, S.; Taguchi, A.; Baba, K.; Nakagomi, M.; Aiba, Y.; Nishizawa, S. Trimethine Cyanine Dyes as Deep-Red Fluorescent Indicators with High Selectivity to the Internal Loop of the Bacterial A-Site RNA. Chem. Commun. 2019, 55, 3183–3186. [Google Scholar] [CrossRef]
- Milanovich, N.; Suh, M.; Jankowiak, R.; Small, G.J.; Hayes, J.M. Binding of TO-PRO-3 and TOTO-3 to DNA: Fluorescence and Hole-Burning Studies. J. Phys. Chem. 1996, 100, 9181–9186. [Google Scholar] [CrossRef]
- Van Hooijdonk, C.A.; Glade, C.P.; Van Erp, P.E. TO-PRO-3 Iodide: A Novel HeNe Laser-Excitable DNA Stain as an Alternative for Propidium Iodide in Multiparameter Flow Cytometry. Cytometry 1994, 17, 185–189. [Google Scholar] [CrossRef]
- Karasawa, A.; Michalski, K.; Mikhelzon, P.; Kawate, T. The P2X7 Receptor Forms a Dye-Permeable Pore Independent of Its Intracellular Domain but Dependent on Membrane Lipid Composition. eLife 2017, 6, e31186. [Google Scholar] [CrossRef]
- Sato, Y.; Aiba, Y.; Yajima, S.; Tanabe, T.; Higuchi, K.; Nishizawa, S. Strong Binding and Off–On Signaling Functions of Deep-Red Fluorescent TO-PRO-3 for Influenza A Virus RNA Promoter Region. ChemBioChem 2019, 20, 2752–2756. [Google Scholar] [CrossRef]
- Mai-Morente, S.P.; Marset, V.M.; Blanco, F.; Isasi, E.E.; Abudara, V. A Nuclear Fluorescent Dye Identifies Pericytes at the Neurovascular Unit. J. Neurochem. 2021, 157, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Holló, Z.; Homolya, L.; Hegedűs, T.; Müller, M.; Szakács, G.; Jakab, K.; Antal, F.; Sarkadi, B. Parallel Functional and Immunological Detection of Human Multidrug Resistance Proteins, P-Glycoprotein and MRP1. Anticancer Res. 1998, 18, 2981–2987. [Google Scholar] [PubMed]
- Homolya, L.; Holló, M.; Müller, M.; Mechetner, E.B.; Sarkadi, B. A New Method for a Quantitative Assessment of P-Glycoprotein-Related Multidrug Resistance in Tumour Cells. Br. J. Cancer 1996, 73, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Elkind, N.B.; Szentpétery, Z.; Apáti, A.; Ozvegy-Laczka, C.; Várady, G.; Ujhelly, O.; Szabó, K.; Homolya, L.; Váradi, A.; Buday, L.; et al. Multidrug Transporter ABCG2 Prevents Tumor Cell Death Induced by the Epidermal Growth Factor Receptor Inhibitor Iressa (ZD1839, Gefitinib). Cancer Res. 2005, 65, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Özvegy-Laczka, C.; Várady, G.; Köblös, G.; Ujhelly, O.; Cervenak, J.; Schuetz, J.D.; Sorrentino, B.P.; Koomen, G.-J.; Váradi, A.; Német, K.; et al. Function-Dependent Conformational Changes of the ABCG2 Multidrug Transporter Modify Its Interaction with a Monoclonal Antibody on the Cell Surface. J. Biol. Chem. 2005, 280, 4219–4227. [Google Scholar] [CrossRef]
- Morisaki, K.; Robey, R.W.; Özvegy-Laczka, C.; Honjo, Y.; Polgar, O.; Steadman, K.; Sarkadi, B.; Bates, S.E. Single Nucleotide Polymorphisms Modify the Transporter Activity of ABCG2. Cancer Chemother. Pharmacol. 2005, 56, 161–172. [Google Scholar] [CrossRef]
- McGrath, T.; Latoud, C.; Arnold, S.T.; Safa, A.R.; Felsted, R.L.; Center, M.S. Mechanisms of Multidrug Resistance in HL60 Cells. Analysis of Resistance Associated Membrane Proteins and Levels of Mdr Gene Expression. Biochem. Pharmacol. 1989, 38, 3611–3619. [Google Scholar] [CrossRef]
- Cserepes, M.; Türk, D.; Tóth, S.; Pape, V.F.S.; Gaál, A.; Gera, M.; Szabó, J.E.; Kucsma, N.; Várady, G.; Vértessy, B.G.; et al. Unshielding Multidrug Resistant Cancer through Selective Iron Depletion of P-Glycoprotein–Expressing Cells. Cancer Res. 2020, 80, 663–674. [Google Scholar] [CrossRef]
- Telford, W.G.; Bradford, J.; Godfrey, W.; Robey, R.W.; Bates, S.E. Side Population Analysis Using a Violet-Excited Cell-Permeable DNA Binding Dye. Stem Cells 2007, 25, 1029–1036. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, E.; Kulin, A.; Jezsó, B.; Kucsma, N.; Sarkadi, B.; Várady, G. Selective Fluorescent Probes for High-Throughput Functional Diagnostics of the Human Multidrug Transporter P-Glycoprotein (ABCB1). Int. J. Mol. Sci. 2022, 23, 10599. https://doi.org/10.3390/ijms231810599
Szabó E, Kulin A, Jezsó B, Kucsma N, Sarkadi B, Várady G. Selective Fluorescent Probes for High-Throughput Functional Diagnostics of the Human Multidrug Transporter P-Glycoprotein (ABCB1). International Journal of Molecular Sciences. 2022; 23(18):10599. https://doi.org/10.3390/ijms231810599
Chicago/Turabian StyleSzabó, Edit, Anna Kulin, Bálint Jezsó, Nóra Kucsma, Balázs Sarkadi, and György Várady. 2022. "Selective Fluorescent Probes for High-Throughput Functional Diagnostics of the Human Multidrug Transporter P-Glycoprotein (ABCB1)" International Journal of Molecular Sciences 23, no. 18: 10599. https://doi.org/10.3390/ijms231810599
APA StyleSzabó, E., Kulin, A., Jezsó, B., Kucsma, N., Sarkadi, B., & Várady, G. (2022). Selective Fluorescent Probes for High-Throughput Functional Diagnostics of the Human Multidrug Transporter P-Glycoprotein (ABCB1). International Journal of Molecular Sciences, 23(18), 10599. https://doi.org/10.3390/ijms231810599






