The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases
Abstract
:1. Introduction
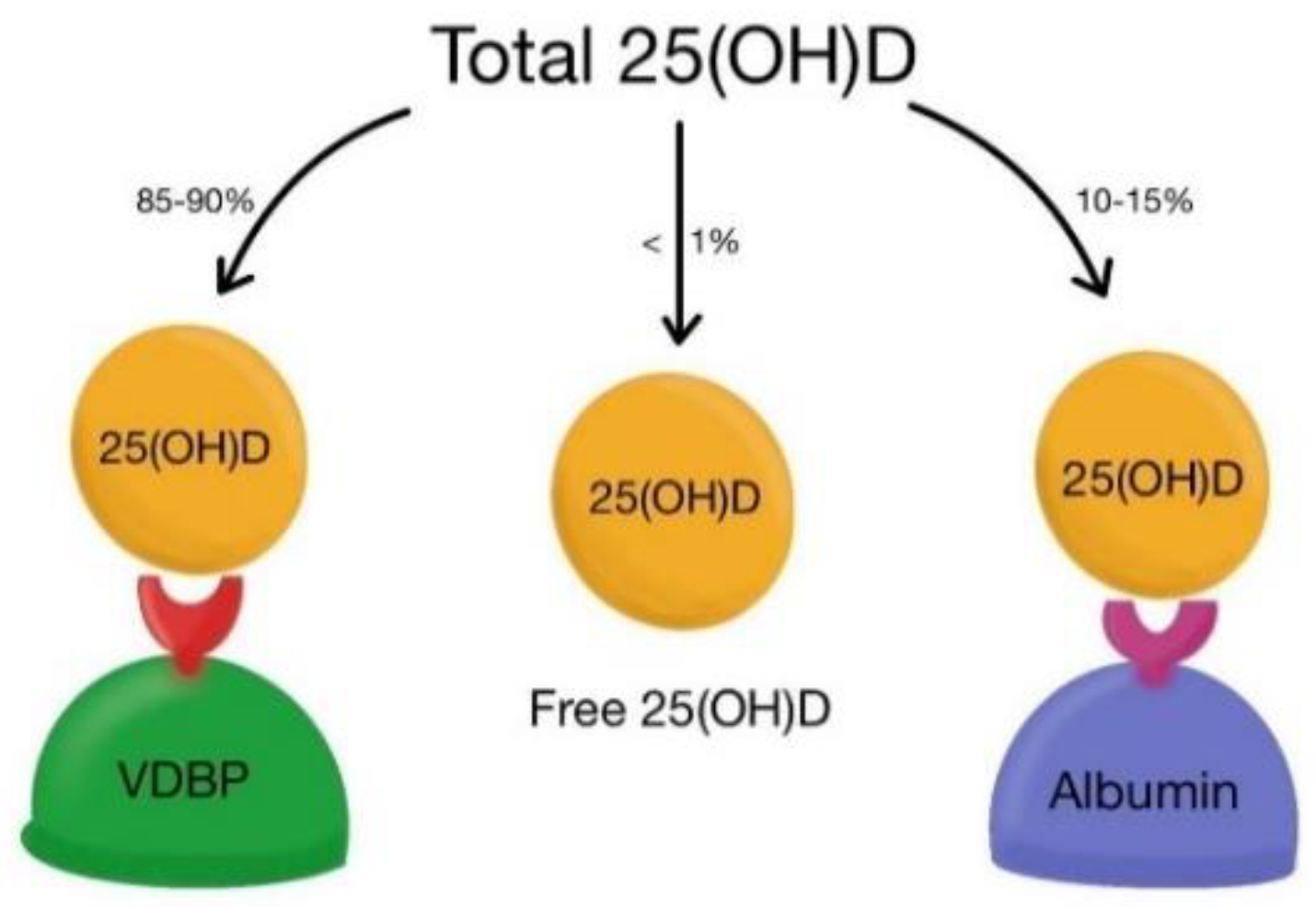
2. Vitamin D Metabolism and Its Action
3. Vitamin D in Liver Diseases
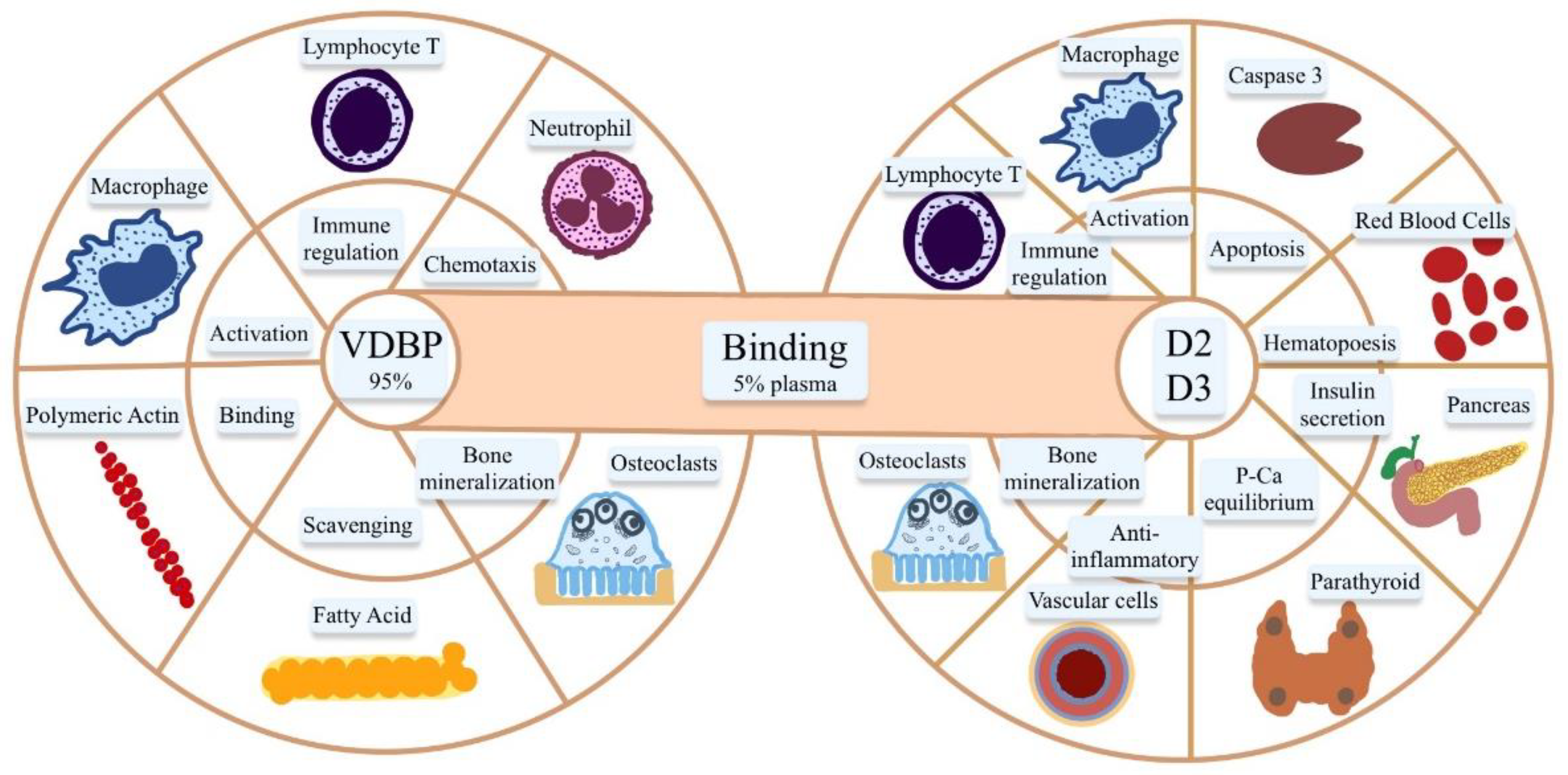
4. VDBP and Liver Disorders
5. Vitamin D Supplementation and Chronic Liver Diseases
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80 (Suppl. S6), 1689S–1696S. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Peng, X.; Porta, A.; Takanaga, H.; Peng, J.-B.; Hediger, M.A.; Fleet, J.C.; Christakos, S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 Dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 2003, 144, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Van Cromphaut, S.; Rummens, K.; Stockmans, I.; Van Herck, E.; Dijcks, F.; Ederveen, A.; Carmeliet, P.; Verhaeghe, J.; Bouillon, R. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin d receptor-independent mechanisms. J. Bone Miner. Res. 2003, 18, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; DeLuca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA. 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Sirajudeen, S.; Shah, I.; Al Menhali, A. A Narrative role of vitamin d and its receptor: With current evidence on the gastric tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef] [PubMed]
- Miraglia Del Giudice, M.; Indolfi, C.; Strisciuglio, C. Vitamin D: Immunomodulatory Aspects. J. Clin. Gastroenterol. 2018, 52, S86–S88. [Google Scholar] [CrossRef]
- Fisk, C.M.; Theobald, H.E.; Sanders, T.A.B. Fortified malted milk drinks containing low-dose ergocalciferol and cholecalciferol do not differ in their capacity to raise serum 25-Hydroxyvitamin D concentrations in healthy men and women not exposed to UV-B. J. Nutr. 2012, 142, 1286–1290. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst. Rev. 2017, 11, CD011564. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. Int. J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, S.; Wang, Y.; Guo, L.; Zhang, J.; Liu, Y.; Yang, Q. A Potential linking between vitamin d and adipose metabolic disorders. Can. J. Gastroenterol. Hepatol. 2020, 2020, 2656321. [Google Scholar] [CrossRef]
- Ng, K.V.; Nguyễn, L.T. The role of vitamin d in primary biliary cirrhosis: Possible genetic and cell signaling mechanisms. Gastroenterol. Res. Pract. 2013, 2013, 602321. [Google Scholar]
- Abramovitch, S.; Dahan-Bachar, L.; Sharvit, E.; Weisman, Y.; Ben Tov, A.; Brazowski, E.; Reif, S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut 2011, 60, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, D.; Płomiński, J.; Augustyn, K.; Cieślińska, A. rs7041 and rs4588 polymorphisms in vitamin D binding protein gene (VDBP) and the risk of diseases. Int. J. Mol. Sci. 2022, 23, 933. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Gressner, O.; Lammert, F.; Gressner, A.M. Gc-globulin: Roles in response to injury. Clin. Chem. 2006, 52, 1247–1253. [Google Scholar] [CrossRef]
- DiMartino, S.J.; Kew, R.R. Initial characterization of the vitamin D binding protein (Gc-globulin) binding site on the neutrophil plasma membrane: Evidence for a chondroitin sulfate proteoglycan. J. Immunol. 1999, 163, 2135–2142. [Google Scholar] [PubMed]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, D.; Ciesielska, A.; Płomiński, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieślińska, A. Vitamin D Binding Protein (VDBP) and its gene polymorphisms-the risk of malignant tumors and other diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D binding protein: A historic overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Schiødt, F.; Ott, P.; Tygstrup, N.; Dahl, B.; Bondesen, S. Temporal profile of total, bound, and free Gc-globulin after acetaminophen overdose. Liver Transplant. 2001, 7, 732–738. [Google Scholar] [CrossRef]
- Dahl, B.; Schiødt, F.V.; Rudolph, S.; Ott, P.; Kiær, T.; Heslet, L. Trauma stimulates the synthesis of Gc-globulin. Intensiv. Care Med. 2001, 27, 394–399. [Google Scholar] [CrossRef]
- Schiødt, F.V. Gc-globulin in liver disease. Dan. Med. Bull. 2008, 55, 131–146. [Google Scholar]
- Lee, W.M.; Emerson, D.L.; Werner, P.A.M.; Arnaud, P.; Goldschmidt-Clermont, P.; Galbraith, R.M. Decreased serum group-specific component protein levels and complexes with actin in fulminant hepatic necrosis. Hepatology 1985, 5, 271–275. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, P.J.; Lee, W.M.; Galbrait, R.M. Proportion of circulating Gc (vitamin D-binding protein) in complexed form: Relation to clinical outcome in fulminant hepatic necrosis. Gastroenterology 1988, 94, 1454–1458. [Google Scholar] [CrossRef]
- Grama, A.; Burac, L.; Aldea, C.O.; Bulata, B.; Delean, D.; Samasca, G.; Abrudan, C.; Sirbe, C.; Pop, T.L. Vitamin D-Binding Protein (Gc-Globulin) in acute liver failure in children. Diagnostics 2020, 10, 278. [Google Scholar] [CrossRef]
- Bagchi, A.; Kumar, S.; Ray, P.C.; Das, B.C.; Gumma, P.K.; Kar, P. Predictive value of serum actin-free Gc-globulin for complications and outcome in acute liver failure. J. Viral Hepat. 2014, 22, 192–200. [Google Scholar] [CrossRef]
- Kalousova, M.; Sulkova, S.D.; Zakiyanov, O.; Kostirova, M.; Safranek, R.; Tesar, V.; Zima, T. Vitamin D binding protein is not involved in vitamin D deficiency in patients with chronic kidney disease. BioMed Res. Int. 2015, 2015, 492365. [Google Scholar] [CrossRef]
- Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-binding protein in pregnancy and reproductive health. Nutrients 2020, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, M.; Zhuang, P.; Chen, G.; Dong, K.; Dong, R.; Zheng, S. Effect and mechanism of vitamin D activation disorder on liver fibrosis in biliary atresia. Sci. Rep. 2021, 11, 19883. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.T.; Elangovan, H.; Stokes, R.A.; Gunton, J.E. Vitamin d and the liver-correlation or cause? Nutrients 2018, 10, 496. [Google Scholar] [CrossRef]
- Arteh, J.; Narra, S.; Nair, S. Prevalence of Vitamin D deficiency in chronic liver disease. Am. J. Dig. Dis. 2009, 55, 2624–2628. [Google Scholar] [CrossRef]
- Elangovan, H.; Chahal, S.; Gunton, J.E. Vitamin D in liver disease: Current evidence and potential directions. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 907–916. [Google Scholar] [CrossRef]
- Stokes, C.S.; Volmer, D.A.; Grünhage, F.; Lammert, F. Vitamin D in chronic liver disease. Liver Int. 2012, 33, 338–352. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Grünhage, F.; Hochrath, K.; Krawczyk, M.; Höblinger, A.; Obermayer-Pietsch, B.; Geisel, J.; Trauner, M.; Sauerbruch, T.; Lammert, F. Common genetic variation in vitamin D metabolism is associated with liver stiffness. Hepatology 2012, 56, 1883–1891. [Google Scholar] [CrossRef]
- Bezerra, J.A.; Wells, R.G.; Mack, C.L.; Karpen, S.J.; Hoofnagle, J.H.; Doo, E.; Sokol, R.J. Biliary atresia: Clinical and research challenges for the twenty-first century. Hepatology 2018, 68, 1163–1173. [Google Scholar] [CrossRef]
- Zhuang, P.; Sun, S.; Dong, R.; Chen, G.; Huang, Y.; Zheng, S. Associations between Vitamin D and liver function and liver fibrosis in patients with biliary atresia. Gastroenterol. Res. Pract. 2019, 2019, 4621372. [Google Scholar] [CrossRef]
- Sakamoto, N.; Muraji, T.; Ohtani, H.; Masumoto, K. The accumulation of regulatory T cells in the hepatic hilar lymph nodes in biliary atresia. Surg. Today 2017, 47, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.-Y.; Dai, Y.-M.; Zhang, R.-Z.; Chen, Y.-H.; Peng, X.-F.; Fu, J.; Chen, Z.-R.; Liu, Y.-F.; Yang, L.-Y.; Wen, Z.; et al. Autoimmune liver disease-related autoantibodies in patients with biliary atresia. World J. Gastroenterol. 2018, 24, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Lages, C.S.; Simmons, J.; Maddox, A.; Jones, K.; Karns, R.; Sheridan, R.; Shanmukhappa, S.K.; Mohanty, S.; Kofron, M.; Russo, P.; et al. The dendritic cell–T helper 17–macrophage axis controls cholangiocyte injury and disease progression in murine and human biliary atresia. Hepatology 2016, 65, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Brindley, S.M.; Lanham, A.M.; Karrer, F.M.; Tucker, R.M.; Fontenot, A.P.; Mack, C.L. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology 2011, 55, 1130–1138. [Google Scholar] [CrossRef]
- Zani, A.; Quaglia, A.; Hadzić, N.; Zuckerman, M.; Davenport, M. Cytomegalovirus-associated biliary atresia: An aetiological and prognostic subgroup. J. Pediatr. Surg. 2015, 50, 1739–1745. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007, 56, 284–292. [Google Scholar] [CrossRef]
- Ng, J.; Paul, A.; Wright, N.; Hadzic, N.; Davenport, M. Vitamin D levels in infants with biliary atresia: Pre- and post-kasai portoenterostomy. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 746–750. [Google Scholar] [CrossRef]
- Dong, R.; Sun, S.; Liu, X.-Z.; Shen, Z.; Chen, G.; Zheng, S. Fat-soluble vitamin deficiency in pediatric patients with biliary atresia. Gastroenterol. Res. Pract. 2017, 2017, 7496860. [Google Scholar] [CrossRef]
- Homchan, K.; Chaiwatanarat, T.; Udomsinprasert, W.; Chongsrisawat, V.; Poovorawan, Y.; Honsawek, S. Low bone mineral density and the severity of cholestasis in biliary atresia. World J. Hepatol. 2017, 9, 746–751. [Google Scholar] [CrossRef]
- Gascon-Barré, M.; Gascon-Barré, M.; Demers, C.; Mirshahi, A.; Néron, S.; Zalzal, S.; Nanci, A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 2003, 37, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Reiter, F.P.; Ye, L.; Bösch, F.; Wimmer, R.; Artmann, R.; Ziesch, A.; Kanitz, V.; Mayr, D.; Steib, C.J.; Trauner, M.; et al. Antifibrotic effects of hypocalcemic vitamin D analogs in murine and human hepatic stellate cells and in the CCl4 mouse model. Lab. Investig. 2019, 99, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2008, 200, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A Vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Jittikoon, J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed. Pharmacother. 2018, 109, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Camma’, C.; Scazzone, C.; Tripodo, C.; Di Marco, V.; Bono, A.; Cabibi, D.; Licata, G.; Porcasi, R.; Marchesini, G.; et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010, 51, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nezu, S.; Uegaki, S.; Kikuchi, K.; Shibuya, A.; Miyakawa, H.; Takahashi, S.-I.; Bianchi, I.; Zermiani, P.; Podda, M.; et al. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J. Hepatol. 2009, 50, 1202–1209. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Koeda, M.; Yoshida, Y.; Okubo, T.; Nakagawa, A.; Itokawa, N.; Kondo, C.; Nakatsuka, K.; et al. Association of vitamin D levels and vitamin D-related gene polymorphisms with liver fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Dig. Liver Dis. 2019, 51, 1036–1042. [Google Scholar] [CrossRef]
- Hochrath, K.; Stokes, C.S.; Geisel, J.; Pollheimer, M.J.; Fickert, P.; Dooley, S.; Lammert, F. Vitamin D modulates biliary fibrosis in ABCB4-deficient mice. Hepatol. Int. 2014, 8, 443–452. [Google Scholar] [CrossRef]
- Ruiz-Gaspã, S.; Guaã±Abens, N.; Enjuanes, A.; Peris, P.; Martinez-Ferrer, A.; de Osaba, M.J.M.; Gonzalez, B.; Alvarez, L.; Monegal, A.; Combalia, A.; et al. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur. J. Clin. Investig. 2009, 40, 25–34. [Google Scholar] [CrossRef]
- Peng, C.-H.; Lee, H.-C.; Jiang, C.-B.; Hsu, C.-K.; Yeung, C.-Y.; Chan, W.-T.; Chang, S.-W.; Weng, S.-C. Serum vitamin D level is inversely associated with liver fibrosis in post Kasai’s portoenterostomy biliary atresia patients living with native liver. PLoS ONE 2019, 14, e0218896. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, M.; Zhang, R.; Shen, C.; Zhang, L.; Ding, Y.; Tang, Z.; Wang, H.; Zhang, W.; Chen, Y.; et al. Influences of Vitamin D levels and vitamin d-binding protein polymorphisms on nonalcoholic fatty liver disease risk in a Chinese Population. Ann. Nutr. Metab. 2022, 78, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation improves adipose tissue inflammation and reduces hepatic steatosis in obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt B, 369–381. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Amin, R.S.; Atteia, H.H.; Ali, M.A. Vitamin D3 intake as regulator of insulin degrading enzyme and insulin receptor phosphorylation in diabetic rats. Biomed. Pharmacother. 2017, 85, 155–159. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): An update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Barchetta, I.; Carotti, S.; Labbadia, G.; Gentilucci, U.V.; Muda, A.O.; Angelico, F.; Silecchia, G.; Leonetti, F.; Fraioli, A.; Picardi, A.; et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: Relationship with liver histology and vitamin D3 levels in patients with non-alcoholic steatohepatitis or hepatitis C virus. Hepatology 2012, 56, 2180–2187. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef]
- Chung, G.E.; Kim, D.; Kwak, M.S.; Yang, J.I.; Yim, J.Y.; Lim, S.H.; Itani, M. The serum vitamin D level is inversely correlated with non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2016, 22, 146–151. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhang, Y.; Yang, Y.; Qin, G. Association between vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Results from a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17221–17234. [Google Scholar] [PubMed]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, L.; Chen, F.H.; Zhou, Y.B. Association of serum vitamin D level and non-alcoholic fatty liver disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Izadi, A.; Aliasghari, F.; Gargari, B.P.; Ebrahimi, S. Strong association between serum Vitamin D and Vaspin Levels, AIP, VAI and liver enzymes in NAFLD patients. Int. J. Vitam. Nutr. Res. 2020, 90, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Rotundo, L.; Kothari, N.; Kim, S.-H.; Pyrsopoulos, N. Vitamin D is associated with severity and mortality of non-alcoholic fatty liver disease: A US population-based study. J. Clin. Transl. Hepatol. 2017, 5, 185–192. [Google Scholar] [CrossRef]
- Bril, F.; Maximos, M.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Subbarayan, S.; Correa, M.; Lo, M.; Suman, A.; Cusi, K. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J. Hepatol. 2015, 62, 405–411. [Google Scholar] [CrossRef]
- Anty, R.; Hastier, A.; Canivet, C.; Patouraux, S.; Schneck, A.-S.; Ferrari, P.; Ben-Amor, I.; Saint-Paul, M.C.; Gugenheim, J.; Gual, P.; et al. Severe vitamin D deficiency is not associated with liver damage in morbidly obese patients. Obes. Surg. 2016, 26, 2138–2143. [Google Scholar] [CrossRef]
- Díez Rodríguez, R.; Ballesteros Pomar, M.D.; Calleja Fernández, A.; Calleja Antolin, S.; Cano Rodríguez, I.; Linares Torres, P.; Jorquera Plaza, F.; Olcoz Goñi, J.L. Vitamin D levels and bone turnover markers are not related to non-alcoholic fatty liver disease in severely obese patients. Nutr. Hosp. 2014, 30, 1256–1262. [Google Scholar]
- Jaruvongvanich, V.; Ahuja, W.; Sanguankeo, A.; Wijarnpreecha, K.; Upala, S. Vitamin D and histologic severity of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 618–622. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Luo, F.; Liu, J.; Xiu, L.; Qin, J.; Wang, T.; Yu, N.; Wu, H.; Zou, T. The level of vitamin d in children and adolescents with nonalcoholic fatty liver disease: A meta-analysis. BioMed Res. Int. 2019, 2019, 7643542. [Google Scholar] [CrossRef]
- Patel, Y.A.; Henao, R.; Moylan, C.A.; Guy, C.D.; Piercy, D.L.; Diehl, A.M.; Abdelmalek, M.F. Vitamin D is not associated with severity in NAFLD: Results of a paired clinical and gene expression profile analysis. Am. J. Gastroenterol. 2016, 111, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Elfers, C.T.; Figlewicz, D.P.; Melhorn, S.J.; Morton, G.J.; Hoofnagle, A.; Yeh, M.M.; Nelson, J.E.; Kowdley, K.V. Vitamin D deficiency in obese rats exacerbates non-alcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology 2012, 55, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-J.; Lee, J.-E.; An, S.-M.; Lee, J.H.; Kwon, H.S.; Kim, B.C.; Kim, S.J.; Kim, J.M.; Hwang, D.Y.; Jung, Y.-J.; et al. The effects of vitamin D3 on lipogenesis in the liver and adipose tissue of pregnant rats. Int. J. Mol. Med. 2015, 36, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and non-alcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Long, Q.; Chen, F.; Zhang, T.; Wang, W. Active vitamin D impedes the progression of non-alcoholic fatty liver disease by inhibiting cell senescence in a rat model. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 513–523. [Google Scholar] [CrossRef]
- Zhu, C.-G.; Liu, Y.-X.; Wang, H.; Wang, B.-P.; Qu, H.-Q.; Wang, B.L.; Zhu, M. Active form of vitamin D ameliorates non-alcoholic fatty liver disease by alleviating oxidative stress in a high-fat diet rat model. Endocr. J. 2017, 64, 663–673. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.-H.; Fu, Y.-C.; Liu, X.-M.; Zhou, X.-H. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann. Clin. Lab. Sci. 2014, 44, 410–418. [Google Scholar]
- Zhang, H.; Shen, Z.; Lin, Y.; Zhang, J.; Zhang, Y.; Liu, P.; Zeng, H.; Yu, M.; Chen, X.; Ning, L.; et al. Vitamin D receptor targets hepatocyte nuclear factor 4α and mediates protective efects of vitamin D in non-alcoholic fatty liver disease. J. Biol. Chem. 2020, 295, 3891–3905. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Chiappetta, C.; Bertoccini, L.; Ceccarelli, V.; Capoccia, D.; Gaggini, M.; Di Cristofano, C.; Della Rocca, C.; Silecchia, G.; et al. Relationship between hepatic and systemic angiopoietin-like 3, hepatic Vitamin D receptor expression and NAFLD in obesity. Liver Int. 2020, 40, 2139–2147. [Google Scholar] [CrossRef]
- Barchetta, I.; Del Ben, M.; Angelico, F.; Di Martino, M.; Fraioli, A.; La Torre, G.; Saulle, R.; Perri, L.; Morini, S.; Tiberti, C.; et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. BMC Med. 2016, 14, 92. [Google Scholar] [CrossRef]
- Kitson, M.T.; Pham, A.; Gordon, A.; Kemp, W.; Roberts, S.K. High-dose vitamin D supplementation and liver histology in NASH. Gut 2015, 65, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Amani, R.; Hajiani, E.; Cheraghian, B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine 2014, 47, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Dadabhai, A.S.; Nanavati, J.; Wang, L.; Shinohara, R.T.; Mullin, G.E. Vitamin D levels do not predict the stage of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A PRISMA compliant systematic review and meta-analysis of pooled data. World J. Hepatol. 2018, 10, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Moosazadeh, M.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Kolahdooz, F.; Samimi, M.; Asemi, Z. The efects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), S975–S982. [Google Scholar] [CrossRef]
- Guo, X.-F.; Wang, C.; Yang, T.; Li, S.; Li, K.-L.; Li, D. Vitamin D and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 7389–7399. [Google Scholar] [CrossRef]
- Farnik, H.; Bojunga, J.; Berger, A.; Allwinn, R.; Waidmann, O.; Kronenberger, B.; Keppler, O.T.; Zeuzem, S.; Sarrazin, C.; Lange, C.M. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology 2013, 58, 1270–1276. [Google Scholar] [CrossRef]
- Hoan, N.X.; Khuyen, N.; Binh, M.T.; Giang, D.P.; Van Tong, H.; Hoan, P.Q.; Trung, N.T.; Anh, D.T.; Toan, N.L.; Meyer, C.G.; et al. Association of vitamin D deficiency with hepatitis B virus—Related liver diseases. BMC Infect. Dis. 2016, 16, 507. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, J.; Wang, J.H.; Habib, S.; Wei, W.; Sun, S.J.; Strobel, H.W.; Jia, J.D. Vitamin D serum level is associated with Child-Pugh score and metabolic enzyme imbalances, but not viral load in chronic hepatitis B patients. Medicine 2016, 95, e3926. [Google Scholar] [CrossRef]
- Chan, H.L.-Y.; Elkhashab, M.; Trinh, H.; Tak, W.Y.; Ma, X.; Chuang, W.-L.; Kim, Y.J.; Martins, E.B.; Lin, L.; Dinh, P.; et al. Association of baseline vitamin D levels with clinical parameters and treatment outcomes in chronic hepatitis B. J. Hepatol. 2015, 63, 1086–1092. [Google Scholar] [CrossRef]
- Boglione, L.; Cusato, J.; De Nicolò, A.; Cariti, G.; Di Perri, G.; D’Avolio, A. Role of CYP27B1+2838 promoter polymorphism in the treatment of chronic hepatitis B HBeAg negative with PEG-interferon. J. Viral Hepat. 2015, 22, 318–327. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Liao, Y.-T.; Chen, W.; Chen, C.-L.; Hu, J.-T.; Liu, C.-J.; Lai, M.-Y.; Chen, P.-J.; Chen, D.-S.; Yang, S.-S.; et al. Vitamin D receptor gene polymorphisms and distinct clinical phenotypes of hepatitis B carriers in Taiwan. Genes Immun. 2009, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Li, H.-Q.; Li, Z.; Liu, Y.; Gao, J.-R.; Zeng, X.-J.; Gou, C.-Y.; Zhu, X.-L.; Guo, X.-H.; Pan, L.; et al. Association of Taq I T/C and Fok I C/T polymorphisms of vitamin D receptor gene with outcome of hepatitis B virus infection. Zhonghua Yi Xue Za Zhi 2006, 86, 1952–1956. [Google Scholar] [PubMed]
- Bellamy, R.; Ruwende, C.; Corrah, T.; McAdam, K.P.W.J.; Thursz, M.; Whittle, H.C.; Hill, A.V.S. Tuberculosis and chronic hepatitis B virus infection in africans and variation in the vitamin D receptor gene. J. Infect. Dis. 1999, 179, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Miroliaee, A.; Nasiri-Toosi, M.; Khalilzadeh, O.; Esteghamati, A.; Abdollahi, A.; Mazloumi, M. Disturbances of parathyroid hormone–vitamin D axis in non-cholestatic chronic liver disease: A cross-sectional study. Hepatol. Int. 2010, 4, 634–640. [Google Scholar] [CrossRef]
- Fisher, L.; Fisher, A. Vitamin D and parathyroid hormone in outpatients with Noncholestatic chronic liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, 513–520. [Google Scholar] [CrossRef]
- Crawford, B.A.L.; Kam, C.; Donaghy, A.J.; Mccaughan, G. The heterogeneity of bone disease in cirrhosis: A multivariate analysis. Osteoporos. Int. 2003, 14, 987–994. [Google Scholar] [CrossRef]
- Avihingsanon, A.; Jitmitraparp, S.; Tangkijvanich, P.; Ramautarsing, R.A.; Apornpong, T.; Jirajariyavej, S.; Putcharoen, O.; Treeprasertsuk, S.; Akkarathamrongsin, S.; Poovorawan, Y.; et al. Advanced liver fibrosis by transient elastography, Fibrosis 4, and alanine aminotransferase/platelet ratio index among Asian hepatitis C with and without human immunodeficiency virus infection: Role of vitamin D levels. J. Gastroenterol. Hepatol. 2014, 29, 1706–1714. [Google Scholar] [CrossRef]
- Lange, C.M.; Bojunga, J.; Ramos-Lopez, E.; von Wagner, M.; Hassler, A.; Vermehren, J.; Herrmann, E.; Badenhoop, K.; Zeuzem, S.; Sarrazin, C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 2011, 54, 887–893. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Jones, K.A.; Flores, R.; Singhania, A.; Woelk, C.H.; Schooley, R.T.; Wyles, D.L. Vitamin D metabolites inhibit hepatitis c virus and modulate cellular gene expression. J. Virol. Antivir. Res. 2014, 3. [Google Scholar] [CrossRef]
- Yuasa, K.; Naganuma, A.; Sato, K.; Ikeda, M.; Kato, N.; Takagi, H.; Mori, M. Zinc is a negative regulator of hepatitis C virus RNA replication. Liver Int. 2006, 26, 1111–1118. [Google Scholar] [CrossRef]
- Kitson, M.T.; Sarrazin, C.; Toniutto, P.; Eslick, G.D.; Roberts, S.K. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: A systematic review and meta-analysis. J. Hepatol. 2014, 61, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, M.; Pineda-Tenor, D.; Jiménez-Sousa, M.; Rodríguez, A.F.; Guzmán-Fulgencio, M.; Resino, S. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: A meta-analysis. Hepatology 2014, 60, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Belle, A.; Gizard, E.; Conroy, G.; Lopez, A.; Bouvier-Alias, M.; Rouanet, S.; Peyrin-Biroulet, L.; Pawlotsky, J.-M.; Bronowicki, J. 25-OH vitamin D level has no impact on the efficacy of antiviral therapy in naïve genotype 1 HCV-infected patients. United Eur. Gastroenterol. J. 2017, 5, 69–75. [Google Scholar] [CrossRef]
- Loftfield, E.; O’Brien, T.R.; Pfeiffer, R.M.; Howell, C.D.; Horst, R.; Prokunina-Olsson, L.; Weinstein, S.J.; Albanes, D.; Morgan, T.R.; Freedman, N.D. Vitamin D Status and Virologic Response to HCV Therapy in the HALT-C and VIRAHEP-C Trials. PLoS ONE 2016, 11, e0166036. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Lapidus, N.; Pol, S.; Serfaty, L.; Ratziu, V.; Asselah, T.; Thibault, V.; Souberbielle, J.C.; Carrat, F.; Cacoub, P. Vitamin D in addition to peg-interferon-alpha/ribavirin in chronic hepatitis C virus infection: ANRS-HC25-VITAVIC study. World J. Gastroenterol. 2015, 21, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Abu-Mouch, S.; Fireman, Z.; Jarchovsky, J.; Zeina, A.R.; Assy, N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J. Gastroenterol. 2011, 17, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Nimer, A.; Mouch, A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J. Gastroenterol. 2012, 18, 800–805. [Google Scholar] [CrossRef]
- Grasso, A.; Malfatti, F.; De Leo, P.; Martines, H.; Fabris, P.; Toscanini, F.; Anselmo, M.; Menardo, G. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J. Hepatol. 2009, 51, 984–990. [Google Scholar] [CrossRef]
- Yokoyama, S.; Takahashi, S.; Kawakami, Y.; Hayes, C.N.; Kohno, H.; Kohno, H.; Tsuji, K.; Aisaka, Y.; Kira, S.; Yamashina, K.; et al. Effect of vitamin D supplementation on pegylated interferon/ribavirin therapy for chronic hepatitis C genotype 1b: A randomized controlled trial. J. Viral Hepat. 2013, 21, 348–356. [Google Scholar] [CrossRef]
- Saron, M.L.G.; Godoy, H.T.; Hessel, G. Nutritional status of patients with biliary atresia and autoimmune hepatitis related to serum levels of vitamins A., D and E. Arq. Gastroenterol. 2009, 46, 62–68. [Google Scholar] [CrossRef]
- Smyk, D.S.; Orfanidou, T.; Invernizzi, P.; Bogdanos, D.P.; Lenzi, M. Vitamin D in autoimmune liver disease. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Kav, T.; Aydin, C.; Cengiz, M.; Imga, N.N.; Purnak, T.; Smyk, D.S.; Torgutalp, M.; Turhan, T.; Ozenirler, S.; et al. Low serum vitamin d levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Am. J. Dig. Dis. 2014, 59, 3035–3042. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Emerging therapeutic biomarkers of autoimmune hepatitis and their impact on current and future management. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Luong, K.V.; Nguyen, L.T. The role of vitamin d in autoimmune hepatitis. J. Clin. Med. Res. 2013, 5, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Nasiroglu, I.N.; Efe, C. Editorial: The role of vitamin D in autoimmune hepatitis. Aliment. Pharmacol. Ther. 2019, 49, 342–343. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, T.; Shinoda, M.; Tanaka, Y.; Kuno, T.; Hattori, A.; Usui, T.; Kuno, N.; Osaka, T. Autoantibodies against CYP2D6 and other drug-metabolizing enzymes in autoimmune hepatitis type 2. Drug Metab. Rev. 2005, 37, 235–252. [Google Scholar] [CrossRef]
- Agarwal, K.; Czaja, A.J.; Donaldson, P.T. A functional Fas promoter polymorphism is associated with a severe phenotype in type 1 autoimmune hepatitis characterized by early development of cirrhosis. Tissue Antigens 2007, 69, 227–235. [Google Scholar] [CrossRef]
- Sirbe, C.; Simu, G.; Szabo, I.; Grama, A.; Pop, T.L. Pathogenesis of autoimmune hepatitis—Cellular and molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 13578. [Google Scholar] [CrossRef]
- Vogel, A.; Strassburg, C.P.; Manns, M.P. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology 2002, 35, 126–131. [Google Scholar] [CrossRef]
- Wildin, R.S.; Smyk-Pearson, S.; Filipovich, A.H. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med Genet. 2002, 39, 537–545. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.K. Function of γδ T cells in tumor immunology and their application to cancer therapy. Exp. Mol. Med. 2021, 53, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Seif, A.A.; Abdelwahed, D.M. Vitamin D ameliorates hepatic ischemic/reperfusion injury in rats. J. Physiol. Biochem. 2014, 70, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, M.; Chang, Y.; Kim, J.; Lee, J. Abstracts of the 37th ESPEN Congress, Lisbon, Portugal, 5–8 September 2015SUN-PP010: The vitamin D analogue paricalcitol attenuates hepatic ischemia/reperfusion injury by modulation of Tlr4-mediated signaling in rats. Clin. Nutr. 2015, 34, S26–S27. [Google Scholar] [CrossRef]
- Trautwein, C.; Possienke, M.; Schlitt, H.J.; Böker, K.H.; Horn, R.; Raab, R.; Manns, M.P.; Brabant, G. Bone density and metabolism in patients with viral hepatitis and cholestatic liver diseases before and after liver transplantation. Am. J. Gastroenterol. 2000, 95, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Bitetto, D.; Fabris, C.; Falleti, E.; Fornasiere, E.; Fumolo, E.; Fontanini, E.; Cussigh, A.; Occhino, G.; Baccarani, U.; Pirisi, M.; et al. Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int. 2010, 30, 417–444. [Google Scholar] [CrossRef]
- Xiao, K.; Zhang, D.-C.; Hu, Y.; Song, L.-C.; Xu, J.-Q.; He, W.-X.; Pan, P.; Wang, Y.-W.; Xie, L.-X. Potential roles of vitamin D binding protein in attenuating liver injury in sepsis. Mil. Med. Res. 2022, 9, 4. [Google Scholar] [CrossRef]
- Liu, H.; Han, T.; Xiao, S.X.; Li, J.; Lee, J.; Li, Y.; Zhu, Z.Y.; Xu, Y.Q. Plasma actin-free gc-globulin in patients with chronic or acute-on-chronic liver failure caused by hepatitis B virus. Gastroenterol. Res. 2009, 2, 213–219. [Google Scholar] [CrossRef]
- Brown, I.; Sood, A.; Carter, N.D. Vitamin D binding globulin levels and affinity in various clinical conditions. J. Clin. Pathol. 1980, 33, 966–970. [Google Scholar] [CrossRef]
- Huang, C.-Z.; Zhang, J.; Zhang, L.; Yu, C.-H.; Mo, Y.; Mo, L.-Y. Serum vitamin D and vitamin-D-binding protein levels in children with chronic hepatitis B. World J. Gastroenterol. 2021, 27, 255–266. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, W.W.; Jiang, W.Y.; Li, C.Q.; Guo, J.C.; Xun, Y.H. Low vitamin D levels are associated with high viral loads in patients with chronic hepatitis B: A systematic review and meta-analysis. BMC Gastroenterol. 2019, 19, 84. [Google Scholar] [CrossRef]
- Gotlieb, N.; Tachlytski, I.; Lapidot, Y.; Sultan, M.; Safran, M.; Ben-Ari, Z. Hepatitis B virus downregulates vitamin D receptor levels in hepatoma cell lines, thereby preventing vitamin D-dependent inhibition of viral transcription and production. Mol. Med. 2018, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Thanapirom, K.; Suksawatamnuay, S.; Sukeepaisarnjaroen, W.; Treeprasertsuk, S.; Tanwandee, T.; Charatcharoenwitthaya, P.; Thongsawat, S.; Leerapun, A.; Piratvisuth, T.; Boonsirichan, R.; et al. Vitamin D-binding protein gene polymorphism predicts pegylated interferon-related HBsAg seroclearance in HBeAg-negative thai chronic hepatitis B patients: A multicentre study. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Falleti, E.; Bitetto, D.; Fabris, C.; Fattovich, G.; Cussigh, A.; Cmet, S.; Ceriani, E.; Fornasiere, E.; Pasino, M.; Ieluzzi, D.; et al. Vitamin D binding protein gene polymorphisms and baseline vitamin D levels as predictors of antiviral response in chronic hepatitis C. Hepatology 2012, 56, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Schiødt, F.V.; Bondesen, S.; Müller, K.; Rasmussen, A.; Hjortrup, A.; Kirkegaard, P.; Hansen, B.A.; Tygstrup, N.; Ott, P. Reconstitution of the actin-scavenger system after orthotopic liver transplantation for end-stage liver disease: A prospective and longitudinal study. Liver Transplant. Surg. 1999, 5, 310–317. [Google Scholar] [CrossRef]
- Moore, M.P.; Cunningham, R.P.; Dashek, R.J.; Mucinski, J.M.; Rector, R.S. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity 2020, 28, 1843–1852. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D incorporation in foods: Formulation strategies, stability, and bioaccessibility as affected by the food matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, T.L.; Sîrbe, C.; Benţa, G.; Mititelu, A.; Grama, A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10705. https://doi.org/10.3390/ijms231810705
Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. International Journal of Molecular Sciences. 2022; 23(18):10705. https://doi.org/10.3390/ijms231810705
Chicago/Turabian StylePop, Tudor Lucian, Claudia Sîrbe, Gabriel Benţa, Alexandra Mititelu, and Alina Grama. 2022. "The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases" International Journal of Molecular Sciences 23, no. 18: 10705. https://doi.org/10.3390/ijms231810705
APA StylePop, T. L., Sîrbe, C., Benţa, G., Mititelu, A., & Grama, A. (2022). The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. International Journal of Molecular Sciences, 23(18), 10705. https://doi.org/10.3390/ijms231810705






