Zygo-Albuside A: New Saponin from Zygophyllum album L. with Significant Antioxidant, Anti-Inflammatory and Antiapoptotic Effects against Methotrexate-Induced Testicular Damage
Abstract
:1. Introduction
2. Results
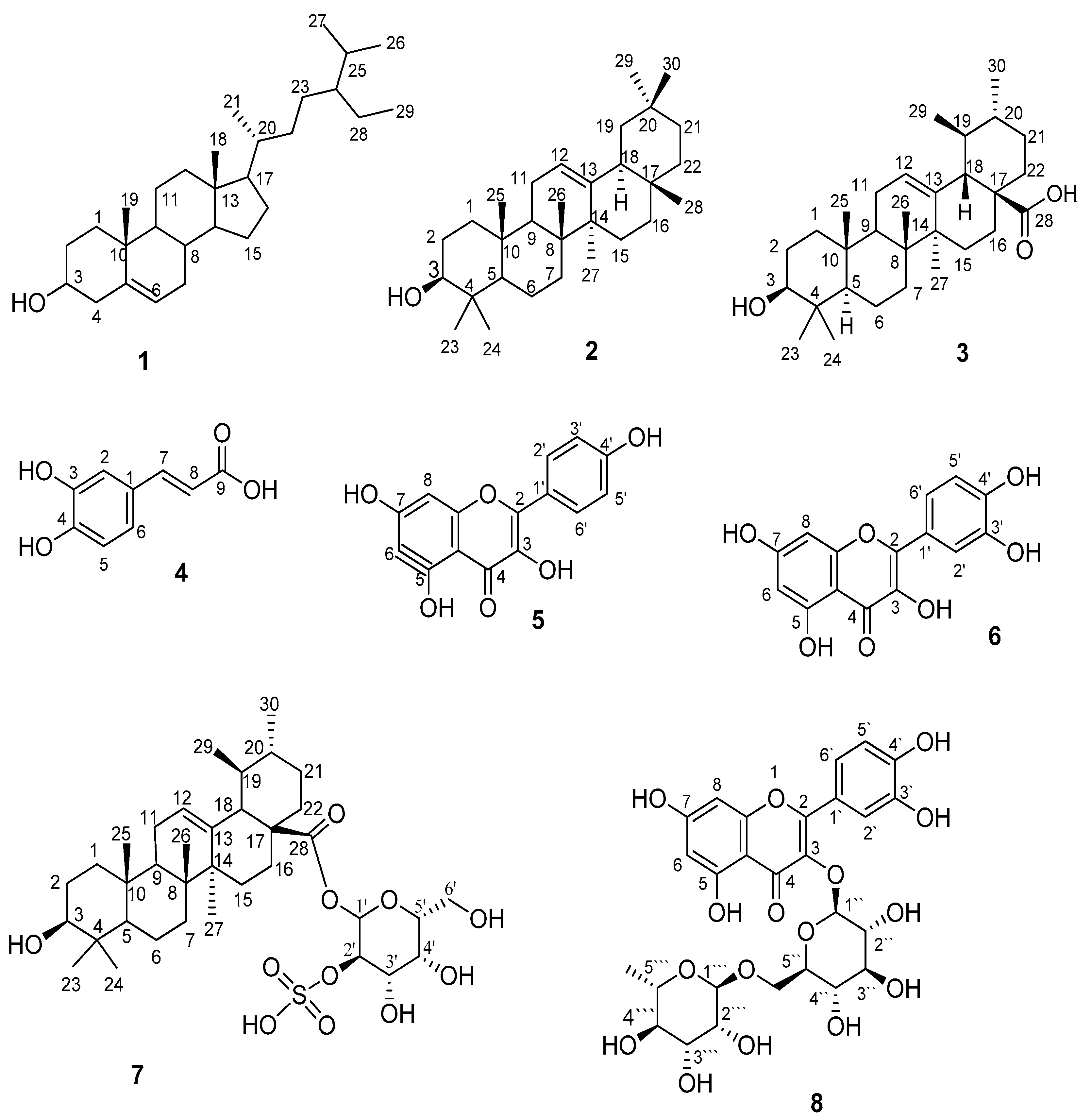
2.1. Structure Elucidation of the Isolated Compounds
2.2. In Vitro Antioxidant Assessment of Compound 7 (Zygo-Albuside A)
2.3. In Vivo Evaluation of Z. album Extract, Compound 7 (Zygo-Albuside A) and Compound 8 (Rutin)
2.3.1. Effect on Liver and Kidney Function Markers (Preliminary Study)
2.3.2. Reversing Serum Testosterone Levels in the MTX-Administrated Mice
2.3.3. Restoring the Antioxidant Activity in the MTX-Administrated Mice
2.3.4. Reduction of Inflammation in the MTX-Administrated Mice
2.3.5. Prevention of Apoptosis in the MTX-Administrated Mice
2.3.6. Inhibition of mi-RNA 29a Expression in MTX-Administrated Mice
2.3.7. Improvement of the Histological Damage in the Testis
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Acid Hydrolysis of Compound 7
4.5. Detection of the Sulfate Group
4.6. Spectroscopic Data of the Isolated Compounds
4.6.1. Compound (1) (Rf = 0.25, 0.41 and 0.83; Mobile Phase: 25% EtOAc in Hexane, CHCl3/MeOH/AcOH (97:2:1) and Petroleum Ether/CHCl3/MeOH (49:50:1), Respectively [80,124,125]
4.6.2. Compound (2) (Rf = 0.39, 0.57 and 0.58; Mobile Phase: 25% EtOAc in Hexane, CHCl3/MeOH (97:3) and Toluene/MeOH (9:1) and, Respectively [80,126,127]
4.6.3. Compound (3) (Rf = 0.30, 0.64 and 0.71; Mobile Phase: Benzene/Toluene (1:4), Toluene/EtOAc/AcOH (6:3:1) and 3% MeOH in CHCl3, Respectively [78,79,80]
4.6.4. Compound (4) (Rf = 0.35, 0.79 and 0.96; Mobile Phase: Toluene/EtOAc/HCOOH (6:3:1), n-Butanol/AcOH/H2O (4:1:5) and EtOAc/AcOH/HCOOH/H2O (100:11:11:26), Respectively [79,82,128]
4.6.5. Compound (5) (Rf = 0.26, 0.55 and 0.58; Mobile Phase: Toluene/EtOAc/HCOOH (7:3:0.1), Forestal = conc. HCl/AcOH/H2O (3:30:10) and PhOH/H2O (3:1), Respectively [82,129]
4.6.6. Compound (6) (Rf = 0.29, 0.41 and 0.9; Mobile Phase: PhOH/H2O (3:1), Forestal = conc. HCl/AcOH/H2O (3:30:10) and EtOAc/AcOH/H2O (7.5:1.5:1.5), Respectively [80,130]
4.6.7. Compound (7) (Rf for the aglycone (Rf = 0.30, 0.64 and 0.71 Mobile Phase: Benzene/Toluene (1:4), Toluene/EtOAc/AcOH (6:3:1) and 3% MeOH in CHCl3, Respectively. [78,79,80]. Rf for the Sugar Part = 0.21 0.38 and 0.39; Mobile Phase: n-Butanol/Benzene/Pyridine/H2O (5:1:3:3), PhOH satd. with H2O and Propanol/H2O (8.5:1.5), respectively) [79,80]
4.6.8. Compound (8) (Rf = 0.24, 0.28 and 0.45; Mobile Phase: EtOAc/AcOH/HCOOH/H2O (100:10:10:18), EtOAc/AcOH/H2O (7.5:1.5:1.5) and EtOAc/AcOH/HCOOH/H2O (100:11:11:26) [130,131,132]
4.7. In Vitro Antioxidant Assay of Compound 7
4.7.1. DPPH Radical Scavenging Activity
4.7.2. Hydrogen Peroxide Radical (H2O2) Scavenging Activity
4.7.3. Total Antioxidant Capacity (TAC)
4.8. In Vivo Investigation of the Protective Effects of Z. album Extract, Compound 7 and Compound 8 against MTX-Induced Testicular Injury
4.8.1. Preliminary Assessment of the Liver and Kidney Toxicity
4.8.2. Animals and Treatment (the Principle In Vivo Study)
4.8.3. Tissue Collection
4.8.4. Biochemical Analysis
Measurement of Testosterone
Determination of the Level of Oxidative Stress in the Testis
Determination of Testicular Inflammation and Apoptosis Mediators
Determination of Testicular Expression of NF-κB, TNF-α, p53, and miR-29a by Quantitative Real-Time Polymerase Chain Reaction
4.8.5. Histological Analysis
4.8.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, Z.A.; Tripathi, R.; Mishra, B. Methotrexate: A detailed review on drug delivery and clinical aspects. Expert Opin. Drug Deliv. 2012, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Armagan, A.; Uzar, E.; Uz, E.; Yilmaz, H.R.; Kutluhan, S.; Koyuncuoglu, H.R.; Soyupek, S.; Cam, H.; Serel, T.A. Caffeic acid phenethyl ester modulates methotrexate-induced oxidative stress in testes of rat. Hum. Exp. Toxicol. 2008, 27, 547. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Ochaoga, S.E.; Odunola, O.A.; Farombi, E.O. Protocatechuic acid inhibits testicular and epididymal toxicity associated with methotrexate in rats. Andrologia 2019, 51, e13350. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, F.; Khazaei, M.; Rabzia, A.; Mansouri, K.; Ghanbari, A. Protective Effects of Thymoquinone against Methotrexate-Induced Germ Cell Apoptosis in Male Mice. Int. J. Fertil. Steril. 2016, 9, 541. [Google Scholar]
- Vardi, N.; Parlakpinar, H.; Ates, B.; Cetin, A.; Otlu, A. Antiapoptotic and antioxidant effects of beta-carotene against methotrexate-induced testicular injury. Fertil. Steril. 2009, 92, 2028. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, T.T.; Zhao, H.Y.; Wang, H. Melatonin protects methotrexate-induced testicular injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7517. [Google Scholar]
- Belhan, S.; Çomaklı, S.; Küçükler, S.; Gülyüz, F.; Yıldırım, S.; Yener, Z. Effect of chrysin on methotrexate-induced testicular damage in rats. Andrologia 2019, 51, e13145. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Q.; Jiang, C.; Chen, C.; Liu, Y.; Chen, Y.; Zeng, Y. MicroRNA-29a is involved lipid metabolism dysfunction and insulin resistance in C2C12 myotubes by targeting PPARδ. Mol. Med. Rep. 2018, 17, 8493. [Google Scholar] [CrossRef]
- Jafarinejad-Farsangi, S.; Farazmand, A.; Mahmoudi, M.; Gharibdoost, F.; Karimizadeh, E.; Noorbakhsh, F.; Faridani, H.; Jamshidi, A.R. MicroRNA-29a induces apoptosis via increasing the Bax:Bcl-2 ratio in dermal fibroblasts of patients with systemic sclerosis. Autoimmunity 2015, 48, 369. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, D.; Wang, L.; Wang, G.; Zhu, Y. Inhibiting MicroRNA-29a Protects Myocardial Ischemia-Reperfusion Injury by Targeting SIRT1 and Suppressing Oxidative Stress and NLRP3-Mediated Pyroptosis Pathway. J. Pharmacol. Exp. Ther. 2020, 372, 128. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O.; Al-Mutabagani, L.A.; Sarhan, O.M. Ginkgo biloba Extract Attenuates Methotrexate-Induced Testicular Injury in Rats: Cross-talk Between Oxidative Stress, Inflammation, Apoptosis, and miRNA-29a Expression. Integr. Cancer Ther. 2020, 19, 1534735420969814. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.S.; Abdelhameed, R.F.A.; Mehanna, E.T.; Wahba, A.S.; Elfaky, M.A.; Koshak, A.E.; Noor, A.O.; Bogari, H.A.; Malatani, R.T.; Goda, M.S. Metabolic Profiling, Chemical Composition, Antioxidant Capacity, and In Vivo Hepato- and Nephroprotective Effects of Sonchus cornutus in Mice Exposed to Cisplatin. Antioxidants 2022, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Elhady, S.S.; Nafie, M.S.; Ahmed, H.A.; Abo-Elmatty, D.M.; Ahmed, S.A.; Badr, J.M.; Abdel-Hamed, A.R. The Antioxidant Carrichtera annua DC. Ethanolic Extract Counteracts Cisplatin Triggered Hepatic and Renal Toxicities. Antioxidants 2021, 10, 825. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Park, J.H.; Lee, S.H.; Lee, D.E.; Kim, I.S.; Lee, K.Y.; Lee, B.M.; et al. Protective effects of Dendropanax morbifera against cisplatin-induced nephrotoxicity without altering chemotherapeutic efficacy. Antioxidants 2019, 8, 256. [Google Scholar] [CrossRef]
- Soliman, A.M.; Desouky, S.; Marzouk, M.; Sayed, A.A. Origanum majorana attenuates nephrotoxicity of cisplatin anticancer drug through ameliorating oxidative stress. Nutrients 2016, 8, 264. [Google Scholar] [CrossRef]
- Heidari-Soreshjani, S.; Asadi-Samani, M.; Yang, Q.; Saeedi-Boroujeni, A. Phytotherapy of nephrotoxicity-induced by cancer drugs: An updated review. J. Nephropathol. 2017, 6, 254. [Google Scholar] [CrossRef]
- Ilyas, S.; Tabasum, R.; Iftikhar, A.; Nazir, M.; Hussain, A.; Hussain, A.; Ali, M.S.; Saleem, F.; Saleem, U.; Froeyen, M.; et al. Effect of Berberis vulgaris L. root extract on ifosfamide-induced in vivo toxicity and in vitro cytotoxicity. Sci. Rep. 2021, 11, 1708. [Google Scholar] [CrossRef]
- Ilari, S.; Lauro, F.; Giancotti, L.A.; Malafoglia, V.; Dagostino, C.; Gliozzi, M.; Condemi, A.; Maiuolo, J.; Oppedisano, F.; Palma, E.; et al. The Protective Effect of Bergamot Polyphenolic Fraction (BPF) on Chemotherapy-Induced Neuropathic Pain. Pharmaceuticals 2021, 14, 975. [Google Scholar] [CrossRef]
- Hannan, M.A.; Zahan, M.S.; Sarker, P.P.; Moni, A.; Ha, H.; Uddin, M.J. Protective Effects of Black Cumin (Nigella sativa) and Its Bioactive Constituent, Thymoquinone against Kidney Injury: An Aspect on Pharmacological Insights. Int. J. Mol. Sci. 2021, 22, 9078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Z.; Cha, L.; Li, L.; Zhu, D.; Fang, Z.; He, Z.; Huang, J.; Pan, Z. Resveratrol Plays a Protective Role against Premature Ovarian Failure and Prompts Female Germline Stem Cell Survival. Int. J. Mol. Sci. 2019, 20, 3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, Y.; Sakai, H. Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef]
- Martins, R.V.L.; Silva, A.M.S.; Duarte, A.P.; Socorro, S.; Correia, S.; Maia, C.J. Natural Products as Protective Agents for Male Fertility. BioChem 2021, 1, 122–147. [Google Scholar] [CrossRef]
- Timar, M.; Banaei, S.; Mehraban, Z.; Salimnejad, R.; Golmohammadi, M.G. Protective effect of saponin on sperm DNA fragmentation of mice treated with cyclophosphamide. Andrologia 2022, 54, e14336. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Go, A.; Kim, D.B.; Park, M.; Jeon, H.W.; Kim, B. Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxidants 2020, 9, 957. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Farczadi, L.; Huțanu, A.; Ősz, B.E.; Mărușteri, M.; Negroiu, A.; Vari, C.E. Tribulus terrestris Efficacy and Safety Concerns in Diabetes and Erectile Dysfunction, Assessed in an Experimental Model. Plants 2021, 10, 744. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; del Valle Soto, M.; Adams, D.P.; González-Bernal, J.J.; Seco-Calvo, J. The Effects of 6 Weeks of Tribulus terrestris L. Supplementation on Body Composition, Hormonal Response, Perceived Exertion, and CrossFit® Performance: A Randomized, Single-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 3969. [Google Scholar] [CrossRef]
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M. Therapeutic Potential of Date Palm against Human Infertility: A Review. Metabolites 2021, 11, 408. [Google Scholar] [CrossRef]
- Tauchen, J.; Jurášek, M.; Huml, L.; Rimpelová, S. Medicinal Use of Testosterone and Related Steroids Revisited. Molecules 2021, 26, 1032. [Google Scholar] [CrossRef]
- Mesbahzadeh, B.; Hassanzadeh-Taheri, M.; Baniasadi, P.; Hosseini, M. The protective effect of crocin on cisplatin-induced testicular impairment in rats. BMC Urol. 2021, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, M.F.; Çilenk, K.T.; Karabulut, D.; Ünalmış, S.; Deligönül, E.; Öztürk, İ.; Kaymak, E. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed. Pharmacother. 2016, 79, 44. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Abdel-Aziz, A.M.; Abdel-Hafez, S.M.N.; Venugopala, K.N.; Nair, A.B.; Abdel-Gaber, S.A. The Possible Contribution of P-Glycoprotein in the Protective Effect of Paeonol against Methotrexate-Induced Testicular Injury in Rats. Pharmaceuticals 2020, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, L.; Ayeleso, A.; Oyedepo, T.; Mukwevho, E. Ameliorative Potential of Hydroethanolic Leaf Extract of Parquetina nigrescens on d-Galactose-Induced Testicular Injury. Molecules 2021, 26, 3424. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, C.; Zhang, M.; Lu, X.; Cao, W.; Xie, C.; Li, X.; Wu, J.; Zhong, C.; Geng, S. Protective effects of ginseng stem-leaf saponins on D-galactose-induced reproductive injury in male mice. Aging 2021, 13, 8916. [Google Scholar] [CrossRef]
- Leng, J.; Hou, J.; Fu, C.; Ren, S.; Jiang, S.; Wang, Y.; Chen, C.; Wang, Z. Platycodon grandiflorum Saponins attenuate scrotal heat-induced spermatogenic damage via inhibition of oxidative stress and apoptosis in mice. J. Funct. Foods 2019, 54, 479. [Google Scholar] [CrossRef]
- Liu, W.; Leng, J.; Hou, J.G.; Jiang, S.; Wang, Z.; Liu, Z.; Gong, X.J.; Chen, C.; Wang, Y.P.; Li, W. Saponins derived from the stems and leaves of Panax ginseng attenuate scrotal heat-induced spermatogenic damage via inhibiting the MAPK mediated oxidative stress and apoptosis in mice. Phytother. Res. 2021, 35, 311. [Google Scholar] [CrossRef]
- Vyas, R.; Kesari, K.K.; Slama, P.; Roychoudhury, S.; Sisodia, R. Differential Activity of Antioxidants in Testicular Tissues Following Administration of Chlorophytum borivilianum in Gamma-Irradiated Swiss Albino Mice. Front. Pharmacol. 2022, 17, 12. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S. Ginseng and male reproductive function. Spermatogenesis 2013, 3, e26391. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Hussein, S.R.; Kawashty, S.A.; Tantawy, M.E.; Saleh, A.M. Nabiel. Chemosystematic studies of Nitraria retusa and selected taxa of Zygophyllaceae in Egypt. Plant Syst. Evol. 2009, 277, 251. [Google Scholar] [CrossRef]
- Sheahan, D.F.; Cutler, F.L.S. Contribution of vegetative anatomy to the systematics of the Zygophyllaceae. Bot. J. Linn. Soc. 1993, 113, 227. [Google Scholar] [CrossRef]
- Shawky, E.; Gabr, N.; El-gindi, M.; Mekky, R. A comprehensive review on genus Zygophyllum. J. Adv. Pharm. Res. 2019, 3, 1. [Google Scholar] [CrossRef]
- Nabiel, A.M.; Saleh, M.N. An approach to the chemosystematics of the Zygophyllaceae. Biochem. Syst. Ecol. 1977, 5, 121–128. [Google Scholar]
- Aboul-Enein, A.M.; El-Ela, F.A.; Shalaby, E.A.; El-Shemy, H.A. Traditional medicinal plants research in Egypt: Studies of antioxidant and anticancer activities. J. Med. Plant Res. 2012, 6, 689. [Google Scholar]
- Kchaou, M.; Ben-Salah, H.; Mnafgui, K.; Abdennabi, R.; Gharsallah, N.; Elfeki, A.; Damak, M.; Allouche, N. Chemical composition and biological activities of Zygophyllum album (L.) essential oil from Tunisia. J. Agric. Sci. Technol. 2016, 18, 1499. [Google Scholar]
- Bourgou, S.; Megdiche, W.; Ksouri, R. The halophytic genus Zygophyllum and Nitraria from North Africa: A phytochemical and pharmacological overview. Med. Aromat. Plants 2017, 3, 345. [Google Scholar]
- Ouffai, K.; Rachid, A.Z.Z.I.; Abboou, F.; Lahfa, F.B. Antihemolytic and antioxidant activities of aerial parts extracts of Zygophyllum album L. and Globularia alypum L. from Algeria. JNPRA 2022, 1, 41. [Google Scholar] [CrossRef]
- Mnafgui, K.; Kchaou, M.; Hamden, K.; Derbali, F.; Slama, S.; Nasri, M.; Salah, H.B.; Allouche, N.; Elfeki, A. Inhibition of carbohydrate and lipid digestive enzymes activities by Zygophyllum album extracts: Effect on blood and pancreas inflammatory biomarkers in alloxan-induced diabetic rats. J. Physiol. Biochem. 2014, 70, 93. [Google Scholar] [CrossRef]
- Mnafgui, K.; Kchaou, M.; Ben-Salah, H.; Hajji, R.; Khabbabi, G.; Elfeki, A.; Allouche, N.; Gharsallah, N. Essential oil of Zygophyllum album inhibits key-digestive enzymes related to diabetes and hypertension and attenuates symptoms of diarrhea in alloxan-induced diabetic rats. Pharm. Biol. 2016, 54, 1326. [Google Scholar] [CrossRef]
- Ghoul, J.E.; Boughattas, N.A.; Ben Attia, M. Antihyperglycemic and antihyperlipidemic activities of ethanolic extract of Zygophyllum album in streptozotocin-induced diabetic mice. Toxicol. Ind. Health 2013, 29, 43. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, K.; Hamden, K.; Ben Salah, H.; Kchaou, M.; Nasri, M.; Slama, S.; Derbali, F.; Allouche, N.; Elfeki, A. Inhibitory Activities of Zygophyllum album: A Natural Weight-Lowering Plant on Key Enzymes in High-Fat Diet-Fed Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 620384. [Google Scholar] [CrossRef] [PubMed]
- Feriani, A.; Tir, M.; Gómez-Caravaca, A.M.; Del Mar Contreras, M.; Talhaoui, N.; Taamalli, A.; Segura-Carretero, A.; Ghazouani, L.; Mufti, A.; Tlili, N.; et al. HPLC-DAD-ESI-QTOF- MS/MS profiling of Zygophyllum album roots extract and assessment of its cardioprotective effect against deltamethrin-induced myocardial injuries in rat, by suppression of oxidative stress-related inflammation and apoptosis via NF-κB signaling pathway. J. Ethnopharmacol. 2020, 247, 112266. [Google Scholar] [PubMed]
- Tigrine-Kordjani, N.; Meklati, B.Y.; Chemat, F. Contribution of microwave accelerated distillation in the extraction of the essential oil of Zygophyllum album L. Phytochem. Anal. 2010, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hasanean, H.A.; El-Shanawany, M.A.; Bishay, D.W.; Franz, G. Saponins from Zygophyllum album L. Bull. Pharm. Sci. Assiut 1989, 12, 117. [Google Scholar] [CrossRef]
- Hassanean, H.H.; Desoky, E.K.; El-Hamouly, M.M.A. Quinovic acid glycosides from Zygophyllum album. Phytochemistry 1993, 33, 663. [Google Scholar] [CrossRef]
- Hassanean, H.; El-Hamouly, M.; El-Moghazy, S.; Bishay, D. 14-decarboxyquinovic and quinovic acid glycosides from Zygophyllum album. Phytochemistry 1993, 33, 667. [Google Scholar] [CrossRef]
- Elgamal, M.H.A.; Shaker, K.H.; Pöllmann, K.; Seifert, K. Triterpenoid saponins from Zygophyllum species. Phytochemistry 1995, 40, 1233. [Google Scholar] [CrossRef]
- Hussein, S.; Marzouk, M.; Ibrahim, L.; Kawashty, S.; Saleh, N. Flavonoids of Zygophyllum album L. and Zygophyllum simplex L. (Zygophyllaceae). Biochem. Syst. Ecol. 2011, 39, 778. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636. [Google Scholar] [CrossRef]
- Tshilanda, D.D.; Onyamboko, D.N.; Babady-Bila, P.; Ngbolua, K.T.N.; Tshibangu, D.S.; Mpiana, P.T. Anti-sickling activity of ursolic acid isolated from the leaves of Ocimum gratissimum L. (Lamiaceae). Nat. Prod. Bioprospect. 2015, 5, 215. [Google Scholar] [CrossRef] [PubMed]
- Abd ELnasser, B.S.; El Dina, M.Y.; Elgindi, M.R.; Elsaid, M.B. Biological studies of isolated triterpenoids and phenolic compounds identified from Wodyetia bifurcata family Arecaceae. J. Pharmacogn. Phytochem. 2015, 3, 67. [Google Scholar]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Ryu, S.Y.; Lee, K.R. Phytochemical constituents of Bistorta manshuriensis. Nat. Prod. Sci. 2009, 15, 234. [Google Scholar]
- Kim, J.W.; Kim, T.B.; Yang, H.; Sung, S.H. Phenolic compounds isolated from Opuntia ficus-indica fruits. Nat. Prod. Sci. 2016, 22, 117. [Google Scholar] [CrossRef]
- Renda, G.; Ozgen, U.; Unal, Z.; Sabuncuoglu, S.; Palaska, E.; Orhan, I.E. Flavonoid derivatives from the aerial parts of Trifolium trichocephalum M. Bieb. and their antioxidant and cytotoxic activity. Rec. Nat. Prod. 2017, 11, 479. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, A. Isolation and identification of flavonoid rutin from Rauwolfia serpentina. Int. J. Chem. Stud. 2015, 3, 113. [Google Scholar]
- Kitajima, J.; Shindo, M.; Tanaka, Y. Two new triterpenoid sulfates from the leaves of Schefflera octophylla. Chem. Pharm. Bull. 1990, 38, 714. [Google Scholar] [CrossRef]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G.; et al. Sulfated Polysaccharide Extracted from the Green Algae Codium bernabei: Physicochemical Characterization and Antioxidant, Anticoagulant and Antitumor Activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef]
- Kellner Filho, L.C.; Picão, B.W.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Dias, G.M.; Copp, B.R.; Bertanha, C.S.; Januario, A.H. Bioactive Aliphatic Sulfates from Marine Invertebrates. Mar. Drugs 2019, 17, 527. [Google Scholar] [CrossRef]
- Chen, S.-K.; Hsu, C.-H.; Tsai, M.-L.; Chen, R.-H.; Drummen, G.P.C. Inhibition of Oxidative Stress by Low-Molecular-Weight Polysaccharides with Various Functional Groups in Skin Fibroblasts. Int. J. Mol. Sci. 2013, 14, 19399. [Google Scholar] [CrossRef] [PubMed]
- Safir, O.; Fkih-Tetouani, S.; De Tommasi, N.; Aquino, R. Saponins from Zygophyllum gaetulum. J. Nat. Prod. 1998, 61, 130. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M. Flavonoids from Prunus serotina ehrh. Acta Pol. Pharm. 2005, 62, 127. [Google Scholar] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Natori, T.; Morita, M.; Akimoto, K.; Koezuka, Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge Agelas mauritianus. Tetrahedron. Lett. 1994, 50, 2771. [Google Scholar] [CrossRef]
- Thomford, A.K.; Abdelhameed, R.F.A.; Yamada, K. Chemical studies on the parasitic plant Thonningia sanguinea Vahl. RSC Adv. 2018, 8, 21002. [Google Scholar] [CrossRef]
- Feng, Y.L.; Wu, B.; Li, H.R.; Li, Y.Q.; Xu, L.Z.; Yang, S.L.; Kitanaka, S. Triterpenoidal saponins from the barks of Zygophyllum fabago L. Chem. Pharm. Bull. 2008, 56, 858. [Google Scholar] [CrossRef]
- Popova, O.I.; Murav’eva, D.A. Ursolic acid from leafy shoots of Viscum album. Chem. Nat. Compd. 1990, 26, 346. [Google Scholar] [CrossRef]
- Lim Ah Tock, M.; Combrinck, S.; Kamatou, G.; Chen, W.; Van Vuuren, S.; Viljoen, A. Antibacterial Screening, Biochemometric and Bioautographic Evaluation of the Non-Volatile Bioactive Components of Three Indigenous South African Salvia Species. Antibiotics 2022, 11, 901. [Google Scholar] [CrossRef]
- Parvez, M.K.; Alam, P.; Arbab, A.H.; Al-Dosari, M.S.; Alhowiriny, T.A.; Alqasoumi, S.I. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm. J. 2018, 26, 685. [Google Scholar] [CrossRef]
- Adachi, S. Thin-Layer Chromatography of carbohydrates in the presence of bisulfite. J. Chromatogr. 1965, 17, 295. [Google Scholar] [CrossRef]
- Harborne, J.B. Sugars and their Derivatives. In Phytochemical Methods, 2nd ed.; Chapman and Hall: London, UK, 1984; pp. 222–242. [Google Scholar]
- Amin, E.; El-Hawary, S.S.; Fathy, M.M.; Mohammed, R.; Ali, Z.; Khan, I.A. Zygophylloside S, a New Triterpenoid Saponin from the Aerial Parts of Zygophyllum coccineum L. Planta Med. 2010, 76, 51. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical Profiling, Antioxidant, Cytotoxic Activities and Molecular Docking Simulation of Carrichtera Annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505. [Google Scholar] [PubMed]
- Belguidoum, M.; Dendougui, H.; Kendour, Z. In vitro antioxidant properties and phenolic contents of Zygophyllum album L. from Algeria. J. Chem. Pharm. Res. 2015, 2015, 510. [Google Scholar]
- Mostafa, R.; Essawy, H. Ecophsiological analysis of Zygophyllum album plant and its allelopathy. In Proceedings of the 6th International Conference, Menoufia University, Al Minufiyah, Egypt, 11–12 May 2016. [Google Scholar]
- Lfitat, A.; Zejli, H.; Bousraf, F.; Bousselham, A.; El Atki, Y.; Gourch, A.; Badiaa, L.; Abdellaoui, A. Comparative Assessment of Total Phenolics Content and in vitro Antioxidant Capacity Variations of Macerated Leaf Extracts of Olea europaea L. and Argania Spinosa (L.) Skeels. Mater. Today Proc. 2021, 45, 7271. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anti-Cancer Agents Med. Chem. 2011, 11, 341. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Sankaranarayanan, J.; Morachis, J.M.; Kim, G.; Almutairi, A. Inflammation responsive logic gate nanoparticles for the delivery of proteins. Bioconjug. Chem. 2011, 22, 1416. [Google Scholar] [CrossRef]
- De Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Pakniyat, H.; Pirasteh-Anosheh, H.; Azooz, M.M. Chapter 20—Role of ROS as Signaling Molecules in Plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; p. 585. [Google Scholar]
- Shen, Y.; White, E. p53-dependent apoptosis pathways. Adv. Cancer Res. 2001, 82, 8255. [Google Scholar]
- Akhtar, R.S.; Ness, J.M.; Roth, K.A. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim. Biophys. Acta 2004, 1644, 189. [Google Scholar] [CrossRef]
- Pınar, N.; Çakırca, G.; Özgür, T.; Kaplan, M. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed. Pharmacother. 2018, 97, 1486. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; El-Sheikh, A.A.K.; Khalaf, H.M.; Abdelrahman, A.M. Protective effect of peroxisome proliferator activator receptor (PPAR)-α and -γ ligands against methotrexate-induced nephrotoxicity. Immunopharmacol. Immunotoxicol. 2014, 36, 130. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, Ie01. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.H.; Sharma, R.K.; Thornton, J.; Mascha, E.; Abdel-Hafez, M.A.; Thomas, A.J.; Agarwal, A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum. Reprod. 2004, 19, 129. [Google Scholar] [CrossRef]
- Lysiak, J.J. The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod. Biol. Endocrinol. 2004, 2, 9. [Google Scholar] [CrossRef]
- Makary, S.; Abdo, M.; Fekry, E. Oxidative stress burden inhibits spermatogenesis in adult male rats: Testosterone protective effect. Can. J. Physiol. Pharmacol. 2018, 96, 372. [Google Scholar] [CrossRef]
- Lysiak, J.J.; Nguyen, Q.A.; Kirby, J.L.; Turner, T.T. Ischemia-reperfusion of the murine testis stimulates the expression of proinflammatory cytokines and activation of c-jun N-terminal kinase in a pathway to E-selectin expression. Biol. Reprod. 2003, 69, 202. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, U.I.; Moore, H.D.; Cooke, I.D. Correlation of testicular sperm extraction with morphological, biophysical and endocrine profiles in men with azoospermia due to primary gonadal failure. Hum. Reprod. 1998, 13, 3066. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Siddeek, B.; Vega, A.; Lakhdari, N.; Inoubli, L.; Bellon, R.P.; Lemaire, G.; Mauduit, C.; Benahmed, M. Perinatal programming of adult rat germ cell death after exposure to xenoestrogens: Role of microRNA miR-29 family in the downregulation of DNA methyltransferases and Mcl-1. Endocrinology 2012, 153, 1936. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.H.; Ha, M.; Nam, J.W.; Kim, V.N. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat. Struct. Mol. Biol. 2009, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, X.; Ren, Y.; Wang, J.; Xu, D.; Hang, Y.; Zhou, T.; Li, F.; Wang, L. MicroRNA-29a regulates lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages through the Akt1/ NF-κB pathway. Exp. Cell Res. 2017, 360, 74. [Google Scholar] [CrossRef] [PubMed]
- González-Madariaga, Y.; Mena-Linares, Y.; Martín-Monteagudo, D.; Valido-Díaz, A.; Guerra-de-León, J.; Nieto-Reyes, L. In vivo anti-inflammatory effect of saponin-enriched fraction from Agave brittoniana Trel subspecie brachypus. Ars Pharm. 2020, 61, 231. [Google Scholar]
- Grabowska, K.; Wróbel, D.; Żmudzki, P.; Podolak, I. Anti-inflammatory activity of saponins from roots of Impatiens parviflora DC. Nat. Prod. Res. 2020, 34, 1581. [Google Scholar] [CrossRef]
- Su, S.; Li, X.; Li, S.; Ming, P.; Huang, Y.; Dong, Y.; Ding, H.; Feng, S.; Li, J.; Wang, X.; et al. Rutin protects against lipopolysaccharide-induced mastitis by inhibiting the activation of the NF-κB signaling pathway and attenuating endoplasmic reticulum stress. Inflammopharmacology 2019, 27, 77. [Google Scholar] [CrossRef]
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-β-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151. [Google Scholar] [CrossRef]
- Sen, S.; Makkar, H.P.; Becker, K. Alfalfa Saponins and Their Implication in Animal Nutrition. J. Agric. Food Chem. 1998, 46, 131. [Google Scholar] [CrossRef] [PubMed]
- Akondi, B.R.; Challa, S.R.; Akula, A. Protective effects of rutin and naringin in testicular ischemia-reperfusion induced oxidative stress in rats. J. Reprod. Infertil. 2011, 12, 209. [Google Scholar] [PubMed]
- Alsharif, N.Z.; Hassoun, E.; Bagchi, M.; Lawson, T.; Stohs, S.J. The Effects of Anti-TNF-Alpha Antibody and Dexamethasone on TCDD-lnduced Oxidative Stress in Mice. Pharmacology 1994, 48, 127. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Kang, X.; Zhou, Z.; Zhang, Z.; Liu, D. Anti-apoptotic function and mechanism of ginseng saponins in Rattus pancreatic β-cells. Biol. Pharm. Bull. 2012, 35, 1568. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, H.E.; Abo-elmatty, D.M.; Wahba, N.S.; Shaheen, M.A.; Sakr, R.T.; Wahba, A.S. Infliximab and/or MESNA alleviate doxorubicin-induced Alzheimer’s disease-like pathology in rats: A new insight into TNF-α/Wnt/β-catenin signaling pathway. Life Sci. 2022, 301, 120613. [Google Scholar] [CrossRef] [PubMed]
- Pollutri, D.; Gramantieri, L.; Bolondi, L.; Fornari, F. TP53/MicroRNA Interplay in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 2029. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B. Current state of phenolic and terpenoidal dietary factors and natural products as non-coding RNA/microRNA modulators for improved cancer therapy and prevention. Non-Coding RNA Res. 2016, 1, 12. [Google Scholar] [CrossRef]
- Lin, Q.; Ma, L.; Liu, Z.; Yang, Z.; Wang, J.; Liu, J.; Jiang, G. Targeting microRNAs: A new action mechanism of natural compounds. Oncotarget 2017, 8, 15961. [Google Scholar] [CrossRef]
- Lv, B.; Liu, Z.; Wang, S.; Liu, F.; Yang, X.; Hou, J.; Hou, Z.; Chen, B. MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int. J. Clin. Exp. Pathol. 2014, 7, 8542. [Google Scholar]
- Hu, Y.; Xie, L.; Yu, J.; Fu, H.; Zhou, D.; Liu, H. Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1. Mol. Med. 2019, 26, 3. [Google Scholar] [CrossRef]
- Noman, O.M.; Nasr, F.A.; Mothana, R.A.; Alqahtani, A.S.; Qamar, W.; Al-Mishari, A.A.; Al-Rehaily, A.J.; Siddiqui, N.A.; Alam, P.; Almarfadi, O.M. Isolation, Characterization, and HPTLC-Quantification of Compounds with Anticancer Potential from Loranthus Acaciae Zucc. Separations 2020, 7, 43. [Google Scholar] [CrossRef]
- Gogoi, J.; Nakhuru, K.S.; Policegoudra, R.S.; Chattopadhyay, P.; Rai, A.K.; Veer, V. Isolation, and characterization of bioactive components from Mirabilis jalapa L. radix. J. Tradit. Complement. Med. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.A.; Al-Yousef, H.M.; Alhowiriny, T.A.; Alam, P.; Hassan, W.H.B.; Amina, M.; Hussain, A.; Abdelaziz, S.; Abdallah, R.H. Con-current analysis of bioactive triterpenes oleanolic acid and β-amyrin in antioxidant active fractions of Hibiscus calyphyllus, Hibiscus deflersii and Hibiscus micranthus grown in Saudi Arabia by applying validated HPTLC method. Saudi Pharm. J. 2018, 26, 266. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Alajmi, M.F.; Siddiqui, N.A.; Al-Rehaily, A.J.; Alharbi, H.; Basudan, O.A.; Hussain, A. Densitometric validation and analysis of biomarker β-amyrin in different Acacia species (leaves) grown in Kingdom of Saudi Arabia by high performance thin-layer chromatography. Pak. J. Pharm. Sci. 2015, 28, 1485. [Google Scholar] [PubMed]
- Nile, S.H.; Park, S.W. HPTLC densitometry method for simultaneous determination of flavonoids in selected medicinal plants. Front. Life Sci. 2015, 8, 103. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Mohammed, M.J.; Yi-Chen, L.; Watson, D.G.; Lakhssassi, N.; Cacciola, F.; Ibrahim, S.A. Optimization of Ultrasonicated Kaempferol Extraction from Ocimum basilicum Using a Box–Behnken Design and Its Densitometric Validation. Foods 2020, 9, 1379. [Google Scholar] [CrossRef]
- Avertseva, I.N.; Suleymanova, F.S.; Nesterova, O.V.; Reshetnyak, V.Y.; Matveenko, V.N.; Zhukov, P.A. Study of Polyphenolic Compounds in Extracts from Flowers and Leaves of Canadian Goldenrod and Dwarf Goldenrod (Solidago canadensis L. and Solidago nana Nitt.). Mosc. Univ. Chem. Bull. 2020, 75, 47–51. [Google Scholar] [CrossRef]
- Głowniak, K.; Skalicka, K.; Ludwiczuk, A.; Jop, K. Phenolic Compounds in the Flowers of Lavatera trimestris L. (Malvaceae). J. Planar Chromat. 2005, 18, 264–268. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Heidelberg, Germany, 2009; pp. 195–245. [Google Scholar]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629. [Google Scholar] [CrossRef]
- Elaasser, M.M.; Morsi, M.K.; Galal, S.M.; Abd El-Rahman, M.K.; Katry, M.A. Antioxidant, anti-inflammatory and cytotoxic activities of the unsaponifiable fraction of extra virgin olive oil. Grasas Aceites 2020, 71, e386. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.T.; Klauring, J.E. Prevention of cytotoxicity and inhibition of intercellular comunication by antioxidant catechins isolated from chinese green tea. Carcinogenesis 1989, 10, 1003. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337. [Google Scholar] [CrossRef] [PubMed]
- Najafi, G.; Atashfaraz, E.; Farokhi, F. Attenuation of Methotrexate-Induced Embryotoxicity and Oxidative Stress by Ethyl Pyruvate. Int. J. Fertil. Steril. 2016, 10, 232. [Google Scholar]
- Ghoul, J.E.; Smiri, M.; Saad, G.; Boughattas, N.A.; Ben-Attia, M. Antihyperglycemic, antihyperlipidemic and antioxidant activities of traditional aqueous extract of Zygophyllum album in streptozotocin diabetic mice. Pathophysiology 2011, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yang, X.; Zhang, X.; Duan, H.; Jin, M.; Sun, Y.; Yuan, H.; Li, J.; Qi, Y.; Qiao, W. Antidepressant-like effect of the saponins part of ethanol extract from SHF. J. Ethnopharmacol. 2016, 191, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.L.; Somashekarappa, H.; Rajashekhar, K. Radiomodulatory role of Rutin and Quercetin in Swiss Albino mice exposed to the whole body gamma radiation. Indian J. Nucl. Med. 2012, 27, 237–242. [Google Scholar] [CrossRef] [Green Version]






| No. | δC | δH (Int., Mult., JHz) | No. | δC | δH (Int., Mult., JHz) |
|---|---|---|---|---|---|
| 1 | 39.0 | 1.10 (1H, m) 1.62 (1H, m) | 19 | 38.6 | 1.11 (1H, m) |
| 2 | 27.5 | 1.25 (1H, m) 1.42 (1H, m) | 20 | 38.5 | 0.95 (1H, m) |
| 3 | 74.9 | 3.64 (1H, dd, 5, 10) | 21 | 30.8 | 1.02 (1H, m), 1.62 (1H, m) |
| 4 | 38.9 | - | 22 | 37.7 | 1.41 (1H, m), 2.06 (1H, m) |
| 5 | 55.6 | 0.95 (1H, m) | 23 | 28.4 | 1.03 (3H, S) |
| 6 | 19.9 | 0.95 (1H, m) 1.35 (1H, m) | 24 | 19.9 | 0.93 (3H, S) |
| 7 | 38.6 | 1.62 (1H, m) 1.73 (1H, m) | 25 | 17.5 | 0.90 (3H, S) |
| 8 | 40.4 | - | 26 | 19.9 | 1.00 (3H, S) |
| 9 | 47.1 | 1.19 (1H, m) | 27 | 13.6 | 1.10 (3H, S) |
| 10 | 39.0 | - | 28 | 172.6 | - |
| 11 | 25.8 | 1.96 (1H, m), 2.15 (1H, m) | 29 | 17.6 | 1.11 (1H, d, 10) |
| 12 | 126.1 | 5.35 (1H, m) | 30 | 21.4 | 0.94 (1H, d, 8) |
| 13 | 138.5 | - | 1′ | 86.8 | 4.75 (1H, d, 10) |
| 14 | 57.1 | - | 2′ | 78.5 | 4.07 (1H, dd, 5, 10) |
| 15 | 28.4 | 1.03 (1H, m) 1.11 (1H, m) | 3′ | 73.3 | 3.89 (1H, t, 5) |
| 16 | 25.8 | 1.39 (1H, m) 2.16 (1H, m) | 4′ | 68.9 | 4.08 (1H, m) |
| 17 | 47.2 | - | 5′ | 74.6 | 3.65 (1H, m) |
| 18 | 54.8 | 1.79 (1H, m) | 6′ | 60.3 | 3.65 (1H, m) 3.89 (1H, m) |
| Sample | DPPH (IC50 in µg/mL) | H2O2 (IC50 in µg/mL) | TAC (mg GAE/g) |
|---|---|---|---|
| Compound 7 | 45.41 ± 2.65 | 65.16 ± 3.22 | 29.83 ± 2.19 |
| Ascorbic acid | 10.64 ± 0.82 | NT | 71.28 ± 4.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhameed, R.F.A.; Fattah, S.A.; Mehanna, E.T.; Hal, D.M.; Mosaad, S.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Ahmed, S.A.; Badr, J.M.; Eltamany, E.E. Zygo-Albuside A: New Saponin from Zygophyllum album L. with Significant Antioxidant, Anti-Inflammatory and Antiapoptotic Effects against Methotrexate-Induced Testicular Damage. Int. J. Mol. Sci. 2022, 23, 10799. https://doi.org/10.3390/ijms231810799
Abdelhameed RFA, Fattah SA, Mehanna ET, Hal DM, Mosaad SM, Abdel-Kader MS, Ibrahim AK, Ahmed SA, Badr JM, Eltamany EE. Zygo-Albuside A: New Saponin from Zygophyllum album L. with Significant Antioxidant, Anti-Inflammatory and Antiapoptotic Effects against Methotrexate-Induced Testicular Damage. International Journal of Molecular Sciences. 2022; 23(18):10799. https://doi.org/10.3390/ijms231810799
Chicago/Turabian StyleAbdelhameed, Reda F. A., Shaimaa A. Fattah, Eman T. Mehanna, Dina M. Hal, Sarah M. Mosaad, Maged S. Abdel-Kader, Amany K. Ibrahim, Safwat A. Ahmed, Jihan M. Badr, and Enas E. Eltamany. 2022. "Zygo-Albuside A: New Saponin from Zygophyllum album L. with Significant Antioxidant, Anti-Inflammatory and Antiapoptotic Effects against Methotrexate-Induced Testicular Damage" International Journal of Molecular Sciences 23, no. 18: 10799. https://doi.org/10.3390/ijms231810799
APA StyleAbdelhameed, R. F. A., Fattah, S. A., Mehanna, E. T., Hal, D. M., Mosaad, S. M., Abdel-Kader, M. S., Ibrahim, A. K., Ahmed, S. A., Badr, J. M., & Eltamany, E. E. (2022). Zygo-Albuside A: New Saponin from Zygophyllum album L. with Significant Antioxidant, Anti-Inflammatory and Antiapoptotic Effects against Methotrexate-Induced Testicular Damage. International Journal of Molecular Sciences, 23(18), 10799. https://doi.org/10.3390/ijms231810799







