AIE-Featured Redox-Sensitive Micelles for Bioimaging and Efficient Anticancer Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
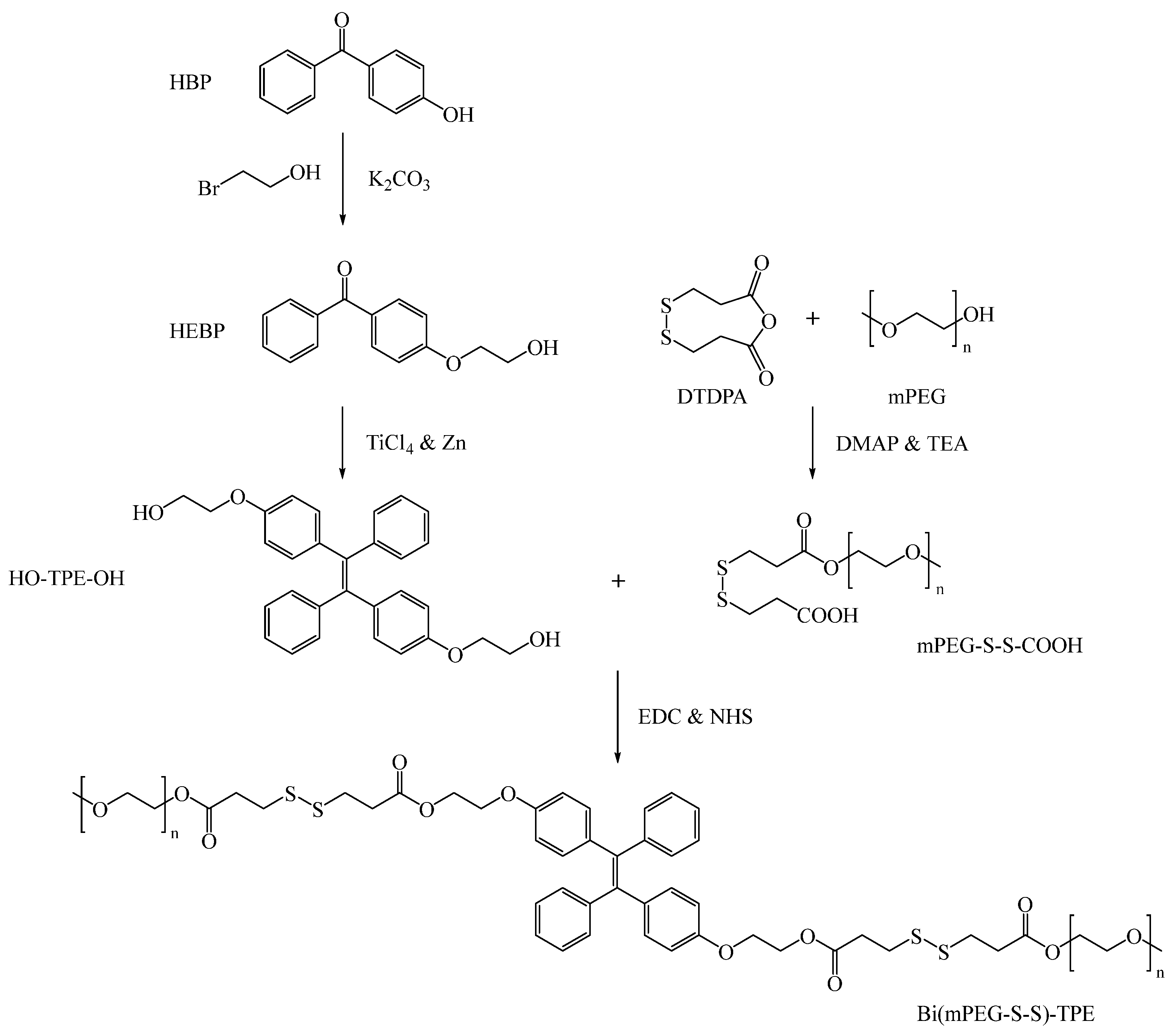
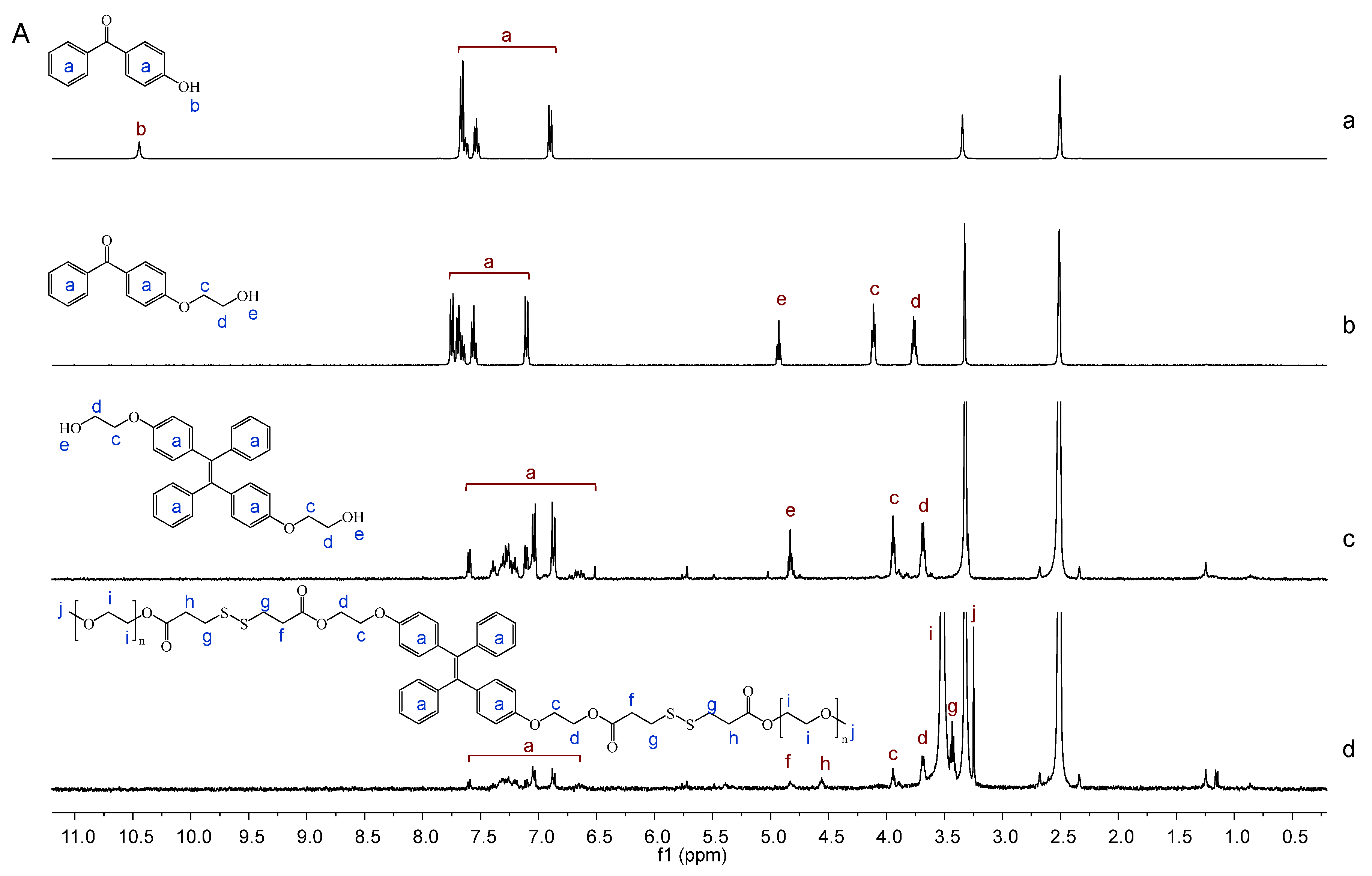
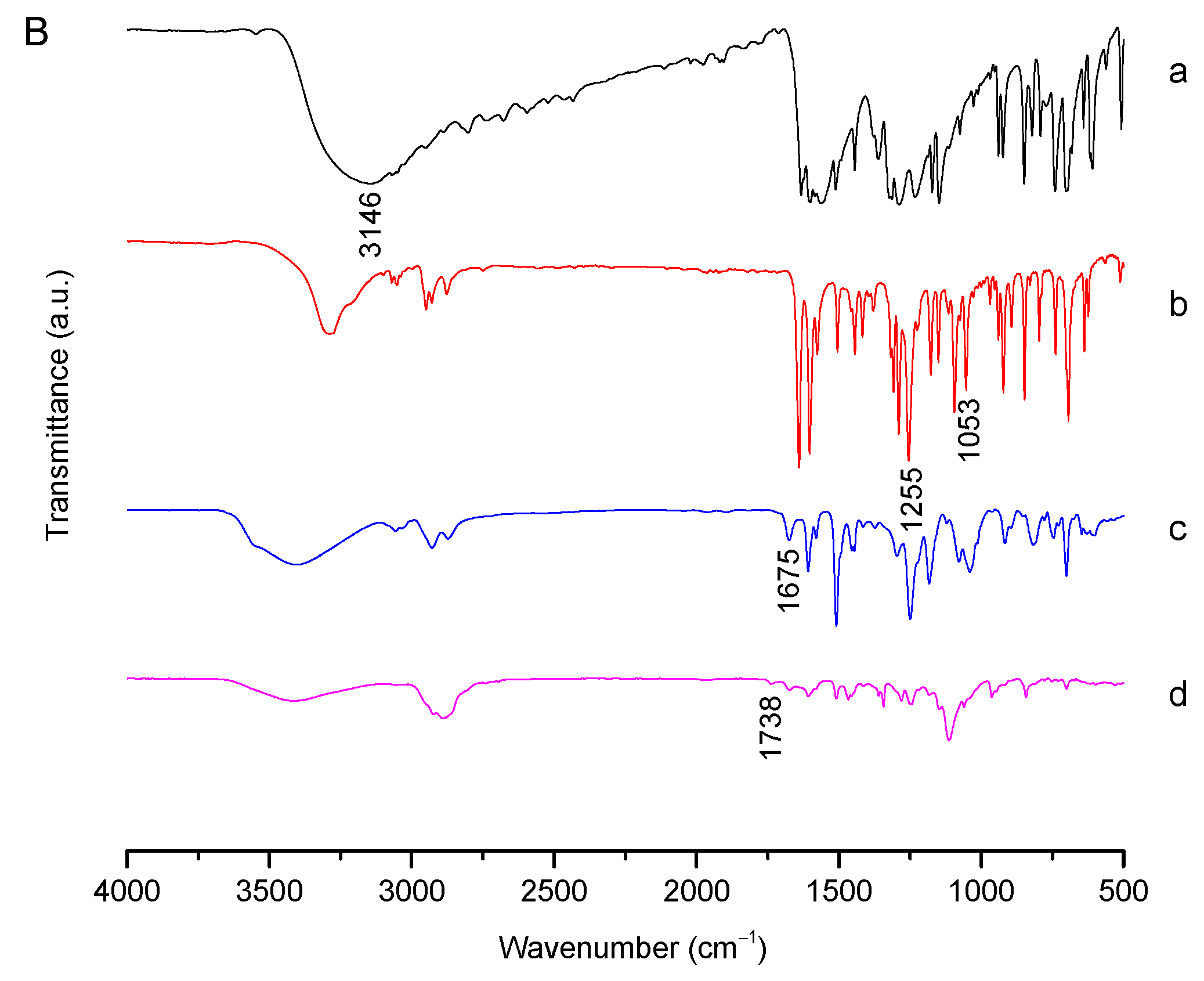
2.1. Synthesis and Characterization of Bi(mPEG-S-S)-TPE
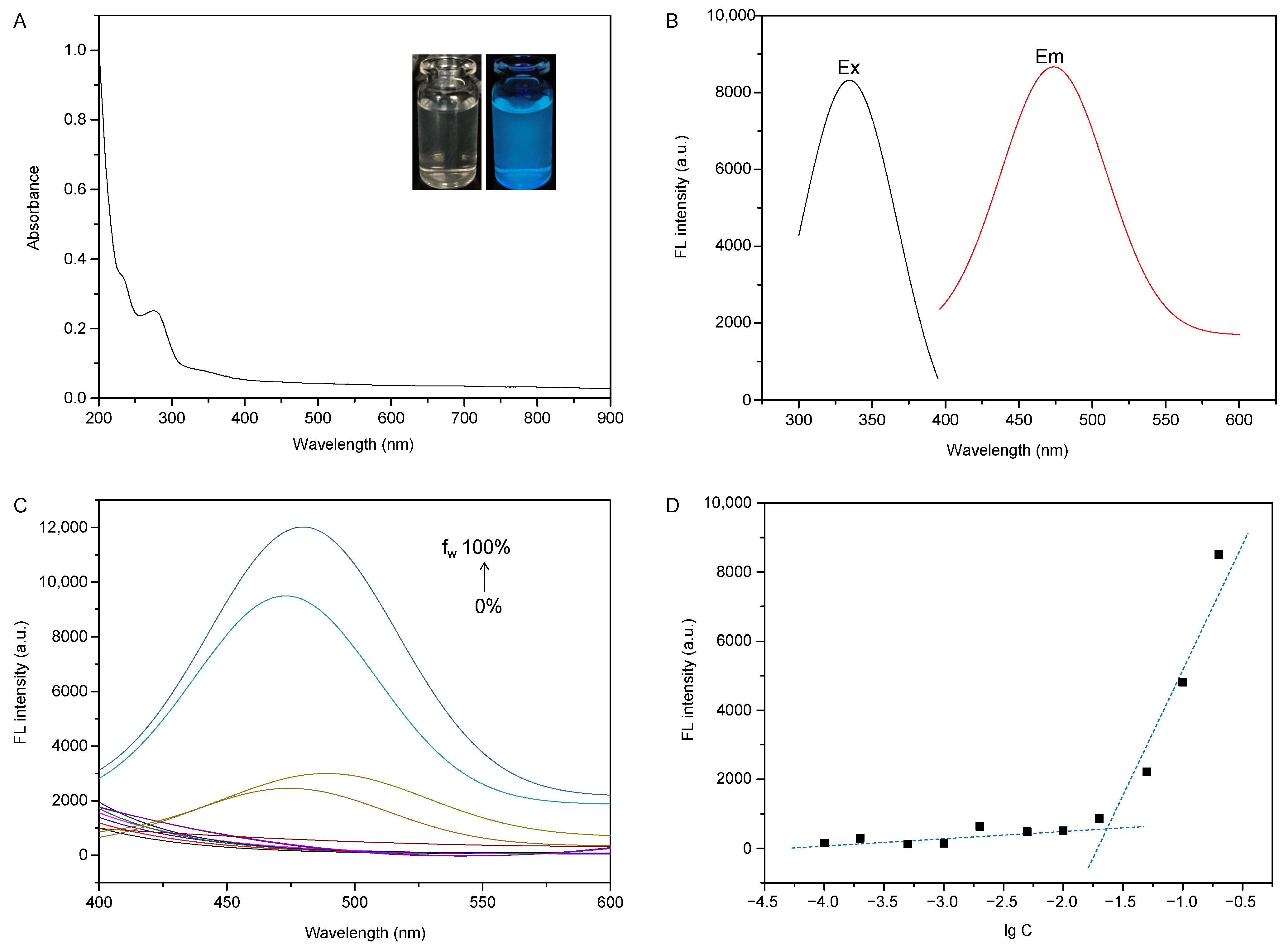
2.2. Determination of Optical Properties
2.3. Characterization of PTX-Loaded Bi(mPEG-S-S)-TPE Micelles
2.4. Protein Adsorption Study
2.5. In Vitro Drug Release Study
2.6. Hemolysis Assay
2.7. Cytotoxicity Evaluation
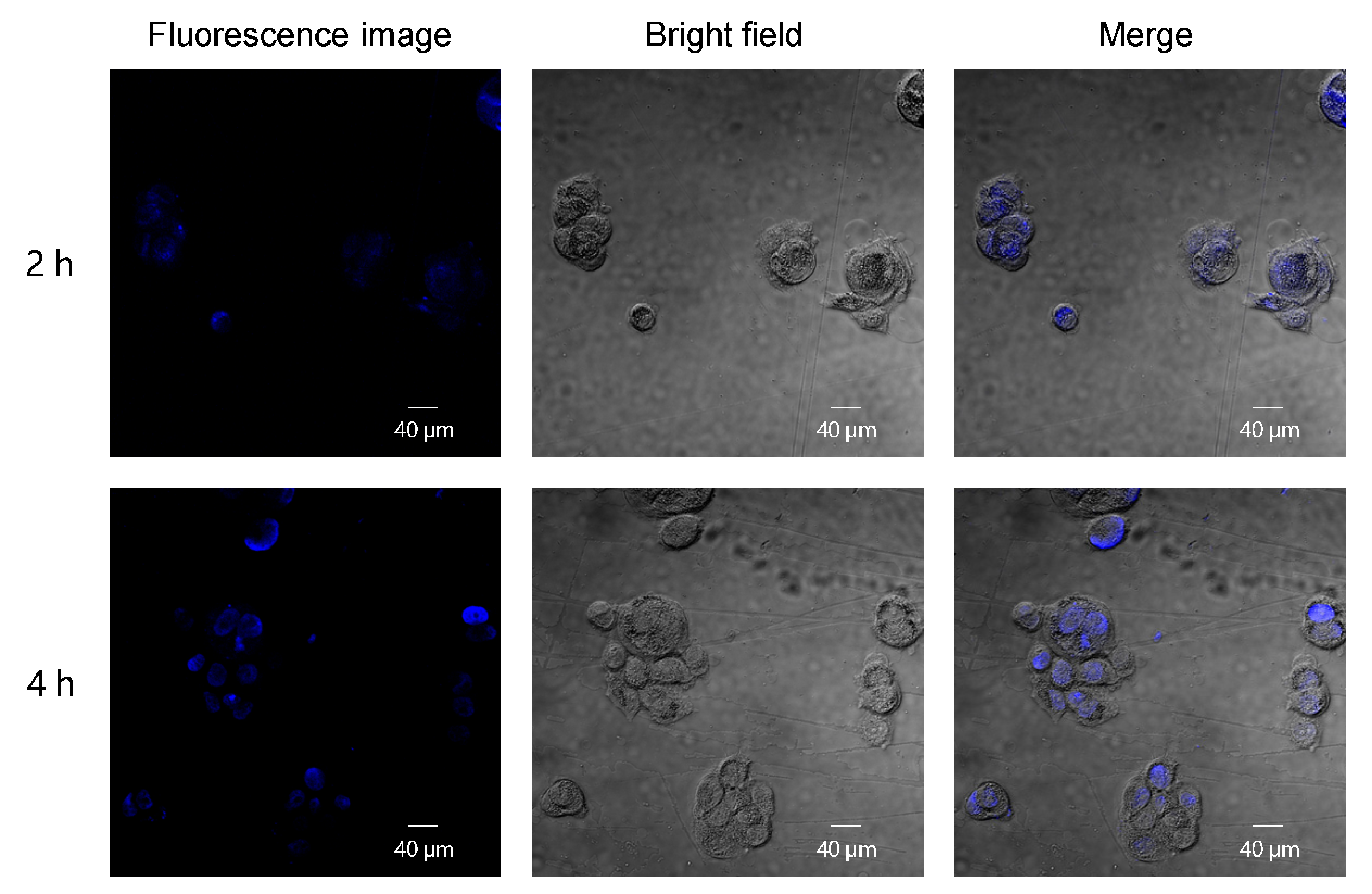
2.8. Cellular Uptake and Cellular Imaging Behavior Study
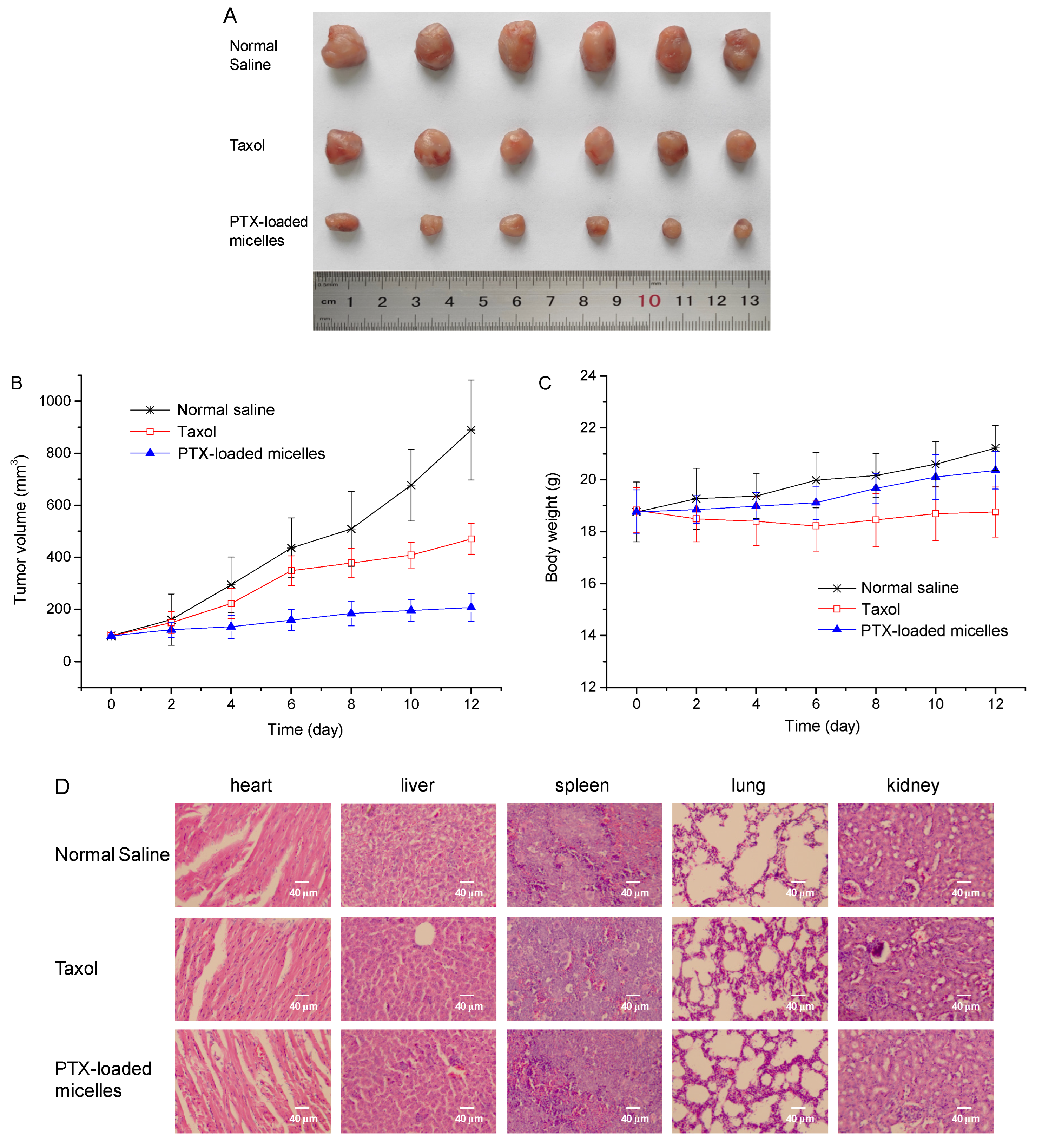
2.9. In Vivo Antitumor Activity Study
3. Materials and Methods
3.1. Materials
3.2. Cells and Animals
3.3. Synthesis of Bi(mPEG-S-S)-TPE
3.3.1. Synthesis of HO-TPE-OH
3.3.2. Synthesis of mPEG-S-S-COOH
3.3.3. Synthesis of Bi(mPEG-S-S)-TPE
3.4. Characterization of Bi(mPEG-S-S)-TPE
3.4.1. 1H NMR and FT-IR Analysis
3.4.2. AIE Behavior Investigation
3.4.3. CMC Measurement
3.5. Preparation and Characterization of Bi(mPEG-S-S)-TPE Micelles
3.6. Protein Adsorption Test
3.7. In Vitro Drug Release Study
3.8. Hemolysis Assay
3.9. In Vitro Cytotoxicity Assay
3.10. Cellular Uptake and Cellular Imaging Study
3.11. In Vivo Antitumor Efficacy Study
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, S.-S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114. [Google Scholar] [CrossRef]
- Jin, R.; Guo, X.; Dong, L.; Xie, E.; Cao, A. Amphipathic dextran-doxorubicin prodrug micelles for solid tumor therapy. Colloids Surf. B 2017, 158, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.; Tan, A.M.; Shi, Y. New and emerging targeted therapies for advanced breast cancer. Int. J. Mol. Sci. 2022, 23, 2288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, S.; Liu, S.; Wang, Y.; Liu, G. Emerging nanomedicines of paclitaxel for cancer treatment. J. Control. Release 2022, 342, 280–294. [Google Scholar] [CrossRef]
- Sabra, S.; Abdelmoneem, M.; Abdelwakil, M.; Mabrouk, T.M.; Anwar, D.; Mohamed, R.; Khattab, S.; Bekhit, A.; Elkhodairy, K.; Freag, M.; et al. Self-assembled nanocarriers based on amphiphilic natural polymers for anti-cancer drug delivery applications. Curr. Pharm. Des. 2017, 23, 5213–5229. [Google Scholar] [CrossRef]
- Zheng, S.; Han, J.; Jin, Z.; Kim, C.-S.; Park, S.; Kim, K.-p.; Park, J.-O.; Choi, E. Dual tumor-targeted multifunctional magnetic hyaluronic acid micelles for enhanced MR imaging and combined photothermal-chemotherapy. Colloids Surf. B 2018, 164, 424–435. [Google Scholar] [CrossRef]
- Wu, W.; Yao, W.; Wang, X.; Xie, C.; Zhang, J.; Jiang, X. Bioreducible heparin-based nanogel drug delivery system. Biomaterials 2015, 39, 260–268. [Google Scholar] [CrossRef]
- Wu, Q.; Du, F.; Luo, Y.; Lu, W.; Huang, J.; Yu, J.; Liu, S. Poly(ethylene glycol) shell-sheddable nanomicelle prodrug of camptothecin with enhanced cellular uptake. Colloids Surf. B 2013, 105, 294–302. [Google Scholar] [CrossRef]
- Fang, X.; Wang, X.; Li, G.; Zeng, J.; Li, J.; Liu, J. SS-mPEG chemical modification of recombinant phospholipase C for enhanced thermal stability and catalytic efficiency. Int. J. Biol. Macromol. 2018, 111, 1032–1039. [Google Scholar] [CrossRef]
- Erdemli, Ö.; Usanmaz, A.; Keskin, D.; Tezcaner, A. Characteristics and release profiles of MPEG-PCL-MPEG microspheres containing immunoglobulin G. Colloids Surf. B 2014, 117, 487–496. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Q.; He, S.; Wu, Y.; Huang, Y. Reduction-responsive disulfide linkage core-cross-linked polymeric micelles for site-specific drug delivery. Polym. Chem. 2020, 11, 7078–7086. [Google Scholar] [CrossRef]
- Shi, C.; Guo, X.; Qu, Q.; Tang, Z.; Wang, Y.; Zhou, S. Actively targeted delivery of anticancer drug to tumor cells by redox-responsive star-shaped micelles. Biomaterials 2014, 35, 8711–8722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, C.; Liu, Y.; Wang, J.; Gao, Y.; Zhang, X.; Jiang, T.; Wang, S. PEGylated mesoporous silica as a redox-responsive drug delivery system for loading thiol-containing drugs. Int. J. Pharm. 2014, 477, 613–622. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; Yu, J.; Fan, D.; Ren, J.; Zhang, L.; Zhao, J. Reduction-sensitive micelles self-assembled from amphiphilic chondroitin sulfate A-deoxycholic acid conjugate for triggered release of doxorubicin. Mater. Sci. Eng. C 2017, 75, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lu, J.; Wang, J.; Hao, P.; Li, C.; Qi, L.; Yang, L.; He, B.; Zhong, Z.; Hao, N. Redox-sensitive polymeric micelles with aggregation-induced emission for bioimaging and delivery of anticancer drugs. J. Nanobiotechnol. 2021, 19, 14. [Google Scholar] [CrossRef]
- Reisch, A.; Klymchenko, A.S. Fluorescent polymer nanoparticles based on dyes: Seeking brighter tools for bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef]
- Mao, L.; Liu, Y.; Yang, S.; Li, Y.; Zhang, X.; Wei, Y. Recent advances and progress of fluorescent bio-/chemosensors based on aggregation-induced emission molecules. Dyes Pigm. 2019, 162, 611–623. [Google Scholar] [CrossRef]
- Long, Z.; Liu, M.; Mao, L.; Zeng, G.; Wan, Q.; Xu, D.; Deng, F.; Huang, H.; Zhang, X.; Wei, Y. Rapid preparation of branched and degradable AIE-active fluorescent organic nanoparticles via formation of dynamic phenyl borate bond. Colloids Surf. B 2017, 150, 114–120. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, Y.; Liu, Y.; Wu, D. Thermostable polymeric nanomicelles of iridium(iii) complexes with aggregation-induced phosphorescence emission characteristics and their recyclable double-strand DNA monitoring. J. Mater. Chem. B 2017, 5, 123–133. [Google Scholar] [CrossRef]
- Wang, W.; Lin, J.; Cai, C.; Lin, S. Optical properties of amphiphilic copolymer-based self-assemblies. Eur. Polym. J. 2015, 65, 112–131. [Google Scholar] [CrossRef]
- Hui, X.; Xu, D.; Wang, K.; Yu, W.; Yuan, H.; Liu, M.; Zhengyu, S.; Zhang, X.; Wei, Y. Supermolecular self assembly of AIE-active nanoprobes: Fabrication and bioimaging applications. RSC Adv. 2015, 5, 107355–107359. [Google Scholar] [CrossRef]
- Yan, K.; Feng, Y.; Gao, K.; Shi, X.; Zhao, X. Fabrication of hyaluronic acid-based micelles with glutathione-responsiveness for targeted anticancer drug delivery. J. Colloid Interface Sci. 2022, 606, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhang, S.; Zhang, K.; Miao, Y.; Qiu, Y.; Zhang, P.; Jia, X.; Zhao, X. Enzyme-responsive polymeric micelles with fluorescence fabricated through aggregation-induced copolymer self-assembly for anticancer drug delivery. Polym. Chem. 2020, 11, 7704–7713. [Google Scholar] [CrossRef]
- Kniess, T.; Laube, M.; Bergmann, R.; Sehn, F.; Graf, F.; Steinbach, J.; Wuest, F.; Pietzsch, J. Radiosynthesis of a 18F-labeled 2,3-diarylsubstituted indole via McMurry coupling for functional characterization of cyclooxygenase-2 (COX-2) in vitro and in vivo. Bioorg. Med. Chem. 2012, 20, 3410–3421. [Google Scholar] [CrossRef]
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.; Wong, K.S.; Peña-Cabrera, E.; et al. Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Cao, Q.-y.; Jiang, R.; Liu, M.; Wan, Q.; Xu, D.; Tian, J.; Huang, H.; Wen, Y.; Zhang, X.; Wei, Y. Preparation of AIE-active fluorescent polymeric nanoparticles through a catalyst-free thiol-yne click reaction for bioimaging applications. Mater. Sci. Eng. C 2017, 80, 411–416. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, R.; Gao, P.; Xu, D.; Mao, L.; Zeng, G.; Liu, M.; Deng, F.; Zhang, X.; Wei, Y. Synthesis and cell imaging applications of amphiphilic AIE-active poly(amino acid)s. Mater. Sci. Eng. C 2017, 79, 563–569. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE luminogens as fluorescent bioprobes. TrAC Trends Anal. Chem. 2020, 123, 115769. [Google Scholar] [CrossRef]
- Wan, Q.; He, C.; Wang, K.; Liu, M.; Huang, H.; Huang, Q.; Deng, F.; Zhang, X.; Wei, Y. Preparation of ultrabright AIE nanoprobes via dynamic bonds. Tetrahedron 2015, 71, 8791–8797. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, Y.; Yang, B.; Zhou, X.; Li, J.; Qin, Z.; Dong, D.; Cui, Y.; Yao, F. Zwitterionic-modified starch-based stealth micelles for prolonging circulation time and reducing macrophage response. ACS Appl. Mater. Interfaces 2016, 8, 4385–4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, Z.; Wang, H.; Su, C.; Lu, Z.; Yu, J.; Dushkin, A.V.; Su, W. Preparation of camptothecin micelles self-assembled from disodium glycyrrhizin and tannic acid with enhanced antitumor activity. Eur. J. Pharm. Biopharm. 2021, 164, 75–85. [Google Scholar] [CrossRef]
- Ding, J.; Chen, J.; Li, D.; Xiao, C.; Zhang, J.; He, C.; Zhuang, X.; Chen, X. Biocompatible reduction-responsive polypeptide micelles as nanocarriers for enhanced chemotherapy efficacy in vitro. J. Mater. Chem. B 2013, 1, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-Y.; Du, J.-Z.; Song, W.-J.; Wang, F.; Yang, X.-Z.; Xiong, M.-H.; Wang, J. Biocompatible and functionalizable polyphosphate nanogel with a branched structure. J. Mater. Chem. 2012, 22, 9322–9329. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wang, F.; Sun, T.-M.; Wang, J. Redox-responsive nanoparticles from the single disulfide bond-bridged block copolymer as drug carriers for overcoming multidrug resistance in cancer cells. Bioconjug. Chem. 2011, 22, 1939–1945. [Google Scholar] [CrossRef]
- Shafiei-Irannejad, V.; Rahimkhoei, V.; Molaparast, M.; Akbari, A. Synthesis and characterization of novel hybrid nanomaterials based on β-cyclodextrine grafted halloysite nanotubes for delivery of doxorubicin to MCF-7 cell line. J. Mol. Struct. 2022, 1262, 133004. [Google Scholar] [CrossRef]
- Xu, J.; Yan, R.; Wang, H.; Du, Z.; Gu, J.; Cheng, X.; Xiong, J. A novel biocompatible zwitterionic polyurethane with AIE effect for cell imaging in living cells. RSC Adv. 2018, 8, 6798–6804. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, H.; Yang, S.; He, W.; Luan, Y. Redox-sensitive mPEG-SS-PTX/TPGS mixed micelles: An efficient drug delivery system for overcoming multidrug resistance. Int. J. Pharm. 2016, 515, 281–292. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Luo, X.; Chen, M.; Chen, Z.; Zhao, Y.; Li, X. Folate-decorated and reduction-sensitive micelles assembled from amphiphilic polymer–camptothecin conjugates for intracellular drug delivery. Mol. Pharm. 2014, 11, 4258–4269. [Google Scholar] [CrossRef]
- Tang, D.; Zhao, X.; Yang, T.; Wang, C. Paclitaxel prodrug based mixed micelles for tumor-targeted chemotherapy. RSC Adv. 2018, 8, 380–389. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Li, Z.; Liang, N.; Liu, J.; Yan, P.; Sun, S. AIE-Featured Redox-Sensitive Micelles for Bioimaging and Efficient Anticancer Drug Delivery. Int. J. Mol. Sci. 2022, 23, 10801. https://doi.org/10.3390/ijms231810801
Zhao W, Li Z, Liang N, Liu J, Yan P, Sun S. AIE-Featured Redox-Sensitive Micelles for Bioimaging and Efficient Anticancer Drug Delivery. International Journal of Molecular Sciences. 2022; 23(18):10801. https://doi.org/10.3390/ijms231810801
Chicago/Turabian StyleZhao, Wei, Zixue Li, Na Liang, Jiyang Liu, Pengfei Yan, and Shaoping Sun. 2022. "AIE-Featured Redox-Sensitive Micelles for Bioimaging and Efficient Anticancer Drug Delivery" International Journal of Molecular Sciences 23, no. 18: 10801. https://doi.org/10.3390/ijms231810801
APA StyleZhao, W., Li, Z., Liang, N., Liu, J., Yan, P., & Sun, S. (2022). AIE-Featured Redox-Sensitive Micelles for Bioimaging and Efficient Anticancer Drug Delivery. International Journal of Molecular Sciences, 23(18), 10801. https://doi.org/10.3390/ijms231810801





