Beneficial Effect of Gastrodia elata Blume and Poria cocos Wolf Administration on Acute UVB Irradiation by Alleviating Inflammation through Promoting the Gut-Skin Axis
Abstract
1. Introduction
2. Results
2.1. Total Polyphenol and Flavonoids Contents
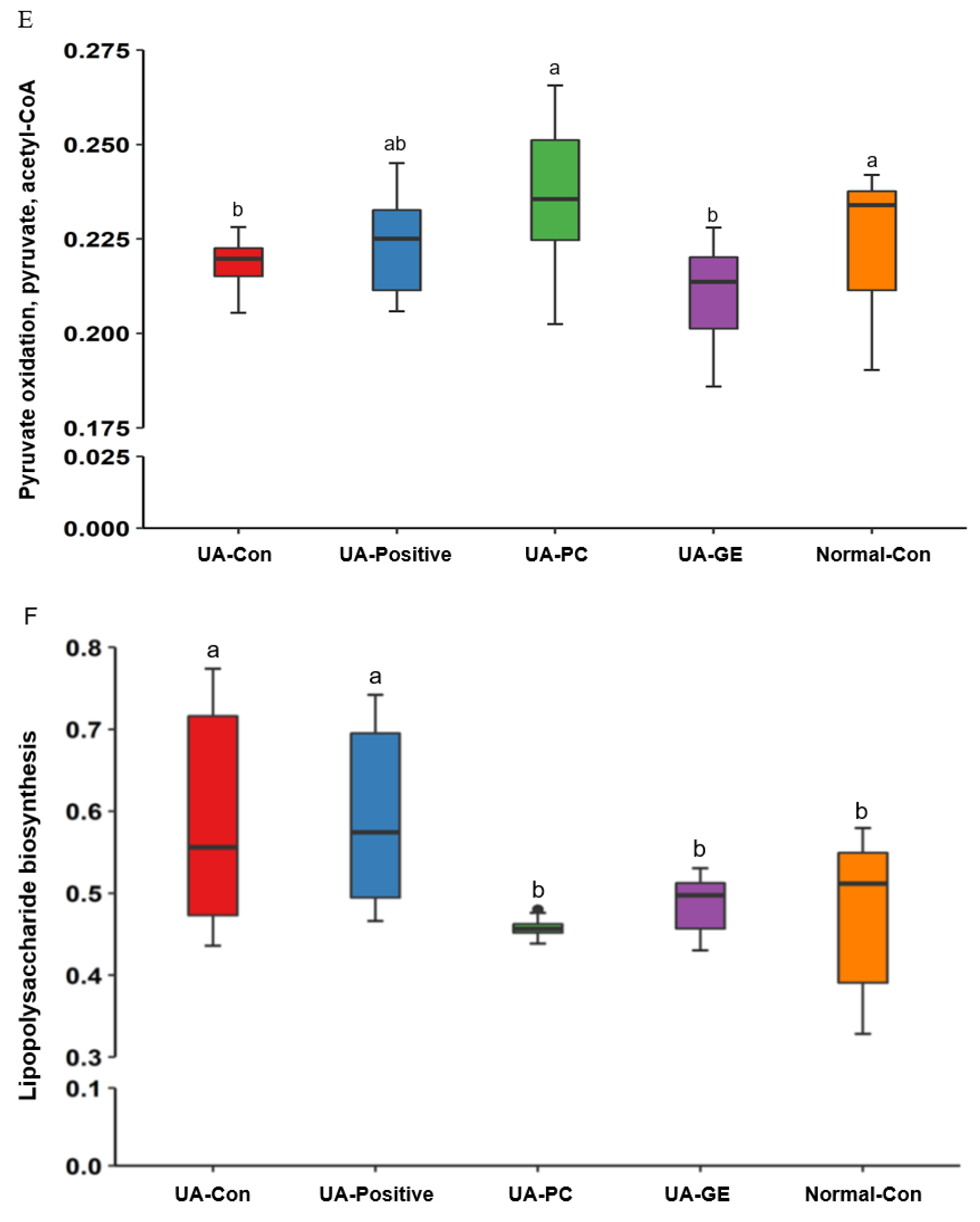
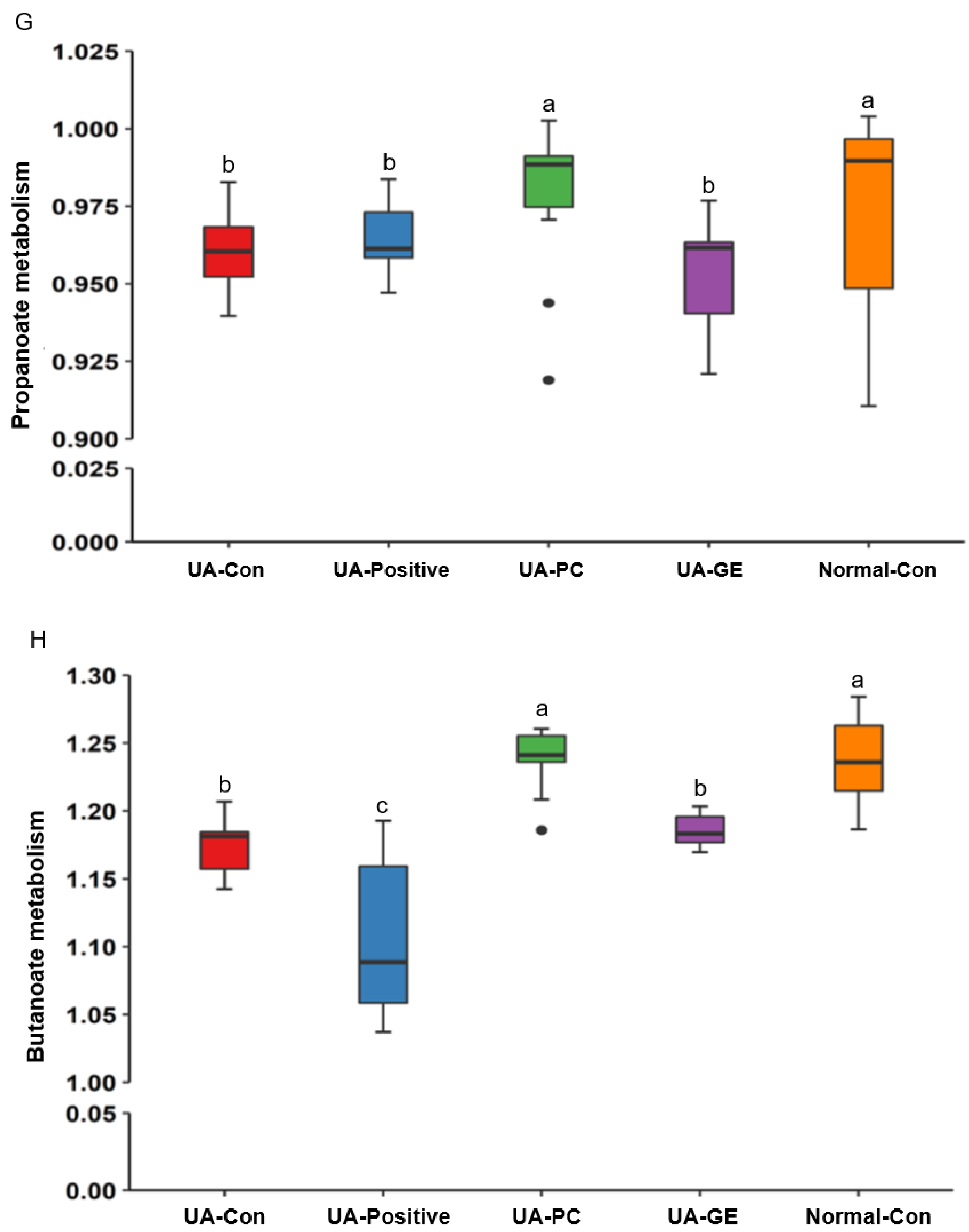
2.2. Energy Metabolism
2.3. Evaluation of Changes in Skin Lesions in Mice
2.4. Histopathology of Skin in UVB-Exposed Mice
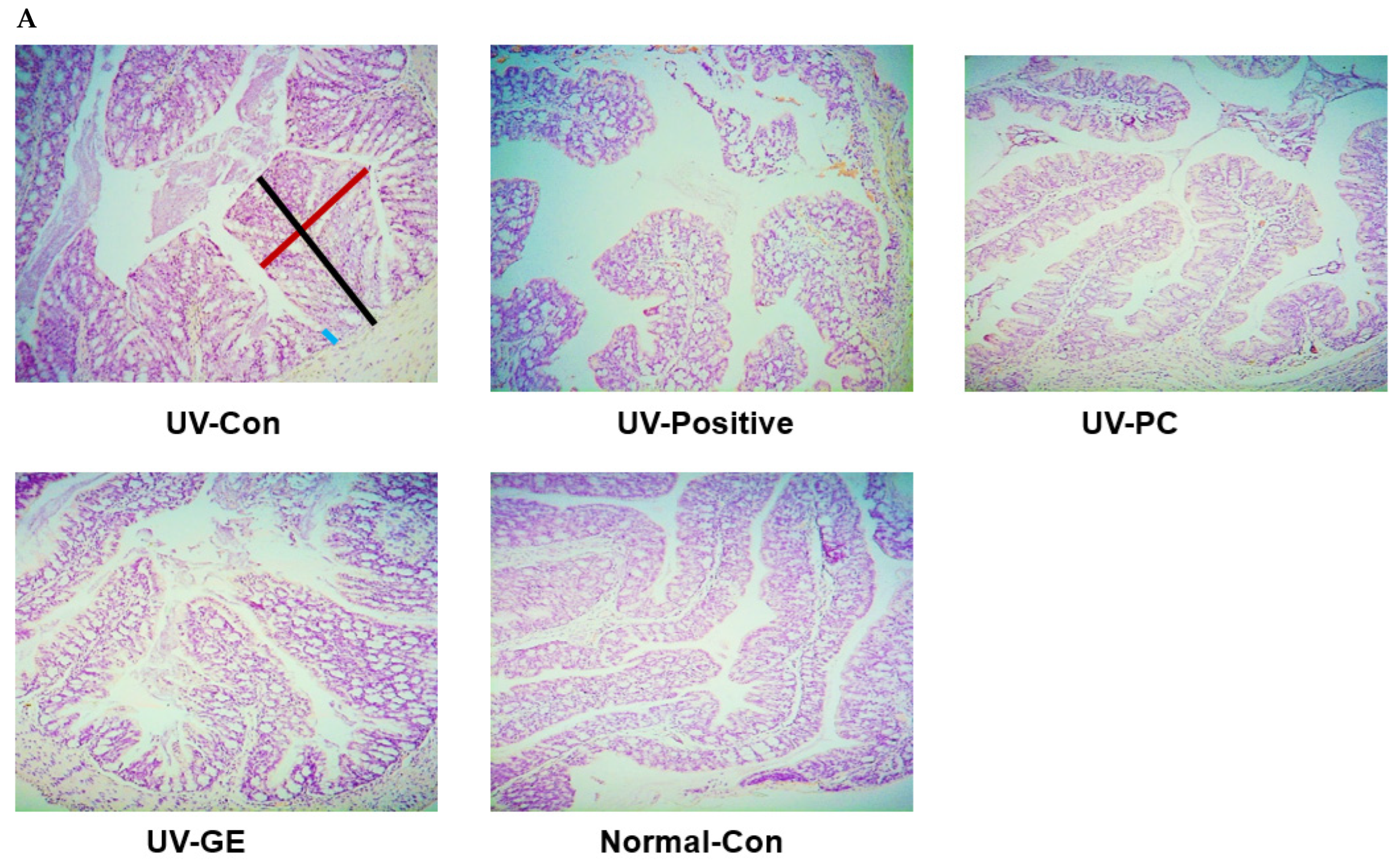
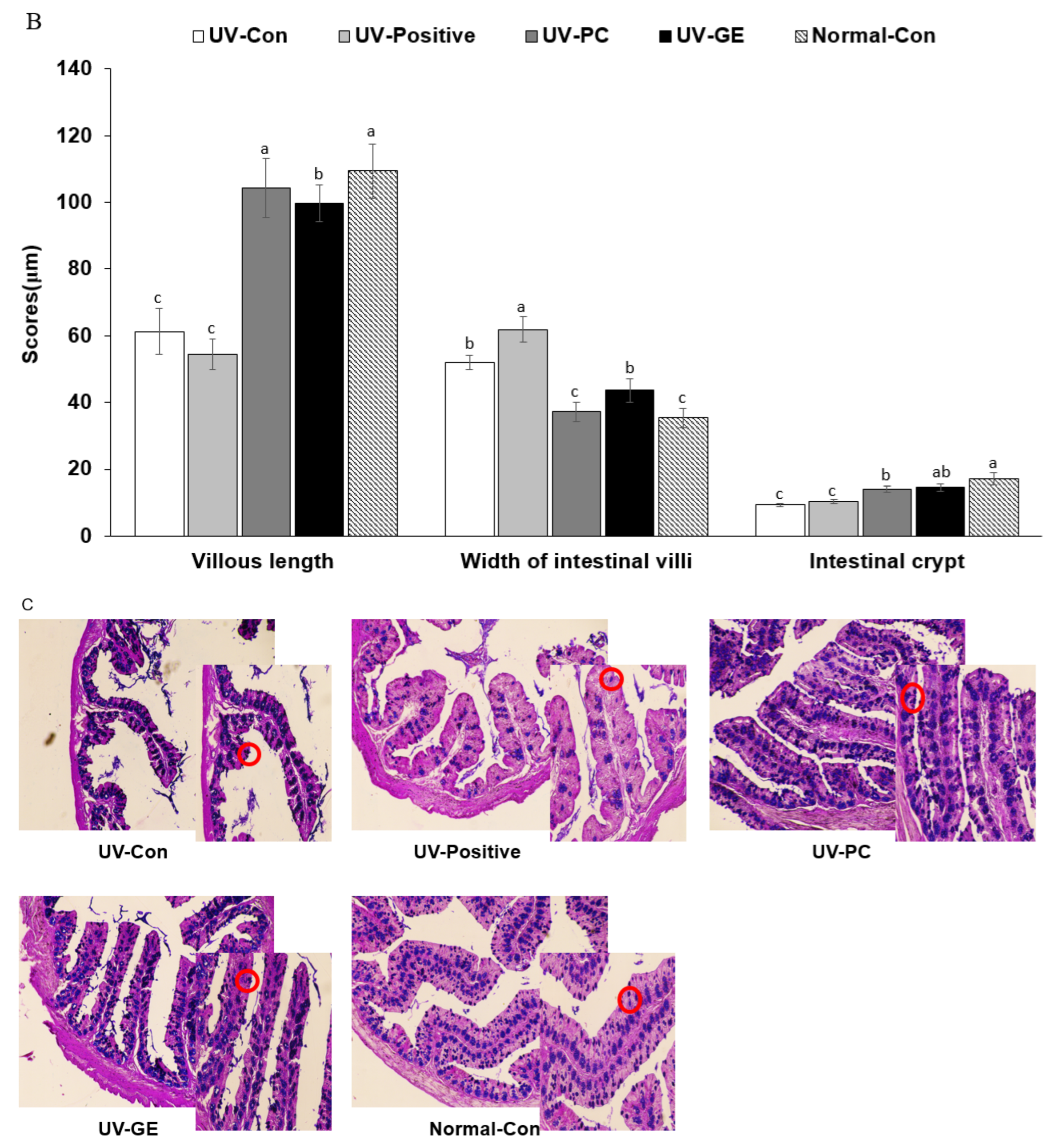
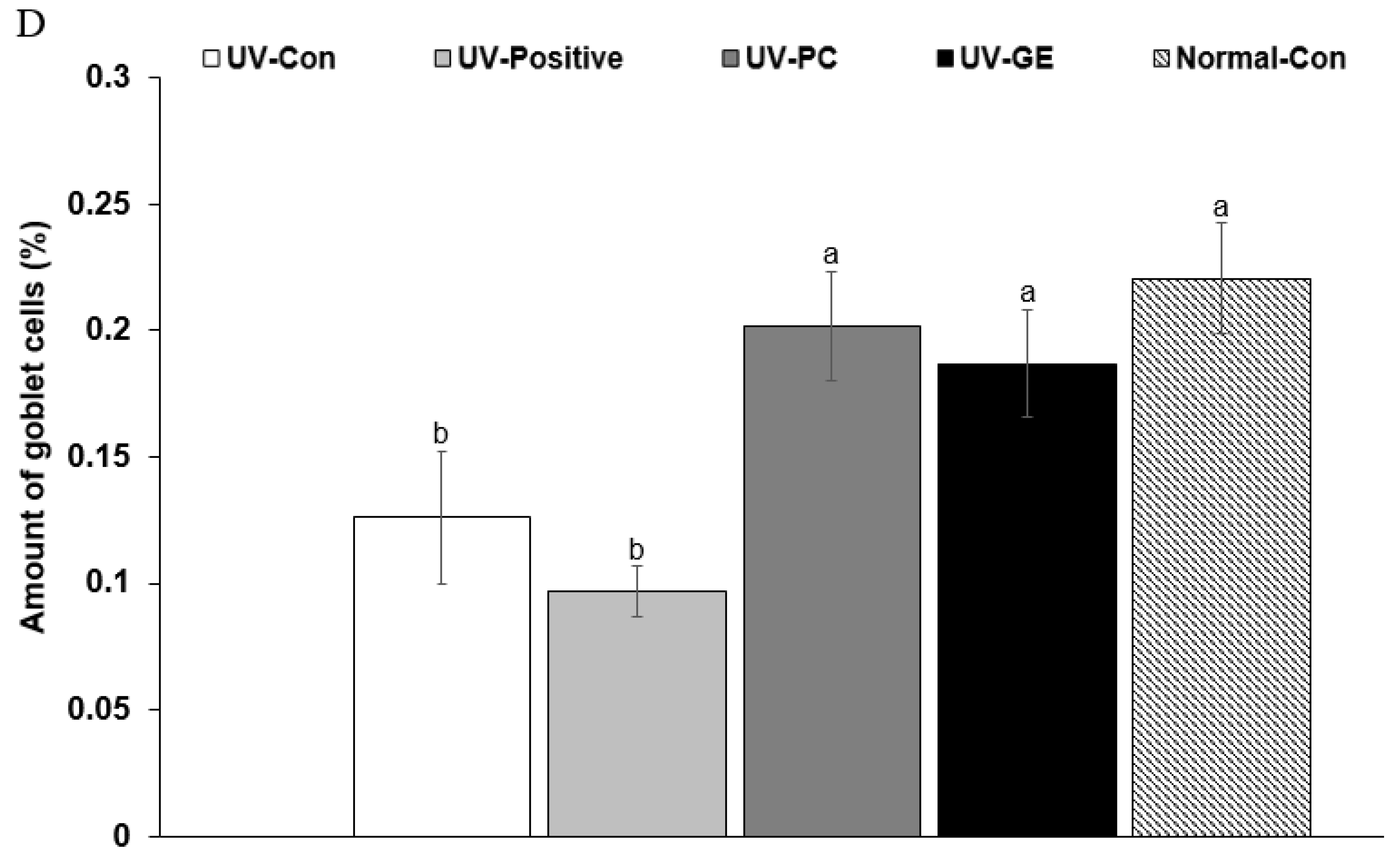
2.5. Histopathological Analysis of the Colon
2.6. Skin and Liver Damage Index
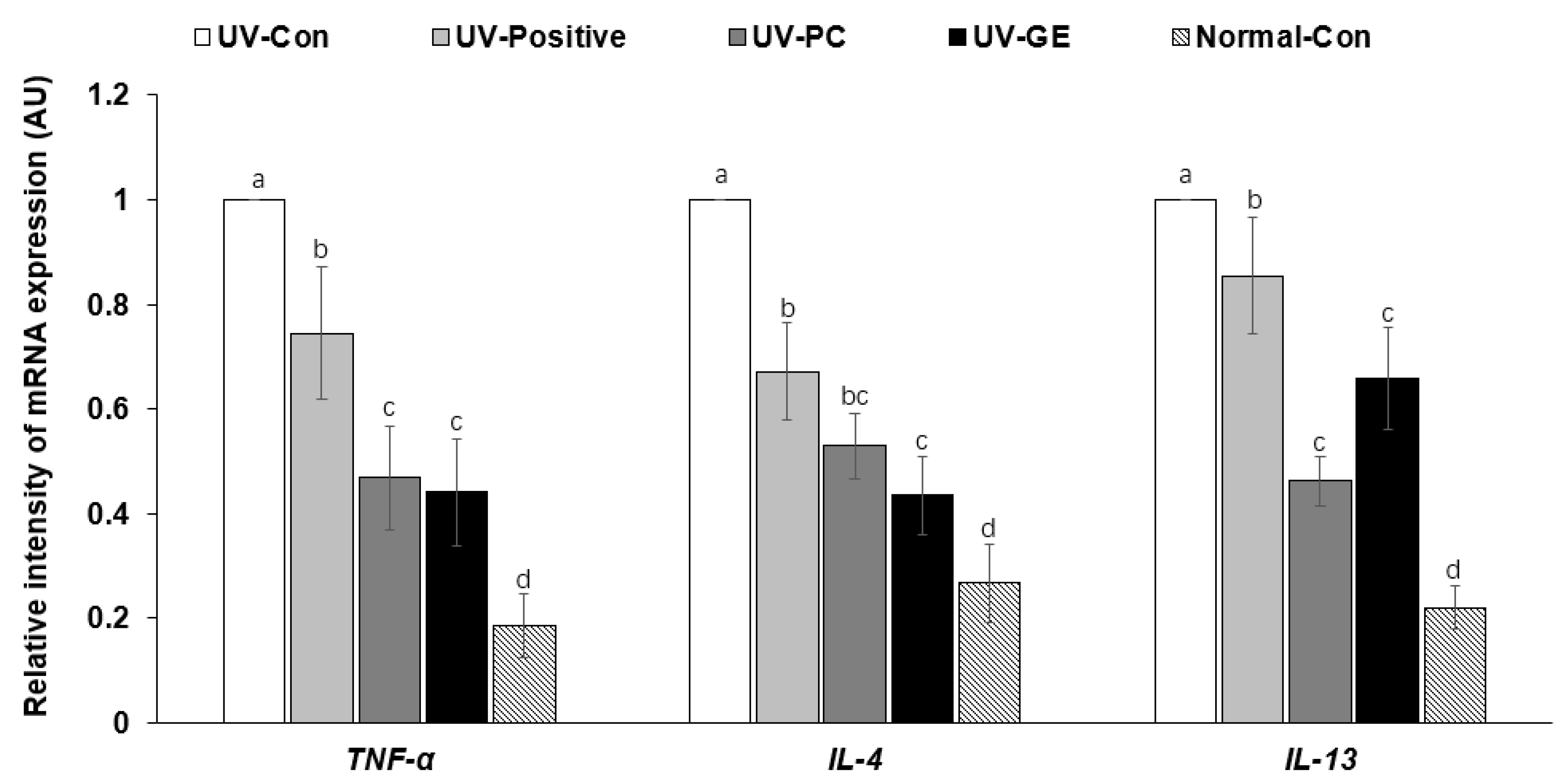
2.7. mRNA Expression Levels of Proinflammatory Cytokines in the Dorsal Skin
2.8. Short-Chain Fatty Acid Concentrations of the Blood from the Portal Vein
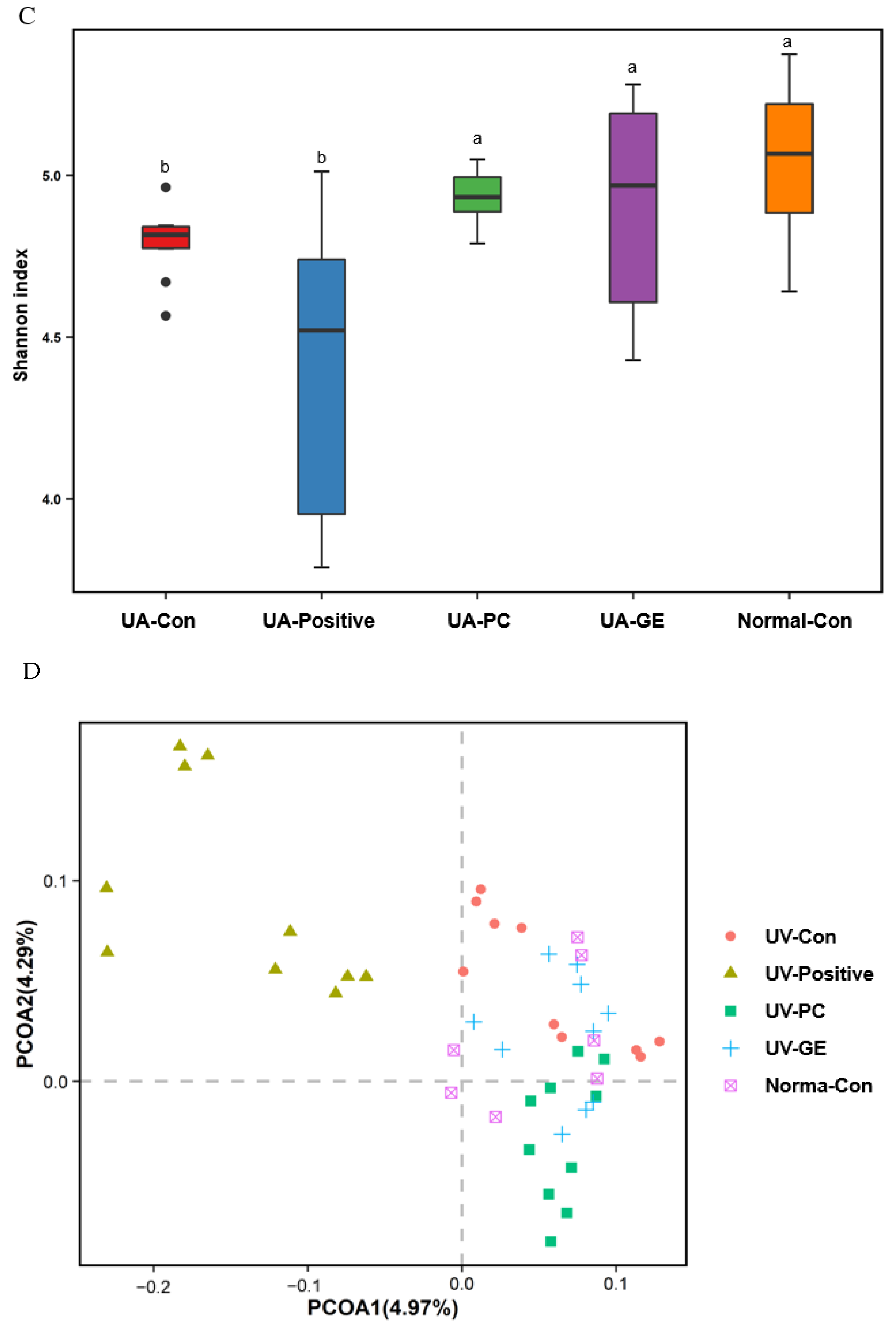
2.9. Gut Microbiota Community
3. Discussion
4. Materials and Methods
4.1. Extraction, Lyophilization, and Quantification of Phenolics
4.2. Animal Care
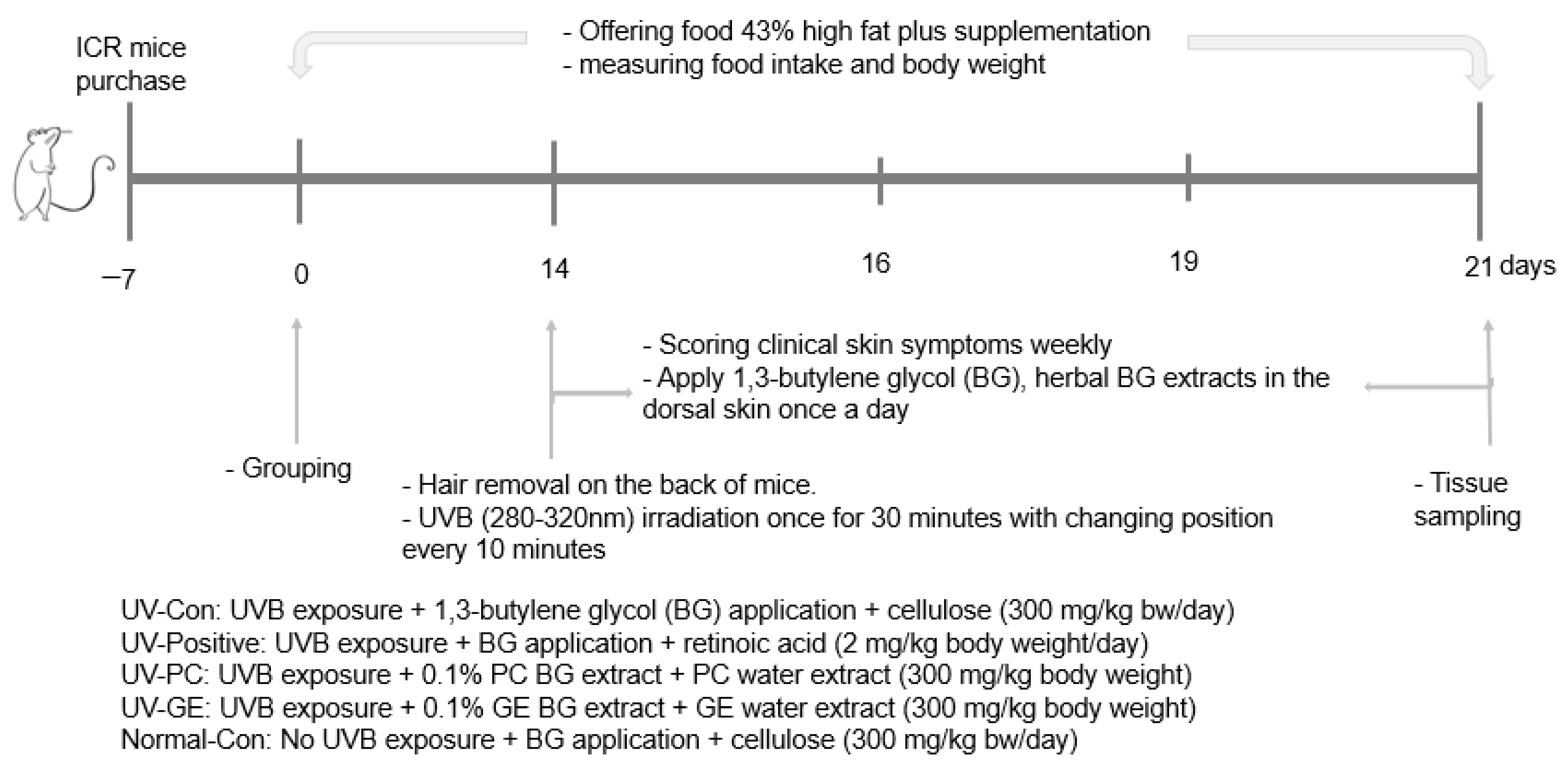
4.3. Experimental Design, UV-B Exposure, and Herbal Treatments
4.4. Evaluation of Clinical Symptoms by Acute UVB Irradiation
4.5. Sample Collection and Tissue Preparation
4.6. Biochemical Test
4.7. Skin Real-Time Quantitative PCR
4.8. NGS of Gut Microbes
4.9. Metagenomic Function Analysis of Gut Microbiome by Picrust2 Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yue, X.; Zhao, Y.; Du, L.; Xie, Z.; Yuan, Y.; Zhang, S.; Li, F.; Feng, J.; Hu, H. Mechanisms of Broad-Band UVB Irradiation—Induced Itch in Mice. J. Investig. Dermatol. 2021, 141, 2499–2508.e2493. [Google Scholar] [CrossRef] [PubMed]
- Arisi, M.; Zane, C.; Caravello, S.; Rovati, C.; Zanca, A.; Venturini, M.; Calzavara-Pinton, P. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front. Med. 2018, 5, 235. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharm. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxid. Med. Cell Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, Y.H.; Hu, S.; Yang, S.H.; Chen, J.L.; Chen, Y.C. Identifying Chinese herbal medicine network for eczema: Implications from a nationwide prescription database. Evid. Based Complement. Altern. Med. 2015, 2015, 347164. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, Y.; Luo, R.C.; Li, A.M. Aspirin for the primary prevention of skin cancer: A meta-analysis. Oncol. Lett. 2015, 9, 1073–1080. [Google Scholar] [CrossRef]
- Xu, J.; Xia, Z. Traditional Chinese Medicine (TCM)—Does its contemporary business booming and globalization really reconfirm its medical efficacy & safety? Med. Drug Discov. 2019, 1, 100003. [Google Scholar]
- Liu, Z.; Hu, Y.; Li, X.; Mei, Z.; Wu, S.; He, Y.; Jiang, X.; Sun, J.; Xiao, J.; Deng, L.; et al. Nanoencapsulation of Cyanidin-3- O-glucoside Enhances Protection Against UVB-Induced Epidermal Damage through Regulation of p53-Mediated Apoptosis in Mice. J. Agric. Food Chem. 2018, 66, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Son, Y.; Kang, S.S.; Bae, C.S.; Kim, J.C.; Kim, S.H.; Shin, T.; Moon, C. Neuropharmacological Potential of Gastrodia elata Blume and Its Components. Evid. Based Complement. Altern. Med. 2015, 2015, 309261. [Google Scholar] [CrossRef]
- Hwang, S.M.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Anti-inflammatory effect of Gastrodia elata rhizome in human umbilical vein endothelial cells. Am. J. Chin. Med. 2009, 37, 395–406. [Google Scholar] [CrossRef]
- Sun, Y. Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives. Int. J. Biol. Macromol. 2014, 68, 131–134. [Google Scholar] [CrossRef]
- Ríos, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Pangestuti, R.; Shin, K.H.; Kim, S.K. Anti-Photoaging and Potential Skin Health Benefits of Seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef]
- Myung, D.B.; Han, H.S.; Shin, J.S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice. Nutrients 2019, 11, 2354. [Google Scholar] [CrossRef]
- Conteville, L.C.; Vicente, A.C.P. Skin exposure to sunlight: A factor modulating the human gut microbiome composition. Gut Microbes. 2020, 11, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Correction: Yoo, J.Y.; et al. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587, Correction in Microorganisms 2020, 8, 2046. [Google Scholar] [CrossRef] [PubMed]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chiou, Y.S.; Tsai, M.L.; Ho, C.T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J. Tradit. Complement. Med. 2011, 1, 8–24. [Google Scholar] [CrossRef]
- Mu, J.; Ma, H.; Chen, H.; Zhang, X.; Ye, M. Luteolin Prevents UVB-Induced Skin Photoaging Damage by Modulating SIRT3/ROS/MAPK Signaling: An in vitro and in vivo Studies. Front. Pharmacol. 2021, 12, 2236. [Google Scholar] [CrossRef]
- Moon, N.R.; Kang, S.; Park, S. Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-β1 and wnt signaling pathways. J. Photochem. Photobiol. B 2018, 178, 92–100. [Google Scholar] [CrossRef]
- Tsai, C.F.; Huang, C.L.; Lin, Y.L.; Lee, Y.C.; Yang, Y.C.; Huang, N.K. The neuroprotective effects of an extract of Gastrodia elata. J. Ethnopharmacol. 2011, 138, 119–125. [Google Scholar] [CrossRef]
- Ojemann, L.M.; Nelson, W.L.; Shin, D.S.; Rowe, A.O.; Buchanan, R.A. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006, 8, 376–383. [Google Scholar] [CrossRef]
- Hoemberg, M.; Schwenzfeur, R.; Berthold, F.; Simon, T.; Hero, B. Hypercalcemia is a frequent side effect of 13-cis-retinoic acid treatment in patients with high-risk neuroblastoma. Pediatr. Blood Cancer 2022, 69, e29374. [Google Scholar] [CrossRef]
- Gorman, S.; Black, L.J.; Feelisch, M.; Hart, P.H.; Weller, R. Can skin exposure to sunlight prevent liver inflammation? Nutrients 2015, 7, 3219–3239. [Google Scholar] [CrossRef]
- Han, S.; Huang, D.; Lan, T.; Wu, Y.; Wang, Y.; Wei, J.; Zhang, W.; Ou, Y.; Yan, Q.; Liu, P.; et al. Therapeutic Effect of Seawater Pearl Powder on UV-Induced Photoaging in Mouse Skin. Evid. Based Complement. Altern. Med. 2021, 2021, 9516427. [Google Scholar] [CrossRef] [PubMed]
- Skopelja-Gardner, S.; Tai, J.; Sun, X.; Tanaka, L.; Kuchenbecker, J.A.; Snyder, J.M.; Kubes, P.; Mustelin, T.; Elkon, K.B. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019097118. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Mahamed, A.H.; Alarcon, J.F.; Al Suwailem, A.; Agustí, S. Adverse Effects of Ultraviolet Radiation on Growth, Behavior, Skin Condition, Physiology, and Immune Function in Gilthead Seabream (Sparus aurata). Front. Mar. Sci. 2020, 7, 306. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, B.C.; Daily, J.W.; Park, S. High genetic risk scores for impaired insulin secretory capacity doubles the risk for type 2 diabetes in Asians and is exacerbated by Western-type diets. Diab. Metab. Res. Rev. 2018, 34, e2944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wei, X.L.; Xiao, S.Q. Effects of glutamine on exercise-induced fatigue, skeletal muscle oxidation and liver cell apoptosis in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2021, 37, 293–296. [Google Scholar]
- Li, Y.; Wang, L.M.; Xu, J.Z.; Tian, K.; Gu, C.X.; Li, Z.F. Gastrodia elata attenuates inflammatory response by inhibiting the NF-κB pathway in rheumatoid arthritis fibroblast-like synoviocytes. Biomed. Pharmacother. 2017, 85, 177–181. [Google Scholar] [CrossRef]
- Kim, M.J.; Yang, H.J.; Moon, B.R.; Kim, J.E.; Kim, K.S.; Park, S. Gastrodia elata Blume Rhizome Aqueous Extract Improves Arterial Thrombosis, Dyslipidemia, and Insulin Response in Testosterone-Deficient Rats. Evid. Based Complem. Altern. Med. 2017, 2017, 2848570. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kim, D.S.; Lee, Y.H.; Kim, J.E.; Park, S. Anti-Diabetic Activities of Gastrodia elata Blume Water Extracts Are Mediated Mainly by Potentiating Glucose-Stimulated Insulin Secretion and Increasing β-Cell Mass in Non-Obese Type 2 Diabetic Animals. Nutrients 2016, 8, 161. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- França, K. Topical Probiotics in Dermatological Therapy and Skincare: A Concise Review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Geng, R.; Kang, S.G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, H.H.; Ni, L.; Ran, J.; Hu, J.; Yu, J.; Zhao, X. Nicotinamide Mononucleotide Combined with Lactobacillus fermentum TKSN041 Reduces the Photoaging Damage in Murine Skin by Activating AMPK Signaling Pathway. Front. Pharmacol. 2021, 12, 643089. [Google Scholar] [CrossRef] [PubMed]
- Bäsler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 2016, 242, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Lin, Y.K.; Yang, S.C.; Alalaiwe, A.; Lin, C.J.; Fang, J.Y.; Lin, C.F. Percutaneous absorption of resveratrol and its oligomers to relieve psoriasiform lesions: In silico, in vitro and in vivo evaluations. Int. J. Pharm. 2020, 585, 119507. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Xu, J.D. Goblet cells and their functions in the intestine. Shijie Huaren Xiaohua Zazhi. 2017, 25, 1279–1286. [Google Scholar]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Ottman, N.A. Host Immunostimulation and Substrate Utilization of the Gut Symbiont Akkermansia Muciniphila. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Reese, A.T.; Dunn, R.R. Drivers of Microbiome Biodiversity: A Review of General Rules, Feces, and Ignorance. MBio 2018, 9, e01294-18. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.R.; Kim, N.R.; Park, S.J.; Lee, M.; Kim, O.K. Combination of Bifidobacterium longum and Galacto-Oligosaccharide Protects the Skin from Photoaging. J. Med. Food. 2021, 24, 606–616. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Yezerska, O.; Shanaida, M.; Horčinová Sedláčková, V.; Wieczorek, P.P. Application of the Folin-Ciocalteu method to the evaluation of Salvia sclarea extracts. Pharmacia 2019, 66, 209. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J. Pharm. Sci. 2012, 74, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Gressel, K.L.; Duncan, F.J.; Oberyszyn, T.M.; La Perle, K.M.; Everts, H.B. Endogenous Retinoic Acid Required to Maintain the Epidermis Following Ultraviolet Light Exposure in SKH-1 Hairless Mice. Photochem. Photobiol. 2015, 91, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Qian, W.; Chen, F.; Jin, Y.; Wang, F.; Lu, X.; Lee, S.R.; Su, D.; Chen, B. ATRA protects skin fibroblasts against UV-induced oxidative damage through inhibition of E3 ligase Hrd1. Mol. Med. Rep. 2019, 20, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Invest. 1998, 101, 1432–1440. [Google Scholar] [CrossRef]
- Oh, S.-T.; Lim, J.-H. Development and Effect Analysis of UVB-LED General Lighting to Support Vitamin D Synthesis. Appl. Sci. 2020, 10, 889. [Google Scholar] [CrossRef]
- Nielsen, F.H. 90th Anniversary Commentary: The AIN-93 Purified Diets for Laboratory Rodents-The Development of a Landmark Article in The Journal of Nutrition and Its Impact on Health and Disease Research Using Rodent Models. J. Nutr. 2018, 148, 1667–1670. [Google Scholar] [CrossRef]
- National Research Council; Board on Agriculture; Committee on Animal Nutrition; Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition; National Academies Press: Washington, DC, USA, 1995.
- Eilander, A.; Harika, R.K.; Zock, P.L. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations? Eur. J. Lipid. Sci. Technol. 2015, 117, 1370–1377. [Google Scholar] [CrossRef]
- Yamano, N.; Kunisada, M.; Nishiaki-Sawada, A.; Ohashi, H.; Igarashi, T.; Nishigori, C. Evaluation of Acute Reactions on Mouse Skin Irradiated with 222 and 235 nm UV-C. Photochem. Photobiol. 2021, 97, 770–777. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, J.; Wu, X.; Huang, S.; Yuan, H.; Park, S. Schizonepeta Tenuifolia with Alpinia Oxyphylla Alleviates Atopic Dermatitis and Improves the Gut Microbiome in Nc/Nga Mice. Pharmaceutics 2020, 12, 722. [Google Scholar] [CrossRef]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2021, 60, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
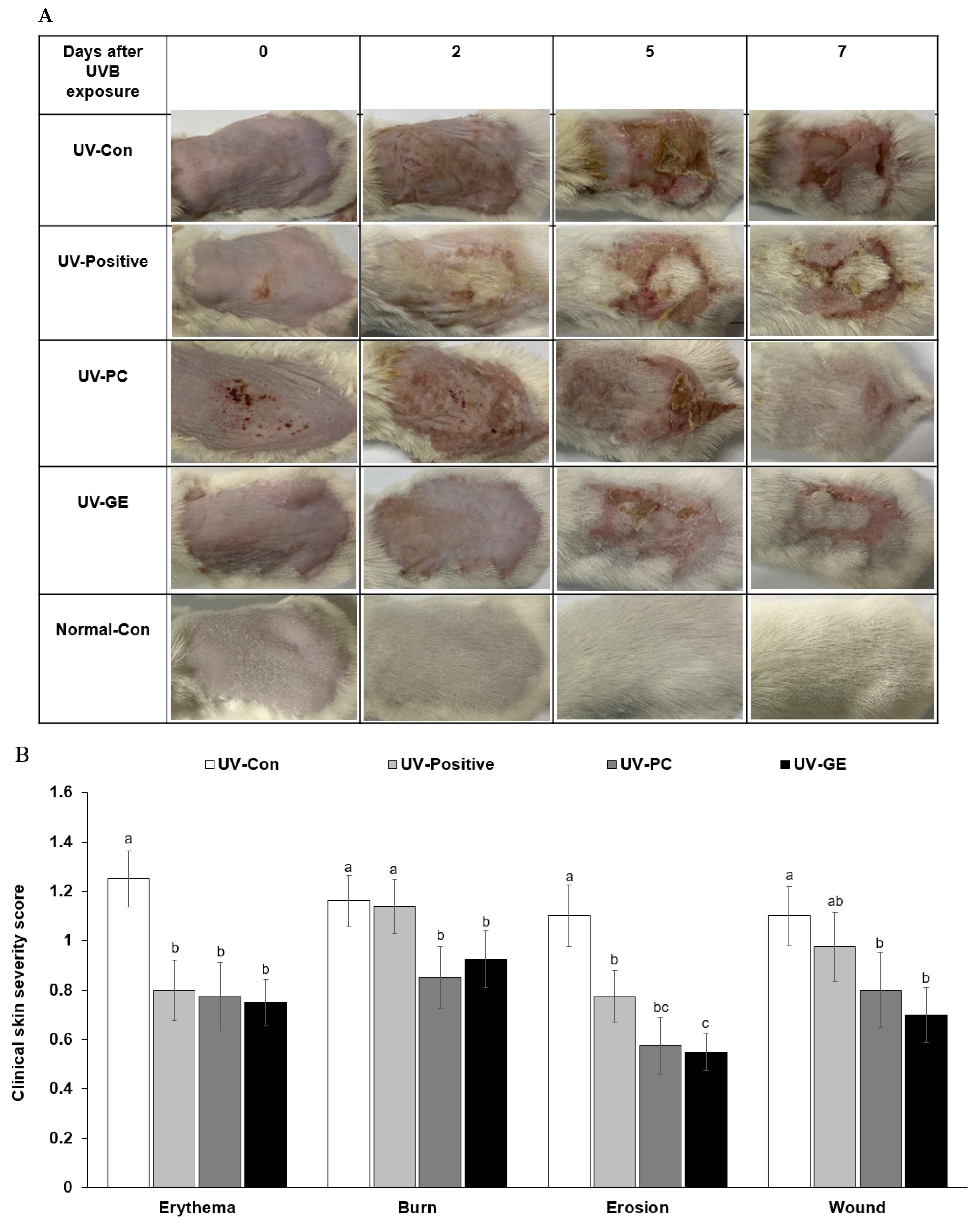
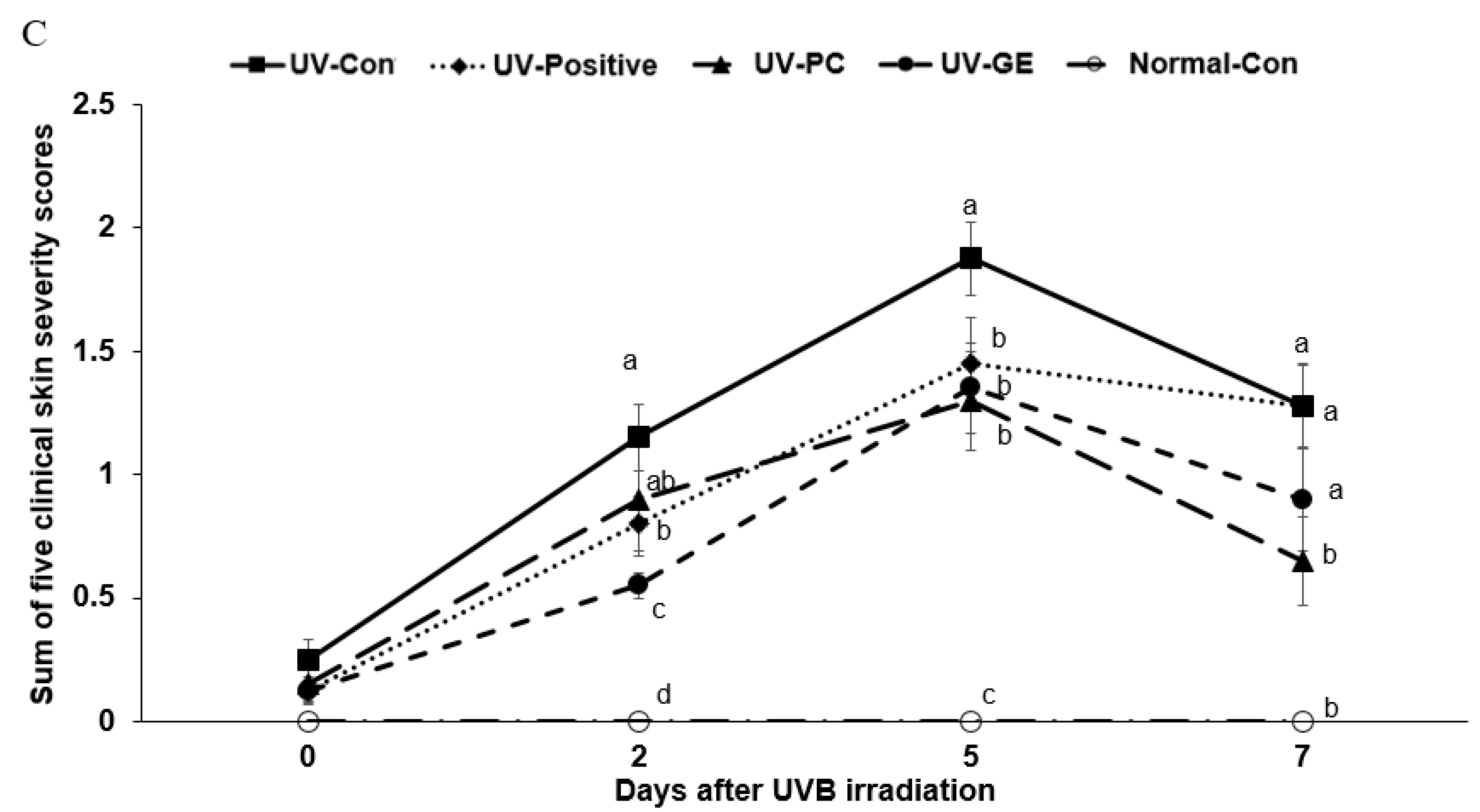
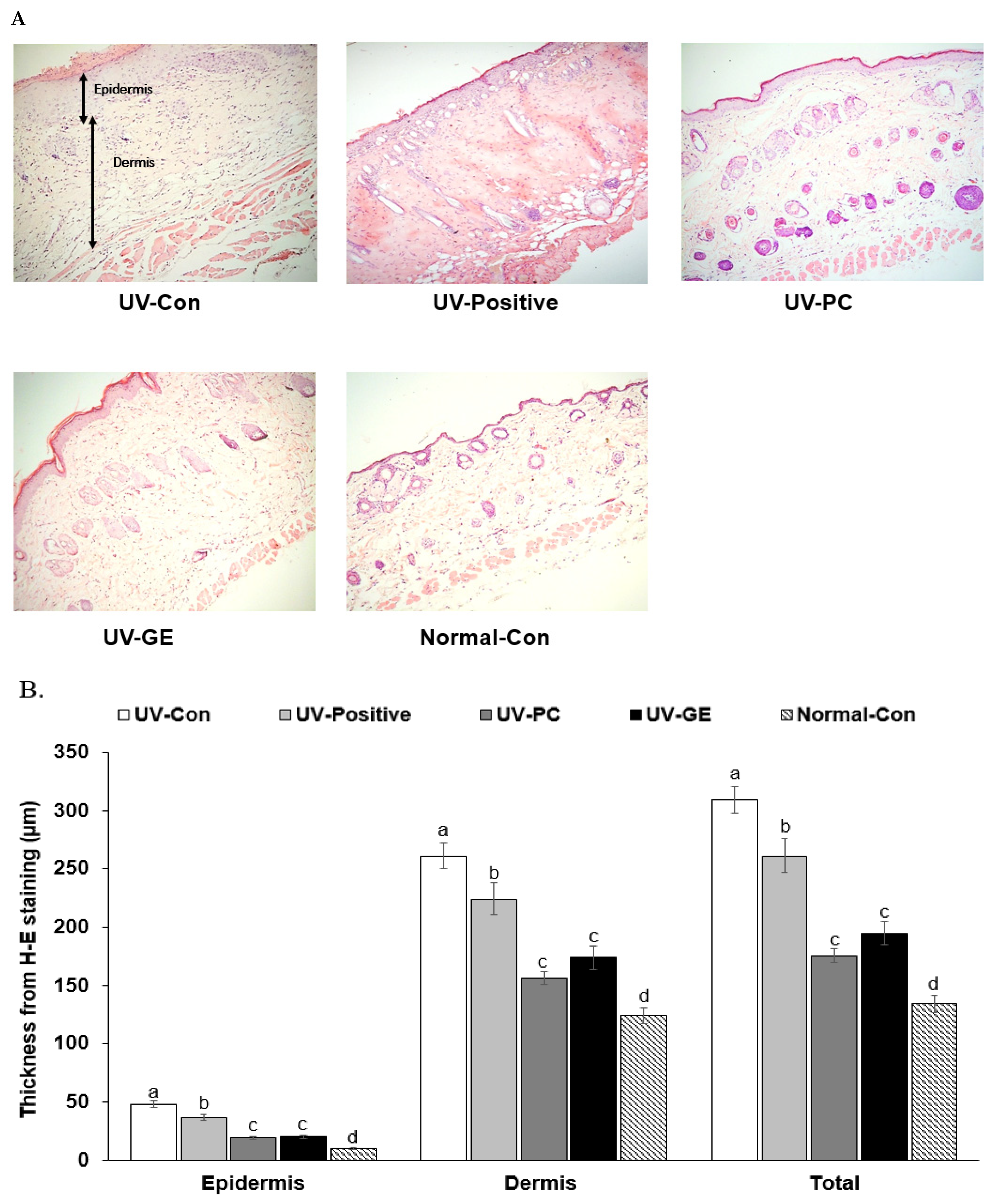
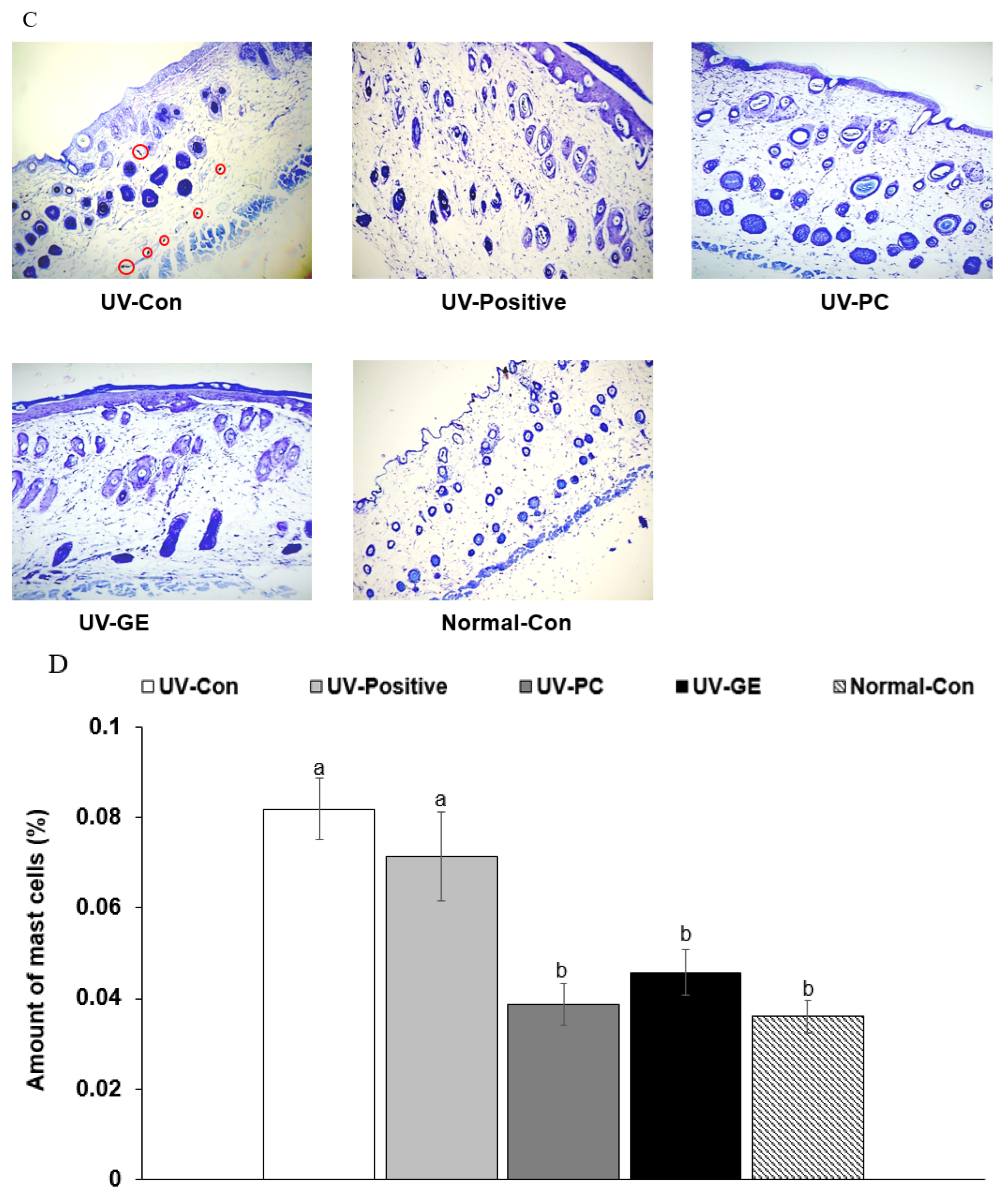

| UV-Con | UV-Positive | UV-PC | UV-GE | Normal-Con | |
|---|---|---|---|---|---|
| Final weight (g) | 33.6 ± 0.84 b | 25.6 ± 0.84 c | 32.2 ± 0.87 b | 36.6 ± 0.85 a | 36.0 ± 1.07 a |
| Weight gain (g) | 3.40 ± 0.82 b | -1.35 ± 0.94 c | 3.10 ± 0.76 b | 4.97 ± 0.91 a | 4.84 ± 0.88 a |
| Food intake (g/day) | 4.66 ± 0.19 | 4.51 ± 0.22 | 4.55 ± 0.38 | 4.89 ± 0.25 | 4.16 ± 0.18 |
| Food efficiency | 0.73 ± 0.27 b | −0.29 ± 0.21 c | 0.71 ± 0.28 b | 1.13 ± 0.24 a | 1.20 ± 0.27 a |
| Epididymal fat (g) | 0.86 ± 0.13 a | 0.38 ± 0.11 b | 0.82 ± 0.13 a | 1.02 ± 0.09 a | 0.88 ± 0.14 a |
| Retroperitoneal fat (g) | 0.38 ± 0.07 a | 0.13 ± 0.03 b | 0.31 ± 0.05 a | 0.41 ± 0.05 a | 0.43 ± 0.08 a |
| Total visceral fat (g) | 1.24 ± 0.18 a | 0.51 ± 0.14 b | 1.12 ± 0.17 a | 1.43 ± 0.14 a | 1.31 ± 0.22 a |
| UV-Con | PA–Positive | UV-PC | UV-GE | Normal-Con | |
|---|---|---|---|---|---|
| Skin TBARs (nmol/mg protein) | 28.51 ± 3.84 a | 31.73 ± 2.48 a | 18.98 ± 1.71 b | 14.37 ± 1.49 c | 16.40 ± 2.80 bc |
| Liver TBARs (nmol/mg protein) | 48.50 ± 2.40 a | 55.83 ± 2.65 a | 41.37 ± 2.74 bc | 38.15 ± 2.50 c | 43.03 ± 3.62 b |
| Serum TNF-α (ng/mL) | 13.2 ± 3.15 a | 12.4 ± 2.82 a | 7.25 ± 1.13 b | 6.16 ± 0.91 b | 5.18 ± 0.69 b |
| Serum AST (mg/dL) | 47.9 ± 1.87 b | 68.5 ± 1.28 a | 40.9 ± 3.00 b | 39.2 ± 1.21 b | 44.9 ± 3.26 b |
| Serum ALT (mg/dL) | 19.6 ± 0.57 a | 20.3 ± 1.69 a | 10.4 ± 1.31 bc | 6.09 ± 0.45 d | 14.8 ± 2.67 b |
| UV-Con | UV-Positive | UV-PC | UV-GE | Normal-Con | |
|---|---|---|---|---|---|
| Acetic acid (mM) | 0.217 ± 0.006 | 0.235 ± 0.011 | 0.254 ± 0.010 | 0.238 ± 0.012 | 0.242 ± 0.021 |
| Propionic acid (mM) | 0.147 ± 0.001 b | 0.159 ± 0.006 ab | 0.161 ± 0.003 a | 0.149 ± 0.002 ab | 0.152 ± 0.004 ab |
| Butyrate acid (mM) | 0.184 ± 0.002 b | 0.200 ± 0.008 ab | 0.208 ± 0.006 a | 0.202 ± 0.006 ab | 0.211 ± 0.012 a |
| Total SCFA (mM) | 0.440 ± 0.057 b | 0.543 ± 0.046 ab | 0.623 ± 0.014 a | 0.591 ± 0.013 a | 0.571 ± 0.054 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Huang, S.; Qiu, J.; Wu, X.; Yuan, H.; Park, S. Beneficial Effect of Gastrodia elata Blume and Poria cocos Wolf Administration on Acute UVB Irradiation by Alleviating Inflammation through Promoting the Gut-Skin Axis. Int. J. Mol. Sci. 2022, 23, 10833. https://doi.org/10.3390/ijms231810833
Zhang T, Huang S, Qiu J, Wu X, Yuan H, Park S. Beneficial Effect of Gastrodia elata Blume and Poria cocos Wolf Administration on Acute UVB Irradiation by Alleviating Inflammation through Promoting the Gut-Skin Axis. International Journal of Molecular Sciences. 2022; 23(18):10833. https://doi.org/10.3390/ijms231810833
Chicago/Turabian StyleZhang, Ting, Shaokai Huang, Jingyi Qiu, Xuangao Wu, Heng Yuan, and Sunmin Park. 2022. "Beneficial Effect of Gastrodia elata Blume and Poria cocos Wolf Administration on Acute UVB Irradiation by Alleviating Inflammation through Promoting the Gut-Skin Axis" International Journal of Molecular Sciences 23, no. 18: 10833. https://doi.org/10.3390/ijms231810833
APA StyleZhang, T., Huang, S., Qiu, J., Wu, X., Yuan, H., & Park, S. (2022). Beneficial Effect of Gastrodia elata Blume and Poria cocos Wolf Administration on Acute UVB Irradiation by Alleviating Inflammation through Promoting the Gut-Skin Axis. International Journal of Molecular Sciences, 23(18), 10833. https://doi.org/10.3390/ijms231810833









