The Endothelial Transcription Factor ERG Mediates a Differential Role in the Aneurysmatic Ascending Aorta with Bicuspid or Tricuspid Aorta Valve: A Preliminary Study
Abstract
:1. Introduction
2. Results
2.1. BAV and TAV Patient Characteristics
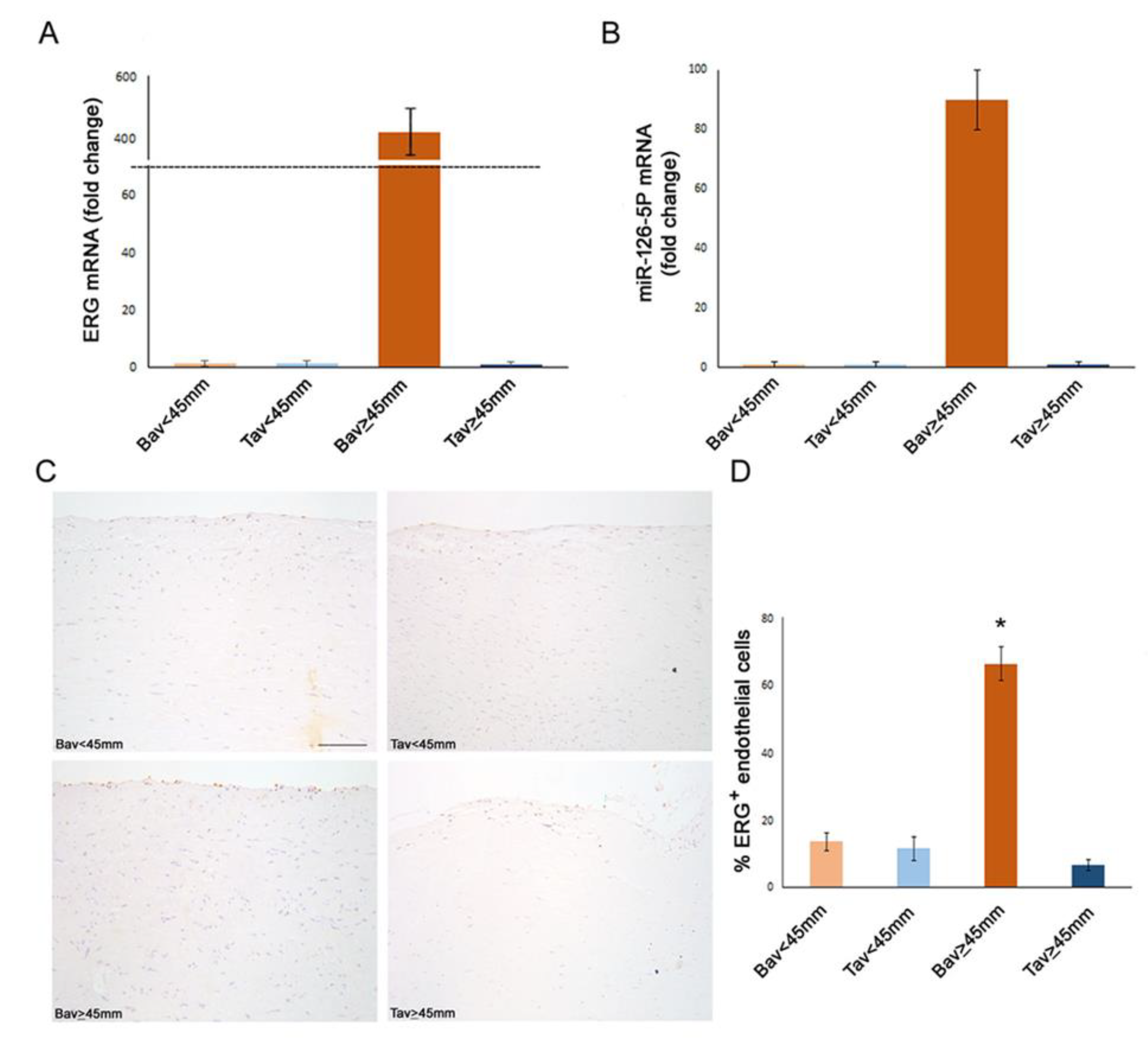
2.2. Differential Expression of Endothelial ERG Transcription Factor in BAV vs. TAV Aortic Intima
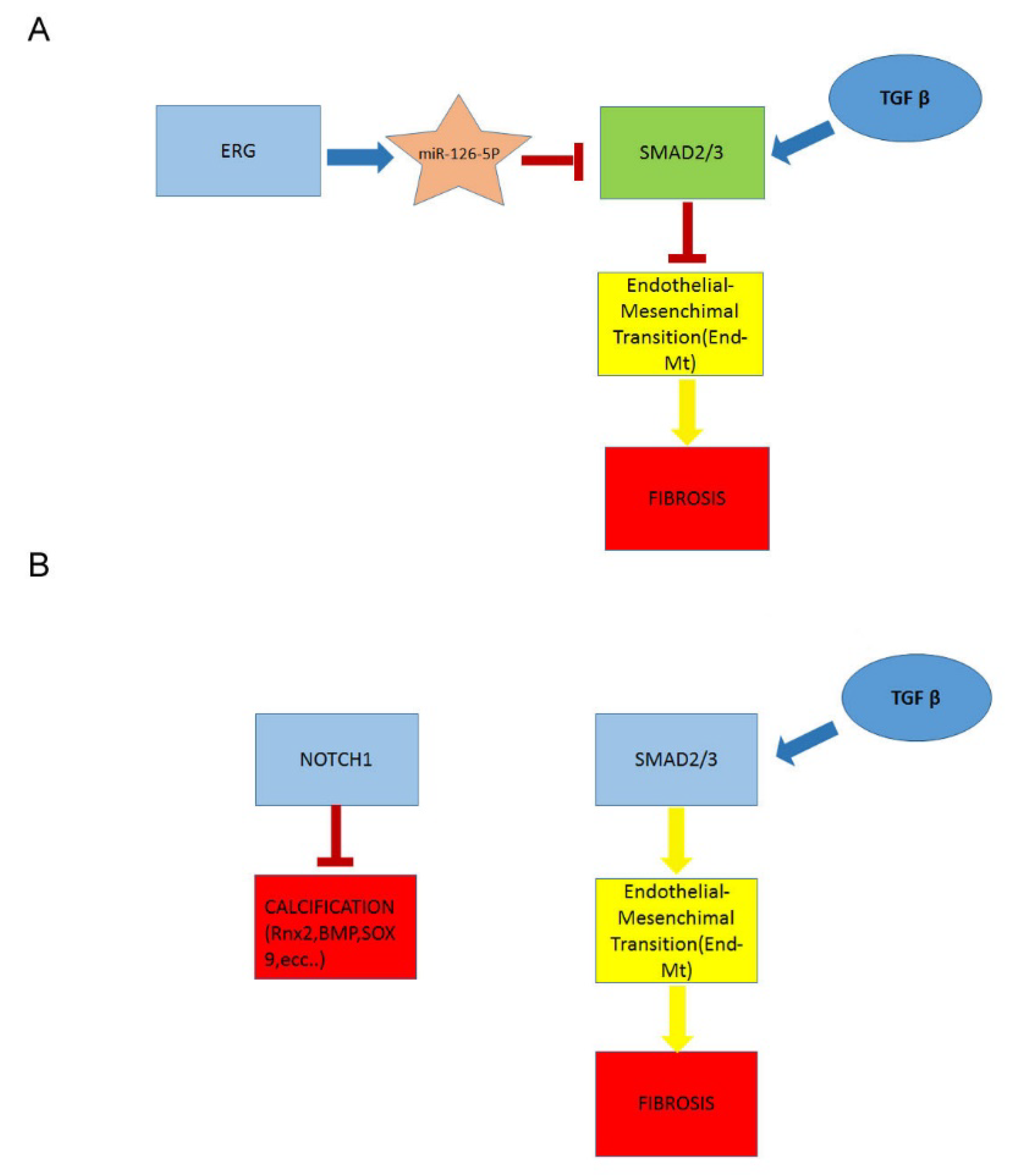
2.3. Upregulation of Tissue ERG Gene Expression in BAV Cases with AAA Correlates with miR126 Levels
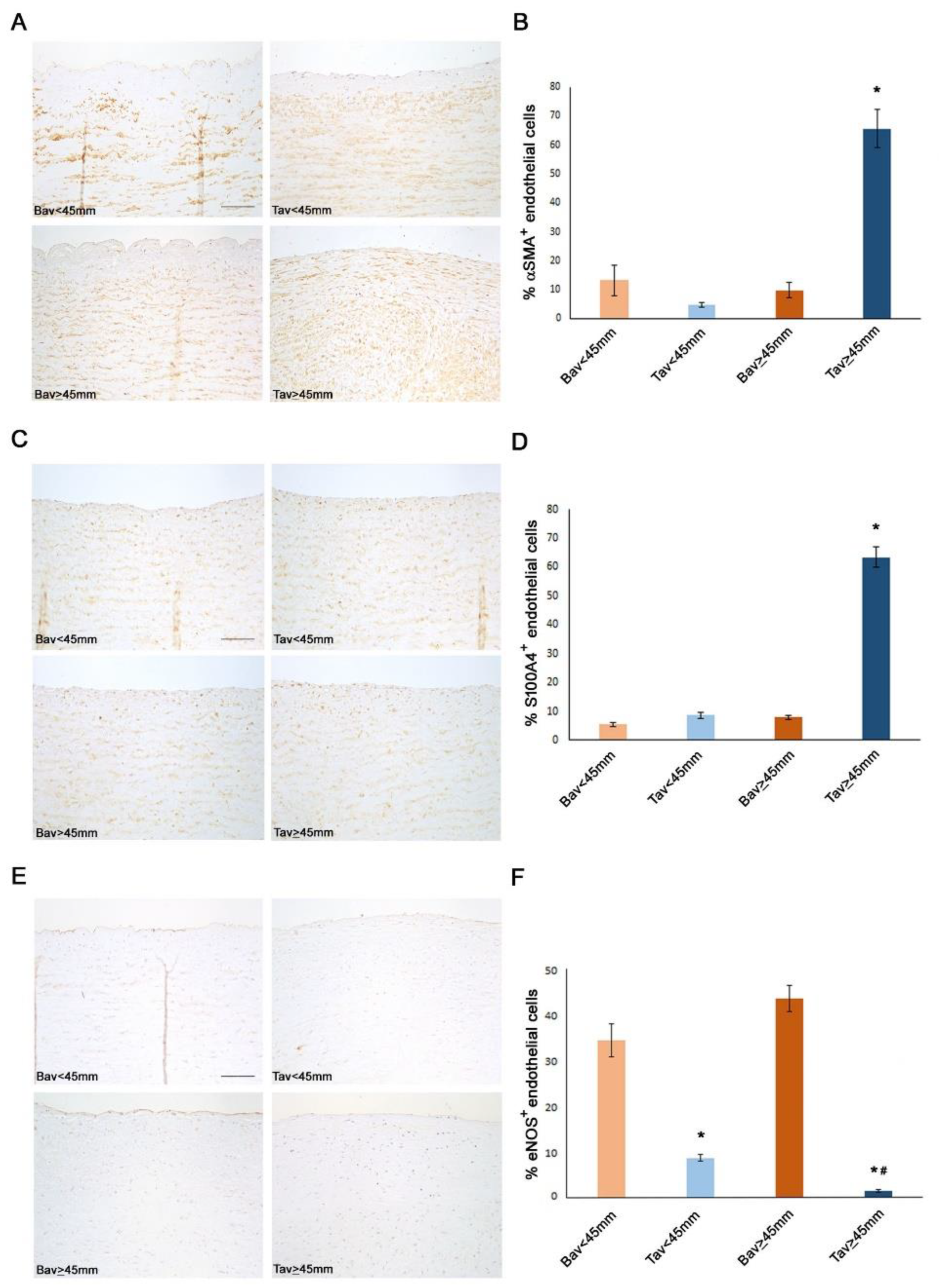
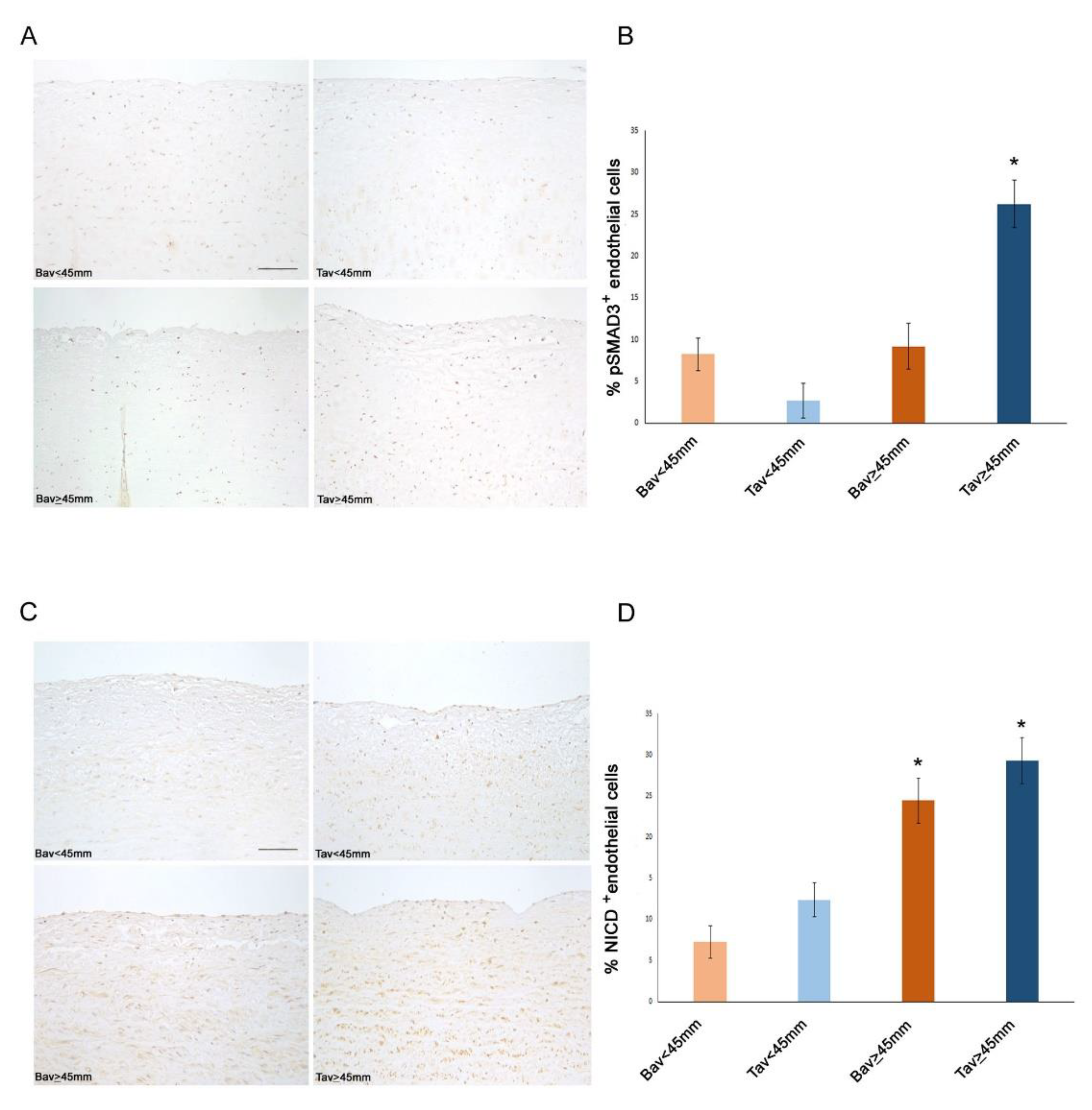
2.4. Downregulation of ERG Gene and miR126 Reciprocally Promotes Higher Expression of SMAD3 in TAV Aortic Tissues with AAA and Higher Levels of αSMA+/S100A4+ EC and EndMTs
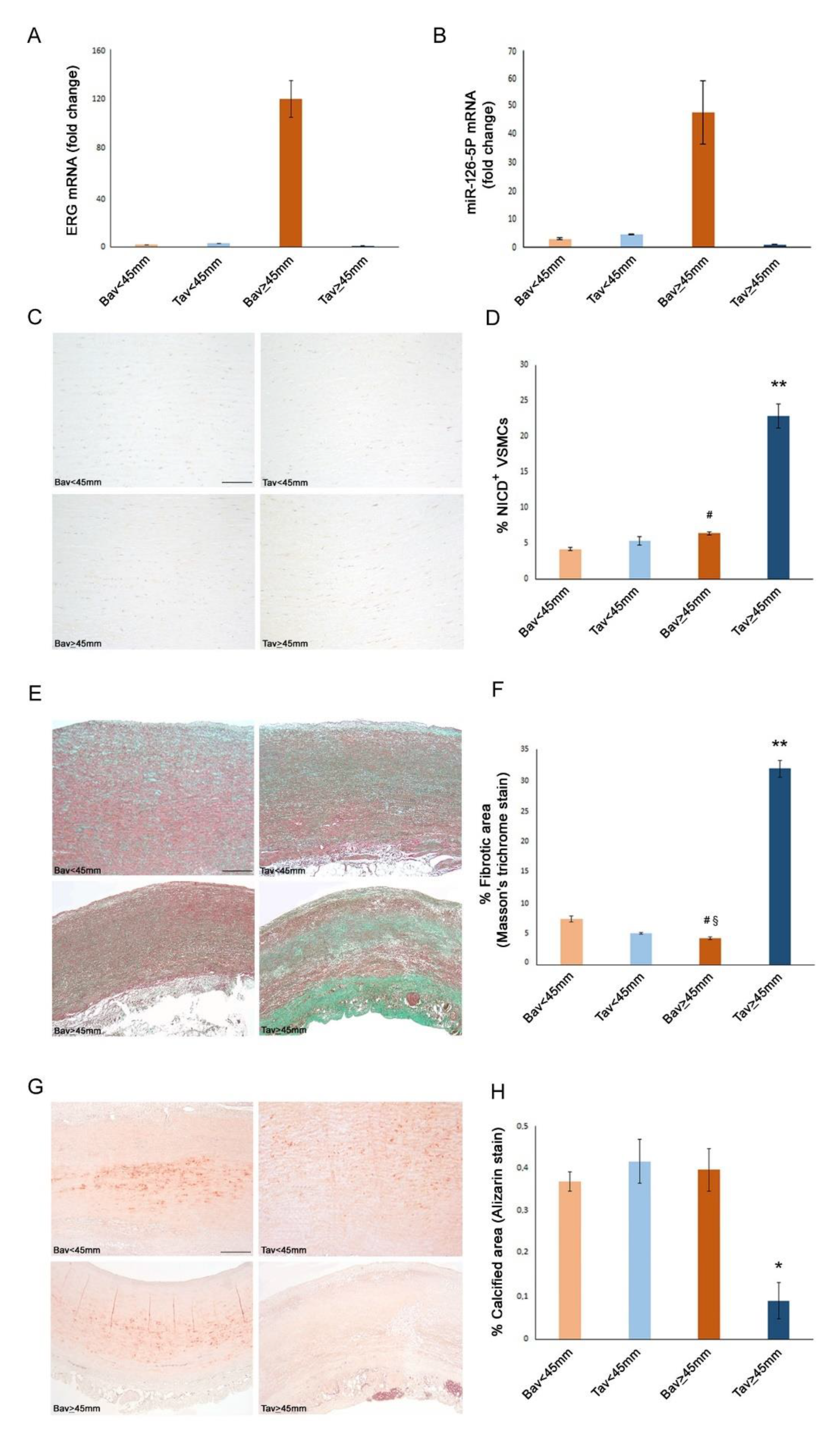
2.5. A Higher Rate of Fibrosis Characterizes TAV Aortic Tissues with AAA, as Well as Increased Calcification in BAV Tissues with AAA
2.6. Decreased Levels of Notch Intracellular Domain (NICD) in EC and VSMCs from BAV vs. TAV Aorta Tissues
2.7. Higher eNOS Levels in BAV vs. TAV Aortic Tissues
2.8. Upregulated Expression of ERG Endothelial Transcription Factor and miR-126-5P in Aortic Medial Tissues
3. Discussion
4. Materials and Methods
4.1. Population Enrolled
4.2. Histochemical and Immunohistochemical Analysis
4.3. Gene Expression Analysis
4.4. Statistical Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paterick, T.E.; Humphries, J.A.; Ammar, K.A.; Jan, M.F.; Loberg, R.; Bush, M.; Khandheria, B.K.; Tajik, A.J. Aortopathies: Etiologies, Genetics, Differential Diagnosis, Prognosis and Management. Am. J. Med. 2013, 126, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Balistreri, C.R.; Ricasoli, A.; Ruvolo, G. Cardiovascular Disease in Ageing: An Overview on Thoracic Aortic Aneurysm as an Emerging Inflammatory Disease. Mediat. Inflamm. 2017, 2017, 1274034. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, M.Y.; Sasidharan, S.; Frattolin, J.; Edgar, L.; Stock, U.; Athanasiou, T.; Moore, J. Regional variation in biomechanical properties of ascending thoracic aortic aneurysms. Eur. J. Cardio-Thoracic Surg. 2022, 62, ezac392. [Google Scholar] [CrossRef]
- Balistreri, C.R. Bicuspid aortic valve disease: A simple embryonic defect or a complex syn¬drome? Paradigm or certainty? Ann. Cardiol. Vasc. Med. 2018, 1, 1004. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Cavarretta, E.; Sciarretta, S.; Frati, G. Light on the molecular and cellular mechanisms of bicuspid aortic valve to unveil phenotypic heterogeneity. J. Mol. Cell. Cardiol. 2019, 133, 113–114. [Google Scholar] [CrossRef]
- Rashed, E.R.; Dembar, A.; Riasat, M.; Zaidi, A.N. Bicuspid Aortic Valves: An Up-to-Date Review on Genetics, Natural History, and Management. Curr. Cardiol. Rep. 2022, 24, 1021–1030. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Pisano, C.; Candore, G.; Maresi, E.; Codispoti, M.; Ruvolo, G. Focus on the unique mechanisms involved in thoracic aortic aneurysm formation in bicuspid aortic valve versus tricuspid aortic valve patients: Clinical implications of a pilot study. Eur. J. Cardio-Thoracic. Surg. 2012, 43, e180–e186. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Buffa, S.; Allegra, A.; Pisano, C.; Ruvolo, G.; Colonna-Romano, G.; Lio, D.; Mazzesi, G.; Schiavon, S.; Greco, E.; et al. A Typical Immune T/B Subset Profile Characterizes Bicuspid Aortic Valve: In an Old Status? Oxidative Med. Cell. Longev. 2018, 2018, 5879281. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Crapanzano, F.; Schirone, L.; Allegra, A.; Pisano, C.; Ruvolo, G.; Forte, M.; Greco, E.; Cavarretta, E.; Marullo, A.G.M.; et al. Deregulation of Notch1 pathway and circulating endothelial progenitor cell (EPC) number in patients with bicuspid aortic valve with and without ascending aorta aneurysm. Sci. Rep. 2018, 8, 13834. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Forte, M.; Greco, E.; Paneni, F.; Cavarretta, E.; Frati, G.; Sciarretta, S. An overview of the molecular mechanisms underlying development and progression of bicuspid aortic valve disease. J. Mol. Cell. Cardiol. 2019, 132, 146–153. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Marullo, A.G.M.; Madonna, M.; Cavarretta, E.; Allegra, A.; Cesarini, V.; Iaccarino, A.; Schiavon, S.; Peruzzi, M.; Greco, E.; et al. Deregulation of TLR4 signaling pathway characterizes Bicuspid Aortic valve syndrome. Sci. Rep. 2019, 9, 11028. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; D’Amico, F.; Balistreri, C.R.; Vacirca, S.R.; Nardi, P.; Altieri, C.; Scioli, M.G.; Bertoldo, F.; Santo, L.; Bellisario, D.; et al. Biomechanical properties and histomorphometric features of aortic tissue in patients with or without bicuspid aortic valve. J. Thorac. Dis. 2020, 12, 2304–2316. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Ruvolo, G.; Lio, D.; Madonna, R. Toll-like receptor-4 signaling pathway in aorta aging and diseases: “its double nature”. J. Mol. Cell. Cardiol. 2017, 110, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Buffa, S.; Borzì, D.; Chiarelli, R.; Crapanzano, F.; Lena, A.M.; Nania, M.; Candi, E.; Triolo, F.; Ruvolo, G.; Melino, G.; et al. Biomarkers for vascular ageing in aorta tissues and blood samples. Exp. Gerontol. 2019, 128, 110741. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, M.; Peters, A.S.; Erhart, P.; Körfer, D.; Böckler, D.; Dihlmann, S. Inflammasomes in the Pathophysiology of Aortic Disease. Cells 2021, 10, 2433. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Chen, J.; Li, B.Y.; Wood, S.C.; Rosebury-Smith, W.; Remmer, H.A.; Jiang, L.; Zhang, M.; Salmon, M.; Ailawadi, G.; et al. Aging Alters the Aortic Proteome in Health and Thoracic Aortic Aneurysm. Arter. Thromb. Vasc. Biol. 2022, 42, 1060–1076. [Google Scholar] [CrossRef]
- Watson, A.M.; Chen, Y.-C.; Peter, K. Vascular Aging and Vascular Disease Have Much in Common! Arter. Thromb. Vasc. Biol. 2022, 42, 1077–1080. [Google Scholar] [CrossRef]
- Flanders, K.C. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004, 85, 47–64. [Google Scholar] [CrossRef]
- Blunder, S.; Messner, B.; Schachner, T.; Zeller, I.; Türkcan, A.; Wiedemann, D.; Andreas, M.; Blüschke, G.; Laufer, G.; Bernhard, D. Characteristics of TAV- and BAV-associated thoracic aortic aneurysms—Smooth muscle cell biology, expression profiling, and histological analyses. Atherosclerosis 2012, 220, 355–361. [Google Scholar] [CrossRef]
- Ruddy, J.M.; Jones, J.A.; Ikonomidis, J.S. Pathophysiology of Thoracic Aortic Aneurysm (TAA): Is It Not One Uniform Aorta? Role of Embryologic Origin. Prog. Cardiovasc. Dis. 2013, 56, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Forte, A.; Galderisi, U.; Cipollaro, M.; De Feo, M.; Della Corte, A. Epigenetic regulation of TGF-β1 signalling in dilative aortopathy of the thoracic ascending aorta. Clin. Sci. 2016, 130, 1389–1405. [Google Scholar] [CrossRef] [PubMed]
- Kostina, A.S.; Uspensky, V.; Irtyuga, O.B.; Ignatieva, E.V.; Freylikhman, O.; Gavriliuk, N.D.; Moiseeva, O.M.; Zhuk, S.; Tomilin, A.; Kostareva, A.; et al. Notch-dependent EMT is attenuated in patients with aortic aneurysm and bicuspid aortic valve. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2016, 1862, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Poujade, F.-A.; Bergman, O.; Gådin, J.R.; Simon, N.; Lång, K.; Franco-Cereceda, A.; Body, S.; Björck, H.M.; Eriksson, P. Endothelial/Epithelial Mesenchymal Transition in Ascending Aortas of Patients with Bicuspid Aortic Valve. Front. Cardiovasc. Med. 2019, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Lyu, X.; Wang, Q.; Hu, M.; Zhang, X. Endothelial to mesenchymal transition in the cardiovascular system. Life Sci. 2017, 184, 95–102. [Google Scholar] [CrossRef]
- Mackay, C.D.A.; Jadli, A.S.; Fedak, P.W.M.; Patel, V.B. Adventitial Fibroblasts in Aortic Aneurysm: Unraveling Pathogenic Contributions to Vascular Disease. Diagnostics 2022, 12, 871. [Google Scholar] [CrossRef]
- Mikołajczyk, K.; Spyt, D.; Zielińska, W.; Żuryn, A.; Faisal, I.; Qamar, M.; Świniarski, P.; Grzanka, A.; Gagat, M. The Important Role of Endothelium and Extracellular Vesicles in the Cellular Mechanism of Aortic Aneurysm Formation. Int. J. Mol. Sci. 2021, 22, 13157. [Google Scholar] [CrossRef]
- Irace, F.G.; Cammisotto, V.; Valenti, V.; Forte, M.; Schirone, L.; Bartimoccia, S.; Iaccarino, A.; Peruzzi, M.; Schiavon, S.; Morelli, A.; et al. Role of Oxidative Stress and Autophagy in Thoracic Aortic Aneurysms. JACC Basic Transl. Sci. 2021, 6, 719–730. [Google Scholar] [CrossRef]
- González-Amor, M.; García-Redondo, A.B.; Jorge, I.; Zalba, G.; Becares, M.; Ruiz-Rodríguez, M.J.; Rodríguez, C.; Bermeo, H.; Rodrigues-Díez, R.; Rios, F.J.; et al. Interferon stimulated gene 15 pathway is a novel mediator of endothelial dysfunction and aneurysms development in angiotensin II infused mice through increased oxidative stress. Cardiovasc. Res. 2021, cvab321. [Google Scholar] [CrossRef]
- Cheng, C.; Nguyen, M.N.; Nayernama, A.; Jones, S.C.; Brave, M.; Agrawal, S.; Amiri-Kordestani, L.; Woronow, D. Arterial aneurysm and dissection with systemic vascular endothelial growth factor inhibitors: A review of cases reported to the FDA Adverse Event Reporting System and published in the literature. Vasc. Med. 2021, 26, 526–534. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Dawson, A.; Vasquez, H.G.; Ageedi, W.; Zhang, C.; Luo, W.; Chen, R.; Li, Y.; Kim, S.; et al. Single-Cell Transcriptome Analysis Reveals Dynamic Cell Populations and Differential Gene Expression Patterns in Control and Aneurysmal Human Aortic Tissue. Circulation 2020, 142, 1374–1388. [Google Scholar] [CrossRef]
- Shah, A.V.; Birdsey, G.M.; Randi, A.M. Regulation of endothelial homeostasis, vascular development and angiogenesis by the transcription factor ERG. Vasc. Pharmacol. 2016, 86, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Birdsey, G.M.; Shah, A.V.; Dufton, N.; Reynolds, L.E.; Almagro, L.O.; Yang, Y.; Aspalter, I.M.; Khan, S.T.; Mason, J.; Dejana, E.; et al. The Endothelial Transcription Factor ERG Promotes Vascular Stability and Growth through Wnt/β-Catenin Signaling. Dev. Cell 2015, 32, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.V.; Birdsey, G.M.; Peghaire, C.; Pitulescu, M.E.; Dufton, N.P.; Yang, Y.; Weinberg, I.; Almagro, L.O.; Payne, L.; Mason, J.C.; et al. The endothelial transcription factor ERG mediates Angiopoietin-1-dependent control of Notch signalling and vascular stability. Nat. Commun. 2017, 8, 16002. [Google Scholar] [CrossRef] [PubMed]
- Peghaire, C.; Dufton, N.P.; Lang, M.; Salles-Crawley, I.I.; Ahnström, J.; Kalna, V.; Raimondi, C.; Pericleous, C.; Inuabasi, L.; Kiseleva, R.; et al. The transcription factor ERG regulates a low shear stress-induced anti-thrombotic pathway in the microvasculature. Nat. Commun. 2019, 10, 5014. [Google Scholar] [CrossRef]
- Dufton, N.; Peghaire, C.R.; Osuna-Almagro, L.; Raimondi, C.; Kalna, V.; Chauhan, A.; Webb, G.; Yang, Y.; Birdsey, G.M.; Lalor, P.; et al. Dynamic regulation of canonical TGFβ signalling by endothelial transcription factor ERG protects from liver fibrogenesis. Nat. Commun. 2017, 8, 895. [Google Scholar] [CrossRef]
- Nagai, N.; Ohguchi, H.; Nakaki, R.; Matsumura, Y.; Kanki, Y.; Sakai, J.; Aburatani, H.; Minami, T. Downregulation of ERG and FLI1 expression in endothelial cells triggers endothelial-to-mesenchymal transition. PLoS Genet. 2018, 14, e1007826. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; de Bonis, M.; de Paulis, R.; et al. ESC/EACTS Scientific Document Group, ESC National Cardiac Societies, 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar]
- Zhang, X.; Hu, C.; Yuan, Y.-P.; Song, P.; Kong, C.-Y.; Wu, H.-M.; Xu, S.-C.; Ma, Z.-G.; Tang, Q.-Z. Endothelial ERG alleviates cardiac fibrosis via blocking endothelin-1-dependent paracrine mechanism. Cell Biol. Toxicol. 2021, 37, 873–890. [Google Scholar] [CrossRef]
- Wythe, J.D.; Dang, L.T.; Devine, W.P.; Boudreau, E.; Artap, S.T.; He, D.; Schachterle, W.; Stainier, D.Y.; Oettgen, P.; Black, B.L.; et al. ETS Factors Regulate Vegf-Dependent Arterial Specification. Dev. Cell 2013, 26, 45–58. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, S.; Mao, Y.; Chen, Z.; Zheng, C.; Li, H.; Sumners, C.; Li, Q.; Yang, P.; Lei, B. Modulating of ocular inflammation with macrophage migration inhibitory factor is associated with notch signalling in experimental autoimmune uveitis. Clin. Exp. Immunol. 2015, 183, 280–293. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Gjølberg, T.T.; Krüger, S.; Haraldsen, G.; Andersen, J.T.; Sundlisæter, E. Targeting the Notch Signaling Pathway in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 668207. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; Valente, S.; Motta, I.; Degiovanni, A.; Ciavarella, C.; Pasquinelli, G. ETS-Related Gene Expression in Healthy Femoral Arteries with Focal Calcifications. Front. Cell Dev. Biol. 2021, 9, 623782. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.; Bundy, K.; Roach, C.; Douglas, H.; Ventura, V.; Segars, M.F.; Schwartz, O.; Simpson, C.L. Mechanisms of the Osteogenic Switch of Smooth Muscle Cells in Vascular Calcification: WNT Signaling, BMPs, Mechanotransduction, and EndMT. Bioengineering 2020, 7, 88. [Google Scholar] [CrossRef]
- Kwon, C.; Qian, L.; Cheng, P.; Nigam, V.; Arnold, J.; Srivastava, D. A regulatory pathway involving Notch1/β-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 2009, 11, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Hans, C.P.; Koenig, S.; Nichols, H.A.; Galindo, C.L.; Garner, H.R.; Merrill, W.H.; Hinton, R.B.; Garg, V. Inhibitory Role of Notch1 in Calcific Aortic Valve Disease. PLoS ONE 2011, 6, e27743. [Google Scholar] [CrossRef] [PubMed]
- Garg, V. Notch Signaling in Aortic Valve Development and Disease. 2016 Jun 25. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Japan, 2016; Chapter 53. [Google Scholar]
- Xia, Z.-Y.; Hu, Y.; Xie, P.-L.; Tang, S.-Y.; Luo, X.-H.; Liao, E.-Y.; Chen, F.; Xie, H. Runx2/miR-3960/miR-2861 Positive Feedback Loop Is Responsible for Osteogenic Transdifferentiation of Vascular Smooth Muscle Cells. BioMed Res. Int. 2015, 2015, 624037. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Y.; Zhang, R. Galectin-3 induces vascular smooth muscle cells calcification via AMPK/TXNIP pathway. Aging 2022, 14, 5086–5096. [Google Scholar] [CrossRef]
- Sulistyowati, E.; Hsu, J.-H.; Lee, S.-J.; Huang, S.-E.; Sihotang, W.Y.; Wu, B.-N.; Dai, Z.-K.; Lin, M.-C.; Yeh, J.-L. Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 5673. [Google Scholar] [CrossRef]
- Pisano, C.; Benedetto, U.; Ruvolo, G.; Balistreri, C.R. Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms. Antioxidants 2022, 11, 182. [Google Scholar] [CrossRef]
- Pisano, C.; Balistreri, C.R.; Nardi, P.; Altieri, C.; Bertoldo, F.; Buioni, D.; Ruvolo, G. Risk of aortic dissection in patients with ascending aorta aneurysm: A new biological, morphological and biochemical networkbehind the aortic diameter. Vessel Plus 2020, 4, 33. [Google Scholar] [CrossRef]
- D’Amico, F.; Doldo, E.; Pisano, C.; Scioli, M.G.; Centofanti, F.; Proietti, G.; Falconi, M.; Sangiuolo, F.; Ferlosio, A.; Ruvolo, G.; et al. Specific miRNA and Gene Deregulation Characterize the Increased Angiogenic Remodeling of Thoracic Aneurysmatic Aortopathy in Marfan Syndrome. Int. J. Mol. Sci. 2020, 21, 6886. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, G.; Pisano, C.; Candore, G.; Lio, D.; Palmeri, C.; Maresi, E.; Balistreri, C.R. Can the TLR-4-mediated signaling pathway be “a key inflammatory promoter for sporadic TAA”? Mediat. Inflamm. 2014, 2014, 349476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Patients (n = 20) |
|---|---|
| Demographic and Clinical Data | |
| Age (years) | 63.7 ± 15.5 |
| Marfan Syndrome | 0 (0%) |
| Hypertension | 15 (75%) |
| Diabetes | 1 (5%) |
| Renal Failure | 2 (10%) |
| Peripheral Vascular Disease | 0 (0%) |
| Family History for Aneurysm | 1 (5%) |
| Other Vascular Disease | 1 (5%) |
| Coronary Artery Disease | 9 (45%) |
| Valsalva Sinuses Prolapse | 5 (25%) |
| Left Ventricular/Aortic Valve disjunction | 2 (10%) |
| Asymmetric Dilation of Ascending Aorta | 6 (30%) |
| Coronary Ostia Dislocation | 19 (95%) |
| Aortic Wall Thickness | 14 (70%) |
| Origin of the epi-aortic vessels from the aorta | 18 (90%) |
| Ejection Fraction (%) | 54.5 ± 8.4 |
| Aortic Root Diameter | 42.3 ± 5.7 |
| Ascending Aorta Diameter | 51.5 ± 7.8 |
| Histological Data | |
| % Endothelial ERG+ cell/tot cells | 24.4 ± 25.8 |
| % vsmcs psmad3+ | 11.08 ± 8.97 |
| % alfa SMA+ endothelial cells | 23.34 ± 26.83 |
| % S100A4+ endothelial cells | 21.33 ± 25.33 |
| % Fibrotic area (Masson standing) | 12.23 ± 11.81 |
| % Calcific area (Alizarin standing) | 0.32 ± 0.19 |
| BAV (n = 10) | TAV (n = 10) | p-Values | |
|---|---|---|---|
| Demographic and Clinical Data | |||
| Age (years) | 56.1 ± 17 | 71.3 ± 9.5 | 0.024 |
| BMI | 27.8 ± 4.1 | 27.5 ± 4.7 | 0.759 |
| Male | 8(80%) | 6(60%) | 0.628 |
| Caucasians | 10(100%) | 10(100%) | 1.000 |
| Marfan Syndrome | 0 (0%) | 0 (0%) | - |
| Hypertension | 9 (90%) | 6 (60%) | 0.303 |
| Diabetes | 0 (0%) | 1 (10%) | 1.000 |
| Renal Failure | 0 (0%) | 2 (20%) | 0.474 |
| Peripheral Vascular Disease | 0 (0%) | 0 (0%) | - |
| Family History for Aneurysm | 0 (0%) | 1 (10%) | 1.000 |
| Other Vascular Disease | 1 (10%) | 0 (0%) | 1.000 |
| Coronary Artery Disease | 4 (40%) | 5 (50%) | 1.000 |
| Valsalva Sinuses Prolapse | 1 (10%) | 4 (40%) | 0.303 |
| Left Ventricular/Aortic Valve disjunction | 0 (0%) | 2 (20%) | 0.474 |
| Asymmetric Dilation of Ascending Aorta | 3 (30%) | 3 (30%) | 1.000 |
| Coronary Ostia Dislocation | 10 (100%) | 9 (90%) | 1.000 |
| Aortic Wall Thickness | 8 (80%) | 6 (60%) | 0.628 |
| Origin of the epiaortic vassels from the ascending aorta | 10 (100%) | 8 (80%) | 0.474 |
| Ejection Fraction (%) | 53.6 ± 8.6 | 55.4 ± 8.7 | 0.646 |
| Aortic Root Diameter | 42.6 ± 6.1 | 41.9 ± 5.6 | 0.792 |
| Ascending Aorta Diameter | 50.2 ± 6.8 | 52.7 ± 8.9 | 0.489 |
| Histological Data | |||
| % Endothelial ERG+ cell/tot cells | 39.8 ± 29 | 9 ± 6.2 | 0.0082 |
| % Vsmcs psmad3+ | 8.7 ± 3.9 | 13.4 ± 11.9 | 0.2581 |
| % alfa SMA+ endothelial cells | 11.5 ± 9 | 35.2 ± 33.6 | 0.0557 |
| % S100A4+ endothelial cells | 6.6 ± 2 | 36 ± 29.5 | 0.0117 |
| % Fibrotic area (Masson staining) | 5.9 ± 1.8 | 18.5 ± 14.3 | 0.0212 |
| % Calcific area (Alizarin staining) | 0.353 ± 0.178 | 0.277 ± 0.208 | 0.3918 |
| % Notch+ Endothelial cells | 13.9 ± 10.1 | 18.6 ± 10.4 | 0.3146 |
| % Vsmcs Notch+ cells | 5.27 ± 1.23 | 14.1 ± 9.6 | 0.0174 |
| % eNOS+ cells | 38.9 ± 8.4 | 4.9 ± 4.06 | <0.001 |
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | p-Value | p-Value Group 1 vs. Group 2 | p-Value Group 1 vs. Group 3 | p-Value Group 1 vs. Group 4 | p-Value Group 2 vs. Group 3 | p-Value Group 2 vs. Group 4 | p-Value Group3 vs. Group 4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic and Clinical Data | ||||||||||||
| Age (years) | 62.6 ± 15.1 | 49.6 ± 17.8 | 66.6 ± 7.7 | 76 ± 9.5 | 0.042 | 0.830 | 1.000 | 0.764 | 0.349 | 0.036 | 1.000 | a |
| Marfan Syndrome | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - | |||||||
| Hypertension | 4 (80%) | 5 (100%) | 2 (40%) | 4 (80%) | 0.291 | |||||||
| Diabetes | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 1.000 | |||||||
| Renal Failure | 0 (0%) | 0 (0%) | 2 (40%) | 0 (0%) | 0.211 | |||||||
| Peripheral Vascular Disease | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | - | |||||||
| Family History for Aneurysm | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) | 1.000 | |||||||
| Other Vascular Disease | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 | |||||||
| Coronary Artery Disease | 1 (20%) | 3 (60%) | 3 (60%) | 2 (40%) | 0.762 | |||||||
| Valsalva Sinuses Prolapse | 0 (0%) | 1 (20%) | 0 (0%) | 4 (80%) | 0.020 | |||||||
| Left Ventricl/Aortic Valve disjuction | 0 (0%) | 0 (0%) | 0 (0%) | 2 (40%) | 0.211 | |||||||
| Asymmetric Dilation of Ascending Aorta | 0 (0%) | 3 (60%) | 0 (0%) | 3 (60%) | 0.033 | |||||||
| Coronary Ostia Dislocation | 5 (100%) | 5 (100%) | 4 (80%) | 5 (100%) | 1.000 | |||||||
| Aortic Wall Thickness | 4 (80%) | 4 (80%) | 3 (60%) | 3 (60%) | 1.000 | |||||||
| Origin of the epiaortic vassels from the aorta | 5 (100%) | 5 (100%) | 5 (100%) | 3 (60%) | 0.211 | |||||||
| Ejection Franction (%) | 53.6 ± 11.8 | 53.6 ± 5 | 51.6 ± 9.1 | 59.2 ± 7.1 | 0.555 | |||||||
| Aortic Root Diameter | 38.2 ± 5.8 | 47 ± 1.4 | 39.6 ± 5.7 | 44.2 ± 5 | 0.037 | 0.064 | 1.000 | 0.398 | 0.163 | 1.000 | 0.902 | a |
| Ascendinng Aorta Diameter | 56.2 ± 2.8 | 44.2 ± 2.4 | 60 ± 6.3 | 45.4 ± 2.5 | <0.001 | <0.001 | 0.827 | 0.002 | <0.001 | 1.000 | <0.001 | a |
| Histological Data | ||||||||||||
| % Endotheliali ERG+ cell/tot cells | 66.1 ± 11.1 | 13.5 ± 5.7 | 6.6 ± 3.7 | 11.5 ± 7.6 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 | 1.000 | 1.000 | a |
| % vsmcs psmad3+ | 9.19 ± 5.03 | 8.25 ± 2.81 | 23.05 ± 9.17 | 3.84 ± 2.43 | 0.008 | 1.000 | 0.184 | 0.363 | 0.184 | 0.363 | 0.002 | k |
| % alfa SMA+ endothelial cells | 9.87 ± 5.98 | 13.2 ± 11.8 | 65.6 ± 14.6 | 4.66 ± 1.78 | 0.006 | 1.000 | 0.049 | 0.930 | 0.085 | 0.657 | 0.002 | k |
| % S100A4+ endothelial cells | 7.77 ± 1.51 | 5.5 ± 1.83 | 63.44 ± 8.03 | 8.62 ± 3.94 | 0.005 | 0.599 | 0.074 | 1.000 | 0.001 | 0.599 | 0.074 | k |
| % Fibrotic area (Masson staining) | 4.38 ± 0.51 | 7.52 ± 1.11 | 31.87 ± 3.35 | 5.16 ± 0.34 | 0.001 | 0.031 | <0.001 | 0.785 | 0.544 | 0.447 | 0.016 | k |
| % Calcific area (Alizarin staining) | 0.34 ± 0.16 | 0.37 ± 0.21 | 0.15 ± 0.22 | 0.41 ± 0.09 | 0.144 | |||||||
| % Notch+ Endothelial cells | 20.5 ± 10.1 | 7.3 ± 4.4 | 24.8 ± 11.1 | 12.4 ± 4.6 | 0.042 | 0.092 | <0.001 | <0.001 | <0.001 | <0.001 | 0.249 | a |
| % vsmcs notch+ cells | 6.4 ± 0.4 | 4.2 ± 0.4 | 22.9 ± 3.7 | 5.3 ± 1.4 | 0.002 | 0.098 | 0.326 | 0.855 | 0.001 | 0.855 | 0.023 | k |
| % eNOS+ cells | 43.4 ± 6.5 | 34.4 ± 8.1 | 1.3 ± 0.5 | 8.6 ± 1.6 | 0.001 | 1.000 | <0.001 | 0.042 | 0.012 | 0.363 | 0.544 | k |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, C.; Terriaca, S.; Scioli, M.G.; Nardi, P.; Altieri, C.; Orlandi, A.; Ruvolo, G.; Balistreri, C.R. The Endothelial Transcription Factor ERG Mediates a Differential Role in the Aneurysmatic Ascending Aorta with Bicuspid or Tricuspid Aorta Valve: A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 10848. https://doi.org/10.3390/ijms231810848
Pisano C, Terriaca S, Scioli MG, Nardi P, Altieri C, Orlandi A, Ruvolo G, Balistreri CR. The Endothelial Transcription Factor ERG Mediates a Differential Role in the Aneurysmatic Ascending Aorta with Bicuspid or Tricuspid Aorta Valve: A Preliminary Study. International Journal of Molecular Sciences. 2022; 23(18):10848. https://doi.org/10.3390/ijms231810848
Chicago/Turabian StylePisano, Calogera, Sonia Terriaca, Maria Giovanna Scioli, Paolo Nardi, Claudia Altieri, Augusto Orlandi, Giovanni Ruvolo, and Carmela Rita Balistreri. 2022. "The Endothelial Transcription Factor ERG Mediates a Differential Role in the Aneurysmatic Ascending Aorta with Bicuspid or Tricuspid Aorta Valve: A Preliminary Study" International Journal of Molecular Sciences 23, no. 18: 10848. https://doi.org/10.3390/ijms231810848
APA StylePisano, C., Terriaca, S., Scioli, M. G., Nardi, P., Altieri, C., Orlandi, A., Ruvolo, G., & Balistreri, C. R. (2022). The Endothelial Transcription Factor ERG Mediates a Differential Role in the Aneurysmatic Ascending Aorta with Bicuspid or Tricuspid Aorta Valve: A Preliminary Study. International Journal of Molecular Sciences, 23(18), 10848. https://doi.org/10.3390/ijms231810848








