Effects of Various Disinfection Methods on the Material Properties of Silicone Dental Impressions of Different Types and Viscosities
Abstract
1. Introduction
2. Results
2.1. Preliminary Comparison of Nondisinfected Materials
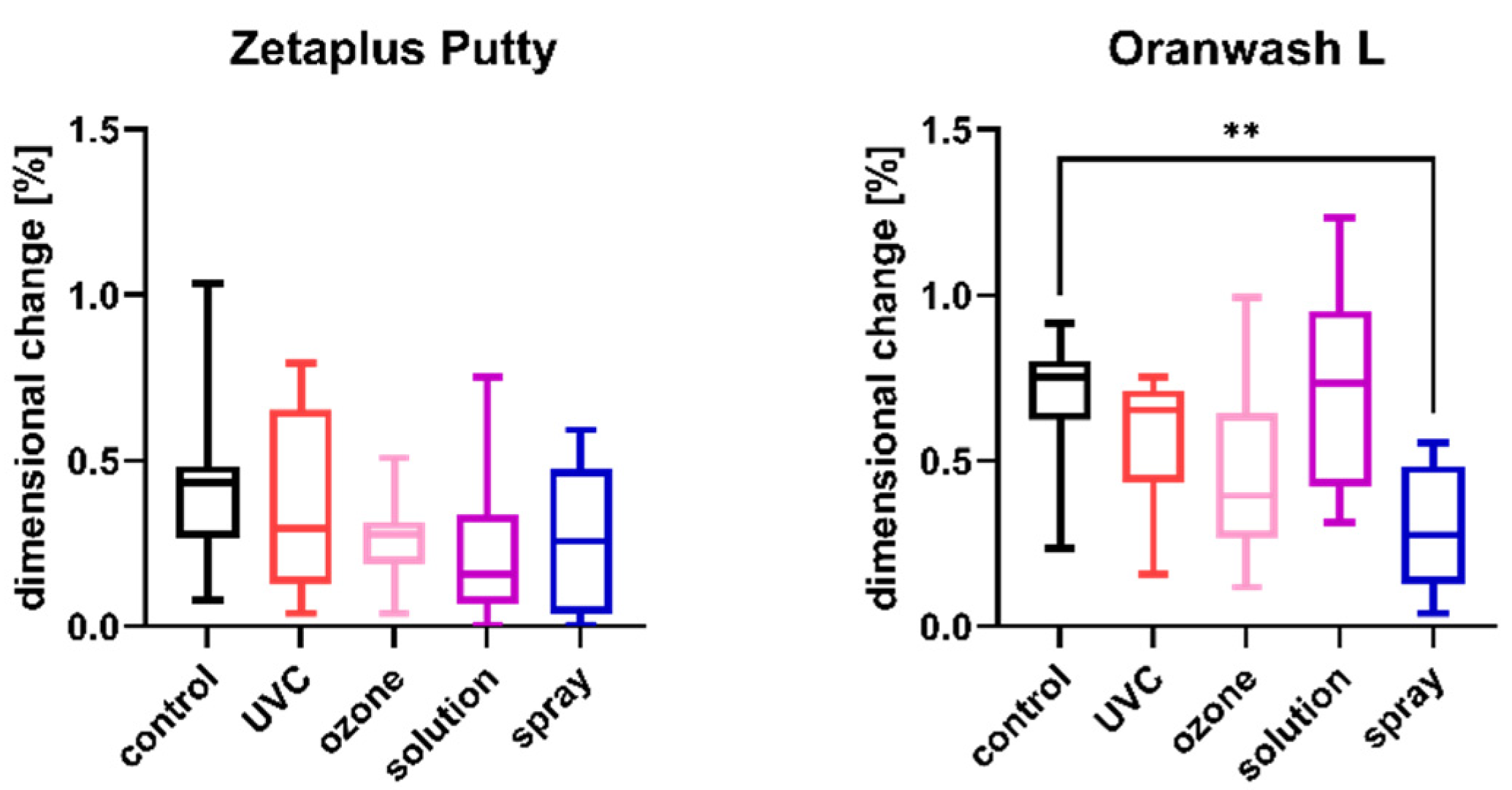
2.2. Linear Dimensional Change
2.3. Tensile Strength
2.4. Shore A Hardness
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Specimens
4.3. Disinfection
4.4. Linear Dimensional Change Test
- L1 is the distance measured between lines d1 and d2 in the test block [mm]; and
- L2 is the distance measured between lines d1 and d2 in the impression material specimen [mm].
4.5. Tensile Strength Test
- Fm is the maximum force [N];
- t is the thickness of the test piece over the test length [mm]; and
- W is the width of the test piece over the test length [mm].
4.6. Hardness Test
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirota, Y.; Tawada, Y.; Komatsu, S.; Watanabe, F. Effect of impression holding time and tray removal speed on the dimensional accuracy of impressions for artificial abutment tooth inclined. Odontology 2021, 109, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kustrzycka, D.; Marschang, T.; Mikulewicz, M.; Grzebieluch, W. Comparison of the Accuracy of 3D Images Obtained from Different Types of Scanners: A Systematic Review. J. Healthc. Eng. 2020, 2020, 8854204. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Fiorillo, L.; D’Amico, C.; Gambino, D.; Amantia, E.M.; Laino, L.; Crimi, S.; Campagna, P.; Bianchi, A.; Herford, A.S.; et al. 3D digital impression systems compared with traditional techniques in dentistry: A recent data systematic review. Materials 2020, 13, 1982. [Google Scholar] [CrossRef]
- Lim, J.-H.; Mangal, U.; Nam, N.-E.; Choi, S.-H.; Shim, J.-S.; Kim, J.-E. A comparison of accuracy of different dental restorative materials between intraoral scanning and conventional impression-taking: An in vitro study. Materials 2021, 14, 2060. [Google Scholar] [CrossRef]
- Lam, W.Y.H.; Mak, K.C.K.; Maghami, E.; Molinero-Mourelle, P. Dental students’ preference and perception on intraoral scanning and impression making. BMC Med. Educ. 2021, 21, 501. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Park, S.J. Digital intraoral scanners and alginate impressions in reproducing full dental arches: A comparative 3D assessment. Appl. Sci. 2020, 10, 7637. [Google Scholar] [CrossRef]
- Ahlholm, P.; Sipilä, K.; Vallittu, P.; Jakonen, M.; Kotiranta, U. Digital Versus Conventional Impressions in Fixed Prosthodontics: A Review. J. Prosthodont. 2018, 27, 35–41. [Google Scholar] [CrossRef]
- Vrbova, R.; Bradna, P.; Bartos, M.; Roubickova, A. The effect of disinfectants on the accuracy, quality and surface structure of impression materials and gypsum casts: A comparative study using light microscopy, scanning electron microscopy and micro computed tomography. Dent. Mater. J. 2020, 39, 500–508. [Google Scholar] [CrossRef]
- Jayaraman, S.; Singh, B.P.; Ramanathan, B.; Pazhaniappan Pillai, M.; MacDonald, L.K.R. Final-impression techniques and materials for making complete and removable partial dentures. Cochrane Database Syst. Rev. 2018, 2018, CD012256. [Google Scholar] [CrossRef]
- Gupta, M.; George, V.T.; Balakrishnan, D. A comparative evaluation of tear strength and tensile strength of autoclavable and non-autoclavable vinylpolysiloxane impression material: An in vitro study. J. Int. Oral Health 2020, 12, 153–157. [Google Scholar]
- Azevedo, M.J.; Correia, I.; Portela, A.; Sampaio-Maia, B. A simple and effective method for addition silicone impression disinfection. J. Adv. Prosthodont. 2019, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Al Mortadi, N.; Al-Khatib, A.; Alzoubi, K.H.; Khabour, O.F. Disinfection of dental impressions: Knowledge and practice among dental technicians. Clin. Cosmet. Investig. Dent. 2019, 11, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.R.; Mohd, I.; Sajjan, S.; Ramaraju, A. Disinfection of Impression Materials: A Comprehensive Review of Disinfection. Int. J. Dent. Mater. 2019, 1, 7–16. [Google Scholar] [CrossRef]
- Adabo, G.L.; Zanarotti, E.; Fonseca, R.G.; Cruz, C.A. Effect of disinfectant agents on dimensional stability of elastomeric impression materials. J. Prosthet. Dent. 1999, 81, 621–624. [Google Scholar] [CrossRef]
- Selvam, S.P.; Rakshagan, V. Day to Day Use of Disinfectant Methods for Different Impression Materials among Dental Practitioners. J. Pharm. Res. Int. 2020, 32, 113–124. [Google Scholar] [CrossRef]
- Duś-Ilnicka, I.; Krala, E.; Cholewińska, P.; Radwan-Oczko, M. The use of saliva as a biosample in the light of COVID-19. Diagnostics 2021, 11, 1769. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef]
- Smith, N.; Wilson, A.; Gandhi, J.; Vatsia, S.; Khan, S. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar]
- Irie, M.S.; Dietrich, L.; de Souza, G.L.; Soares, P.B.F.; Moura, C.C.G.; da Silva, G.R.; Paranhos, L.R. Ozone disinfection for viruses with applications in healthcare environments: A scoping review. Braz. Oral Res. 2022, 36, 1–16. [Google Scholar] [CrossRef]
- Celebi, H.; Büyükerkmen, E.B.; Torlak, E. Disinfection of polyvinyl siloxane impression material by gaseous ozone. J. Prosthet. Dent. 2018, 120, 138–143. [Google Scholar] [CrossRef]
- Fonseca, P.M.M.; Palacios, D.A.B.; Júnior, P.L.D.S.; Miyakawa, W.; Damião, J.; Fernandes, A.B.; de Lima, C.J. Preliminary Study: Comparative Analysis of the Effects of Ozone and Ultrasound on Streptococcus Mutans. Ozone Sci. Eng. 2021, 43, 263–275. [Google Scholar] [CrossRef]
- Aeran, H.; Sharma, S.; Kumar, V.; Gupta, N. Use of clinical UV chamber to disinfect dental impressions: A comparative study. J. Clin. Diagn. Res. 2015, 9, ZC67–ZC70. [Google Scholar] [CrossRef]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, M.; Czarny, A.; Malysa, A.; Seweryn, P.; Zietek, M.; Paradowska-Stolarz, A. Evaluation of Antimicrobial Efficacy of UVC Radiation, Gaseous Ozone, and Liquid Chemicals Used for Disinfection of Silicone Dental Impression Materials. Materials 2022, 15, 2553. [Google Scholar] [CrossRef] [PubMed]
- AlZain, S. Effect of chemical, microwave irradiation, steam autoclave, ultraviolet light radiation, ozone and electrolyzed oxidizing water disinfection on properties of impression materials: A systematic review and meta-analysis study. Saudi Dent. J. 2020, 32, 161–170. [Google Scholar] [CrossRef] [PubMed]
- PN-EN 13727+A2:2015-12; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity in the Medical Area—Test Method and Requirements (Phase 2, Step 1). ISO: Geneva, Switzerland, 2015.
- PN-EN 13624:2013-12; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Fungicidal or yeasticidal Activity in the Medical Area—Test Method and Requirements (Phase 2, Step 1). ISO: Geneva, Switzerland, 2013.
- Amin, W. The Effects of Disinfectants on Dimensional Accuracy and Surface Quality of Impression Materials and Gypsum Casts. J. Clin. Med. Res. 2009, 1, 81–89. [Google Scholar] [CrossRef][Green Version]
- Özdemir, H.; Pekince, K.A. Evaluation of the effect of storage time and disinfectant solutions on the dimensional accuracy of impression materials with digital radiography. Dent. Med. Probl. 2019, 56, 67–74. [Google Scholar] [CrossRef]
- Hiraguchi, H.; Kaketani, M.; Hirose, H.; Kikuchi, H.; Yoneyama, T. Dimensional changes in stone casts resulting from long-term immersion of addition-type silicone rubber impressions in disinfectant solutions. Dent. Mater. J. 2013, 32, 361–366. [Google Scholar] [CrossRef][Green Version]
- Martin, N.; Martin, M.V.; Jedynakiewicz, N.M. The dimensional stability of dental impression materials following immersion in disinfecting solutions. Dent. Mater. 2007, 23, 760–768. [Google Scholar] [CrossRef]
- Abinaya, K.; Kumar, B.M.; Ahila, S.C. Evaluation of Surface Quality of Silicone Impression Materials after Disinfection with Ozone Water: An In vitro Study. Contemp. Clin. Dent. 2018, 9, 60–64. [Google Scholar]
- Guiraldo, R.D.; Berger, S.B.; Siqueira, R.M.; Grandi, V.H.; Lopes, M.B.; Gonini-Júnior, A.; Caixeta, R.V.; de Carvalho, R.; Sinhoreti, M.A. Surface detail reproduction and dimensional accuracy of molds: Influence of disinfectant solutions and elastomeric impression materials. Acta Odontol. Latinoam. 2017, 30, 13–18. [Google Scholar]
- Sinobad, T.; Obradovic-Djuricic, K.; Nikolic, Z.; Dodic, S.; Lazic, V.; Sinobad, V.; Jesenko-Rokvic, A. The effect of disinfectants on dimensional stability of addition and condensation silicone impressions. Vojnosanit. Pregl. 2014, 71, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Khinnavar, P.K.; Dhanya Kumar, B.H.; Nandeeshwar, D.B. An in vitro study to evaluate the effect on dimensional changes of elastomers during cold sterilization. J. Indian Prosthodont. Soc. 2015, 15, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.S.; Khandeparker, R.V.; Somasundaram, P.; Raghav, S.; Babaji, R.P.; Varghese, T.J. Comparative Evaluation of Dimensional Accuracy of Elastomeric Impression Materials when Treated with Autoclave, Microwave, and Chemical Disinfection. J. Int. Oral Health 2015, 7, 22–24. [Google Scholar]
- Samra, R.K.; Bhide, S.V. Comparative evaluation of dimensional stability of impression materials from developing countries and developed countries after disinfection with different immersion disinfectant systems and ultraviolet chamber. Saudi Dent. J. 2018, 30, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Godbole, S.R.; Dahane, T.M.; Patidar, N.A.; Nimonkar, S. Evaluation of the Effect of Ultraviolet Disinfection on Dimensional Stability of the Polyvinyl Silioxane Impressions an in-Vitro Study. J. Clin. Diagn. Res. 2014, 8, 73–76. [Google Scholar]
- Nimonkar, S.V.; Belkhode, V.M.; Nimonkar, P.V.; Dahane, T.S.S. Comparative Evaluation of the Effect of Chemical Disinfectants and Ultraviolet Disinfection on Dimensional Stability of the Polyvinyl Siloxane Impressions. J. Int. Soc. Prev. Community Dent. 2019, 9, 152–158. [Google Scholar] [CrossRef]
- Poulis, N.; Prombonas, A.; Yannikakis, S.; Karampotsos, T.; Katsarou, M.S.; Drakoulis, N. Preliminary SEM observations on the surface of elastomeric impression materials after immersion or ozone disinfection. J. Clin. Diagn. Res. 2016, 10, ZC01–ZC05. [Google Scholar] [CrossRef]
- Poulis, N. Effectiveness of Low-flow High-ozone Concentration Disinfection of Dental Impressions: A Comparative Study to Immersion Disinfection. Br. J. Appl. Sci. Technol. 2014, 4, 2528–2537. [Google Scholar] [CrossRef]
- Meincke, D.K.; Ogliari, A.D.O.; Ogliari, F.A. Influence of different fillers on the properties of an experimental vinyl polysiloxane. Braz. Oral Res. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Kotha, S.B.; Divakar, D.D.; Celur, S.L.; Bin Qasim, S.S.; Matinlinna, J.P.; Ramakrishnaiah, R. Effect of disinfection and sterilization on the tensile strength, surface roughness, and wettability of elastomers. J. Investig. Clin. Dent. 2017, 8, e12244. [Google Scholar] [CrossRef]
- Goiato, M.C.; Haddad, M.F.; Santos, D.M.; Pesqueira, A.A.; Moreno, A. Hardness evaluation of prosthetic silicones containing opacifiers following chemical disinfection and accelerated aging. Braz. Oral Res. 2010, 24, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Hussein, B.M.; Ismail, I.J.; Khalaf, H.A. Effect of some disinfectant solutions on the hardness property of selected soft denture liners after certain immersion periods. J. Fac. Med. Baghdad 2009, 51, 259–264. [Google Scholar]
- Karaman, T.; Oztekin, F.; Tekin, S. Effect of Application Time of Two Different Disinfectants on the Surface Roughness of an Elastomeric Impression Material. J. Clin. Diagn. Res. 2020, 14, ZC10–ZC13. [Google Scholar] [CrossRef]
- Mahalakshmi, A.S.; Jeyapalan, V.; Krishnan, C.S.; Azhagarasan, N.S. Comparative evaluation of the effect of electrolyzed oxidizing water on surface detail reproduction, dimensional stability and Surface texture of poly vinyl siloxane impressions. J Indian Prosthodont. Soc. 2019, 19, 33–41. [Google Scholar]
- ISO 37:2017(E); Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- PN-EN ISO 4823:2015-12; Dentistry—Elastomeric Impression Materials. ISO: Geneva, Switzerland, 2015.
- PN-EN ISO 868:2005; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). ISO: Geneva, Switzerland, 2005.






| Material | Linear Dimensional Change [%] | Tensile Strength [MPa] | Shore A Hardness |
|---|---|---|---|
| Zetaplus Putty | 0.4190 (0.2626) ab | 1.691 (0.1345) d | 58.08 (1.383) c |
| Oranwash L | 0.6933 (0.1977) a | 1.159 (0.2901) e | 19.98 (0.7690) e |
| Panasil Putty Soft | 0.1179 (0.1126) c | 3.196 (0.2547) b | 61.02 (2.025) b |
| Panasil monophase Medium | 0.2039 (0.1236) bc | 3.607 (0.3273) a | 64.66 (1.201) a |
| Panasil initial contact Light | 0.3368 (0.1487) ac | 2.337 (0.1231) c | 54.04 (0.6987) d |
| Type of Material | Linear Dimensional Change [%] | Tensile Strength [MPa] | Shore A Hardness |
|---|---|---|---|
| C-silicones | 0.5561 (0.2664) | 1.425 (0.3506) | 39.03 (19.18) |
| A-silicones | 0.2196 (0.1547) | 3.043 (0.5660) | 59.91 (4.640) |
| Significance | p = 0.0082 | p < 0.0001 | p < 0.0001 |
| Type of Viscosity of Material | Linear Dimensional Change [%] | Tensile Strength [MPa] | Shore A Hardness |
|---|---|---|---|
| Putty | 0.2684 (0.2501) b | 2.542 (0.7904) b | 59.55 (2.271) b |
| Medium-bodied | 0.2039 (0.1236) b | 3.607 (0.3273) a | 64.66 (1.201) a |
| Light-bodied | 0.5151 (0.2499) a | 1.748 (0.6420) c | 37.01 (17.13) c |
| Type of Material | Viscosity | Name | Manufacturer | Mixing Technique | Intraoral Setting Time at 35 °C |
|---|---|---|---|---|---|
| C-silicone (condensation polysiloxane) | Putty | Zetaplus Putty | Zhermack (Badia Polesine, Italy) | Manual (hand mix) | 3 min 15 s |
| Light-bodied | Oranwash L | Manual (with spatula) | 3 min 30 s | ||
| A-silicone (vinyl polysiloxane) | Putty | Panasil Putty Soft | Kettenbach (Eschenburg, Germany) | Manual (hand mix) | 2 min |
| Medium-bodied | Panasil monophase Medium | Dispensing gun with mixing tip | 2 min | ||
| Light-bodied | Panasil initial contact Light | Dispensing gun with mixing tip | 2 min 30 s |
| Method | Material or Equipment | Description |
|---|---|---|
| UVC | UV-C Blue (Activeshop, Wroclaw, Poland) | Irradiation for 40 min at 254 nm |
| Ozone | Ozox Professional G168 (MediaSklep24, Bojszowy, Poland) | Putting in an 8-L box with 15 ppm ozone concentration, and ozonation for 10 min at an ozone flow rate of 800 mg/h |
| Solution | Zeta 7 Solution (Zhermack, Badia Polesine, Italy); active ingredients: quaternary ammonium salts, phenoxyethanol | Immersion for 10 min in 100-time diluted solution and rinsing with distilled water |
| Spray | Zeta 7 spray (Zhermack, Badia Polesine, Italy); active ingredients: alcohols | Spraying all surfaces of the specimen and allowing to dry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wezgowiec, J.; Paradowska-Stolarz, A.; Malysa, A.; Orzeszek, S.; Seweryn, P.; Wieckiewicz, M. Effects of Various Disinfection Methods on the Material Properties of Silicone Dental Impressions of Different Types and Viscosities. Int. J. Mol. Sci. 2022, 23, 10859. https://doi.org/10.3390/ijms231810859
Wezgowiec J, Paradowska-Stolarz A, Malysa A, Orzeszek S, Seweryn P, Wieckiewicz M. Effects of Various Disinfection Methods on the Material Properties of Silicone Dental Impressions of Different Types and Viscosities. International Journal of Molecular Sciences. 2022; 23(18):10859. https://doi.org/10.3390/ijms231810859
Chicago/Turabian StyleWezgowiec, Joanna, Anna Paradowska-Stolarz, Andrzej Malysa, Sylwia Orzeszek, Piotr Seweryn, and Mieszko Wieckiewicz. 2022. "Effects of Various Disinfection Methods on the Material Properties of Silicone Dental Impressions of Different Types and Viscosities" International Journal of Molecular Sciences 23, no. 18: 10859. https://doi.org/10.3390/ijms231810859
APA StyleWezgowiec, J., Paradowska-Stolarz, A., Malysa, A., Orzeszek, S., Seweryn, P., & Wieckiewicz, M. (2022). Effects of Various Disinfection Methods on the Material Properties of Silicone Dental Impressions of Different Types and Viscosities. International Journal of Molecular Sciences, 23(18), 10859. https://doi.org/10.3390/ijms231810859








