Recent Advances in the Emerging Therapeutic Strategies for Diabetic Kidney Diseases
Abstract
:1. Introduction
2. Lifestyle Improvements
3. Pharmacological Therapies
3.1. Glycemic Control
3.2. Blood Lipid Control
3.3. Blood Pressure Control
3.4. Aldosterone Antagonists
3.5. Diuretics
3.6. SGLT2 Inhibitors
3.7. Incretin Mimetics
3.7.1. GLP-1R Agonists
3.7.2. DPP-4 Inhibitors
3.8. Endothelin-1 Receptor Antagonists
4. Surgical Treatment
5. New Potential Therapeutic Strategies
5.1. Protein Kinase C Inhibition
5.2. Adiponectin
5.3. Anti-Inflammatory Treatments
5.3.1. Agents Inhibiting Inflammatory Factors
5.3.2. JAK/STAT Inhibitors
5.3.3. ASK1 Inhibitors
5.4. Antioxidant Treatments
5.4.1. Nrf2 Activators
5.4.2. NOX Inhibitors
5.4.3. Bioactive Antioxidants
5.5. Antifibrotic Treatments
5.6. Treatments Targeting Autophagy
6. Interventions for Nonalbuminuric DKD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valencia, W.M.; Florez, H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ 2017, 356, i6505. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Gui, D.; Zheng, L.; Zhai, R.; Wang, F.; Wang, N. Mechanistic Insight and Management of Diabetic Nephropathy: Recent Progress and Future Perspective. J. Diabetes Res. 2017, 2017, 1839809. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26. [Google Scholar] [CrossRef]
- Block, T.J.; Batu, D.; Cooper, M.E. Recent advances in the pharmacotherapeutic management of diabetic kidney disease. Expert Opin. Pharmacother. 2022, 23, 791–803. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef]
- Kotsis, V.; Martinez, F.; Trakatelli, C.; Redon, J. Impact of Obesity in Kidney Diseases. Nutrients 2021, 13, 4482. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Carlsson, L.M.; Romeo, S.; Jacobson, P.; Burza, M.A.; Maglio, C.; Sjöholm, K.; Svensson, P.A.; Haraldsson, B.; Peltonen, M.; Sjöström, L. The incidence of albuminuria after bariatric surgery and usual care in Swedish Obese Subjects (SOS): A prospective controlled intervention trial. Int. J. Obes. 2015, 39, 169–175. [Google Scholar] [CrossRef]
- Jiang, S.; Quan, D.V.; Sung, J.H.; Lee, M.Y.; Ha, H. Cigarette smoke inhalation aggravates diabetic kidney injury in rats. Toxicol. Res. 2019, 8, 964–971. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, J.; Shen, X.; Lu, W.; Wang, Y.; Li, W.; Gao, Z.; Xu, J.; Li, X.; Liu, R.; et al. Establishment and Validation of a Risk Prediction Model for Early Diabetic Kidney Disease Based on a Systematic Review and Meta-Analysis of 20 Cohorts. Diabetes Care 2020, 43, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Taler, S.J.; Agarwal, R.; Bakris, G.L.; Flynn, J.T.; Nilsson, P.M.; Rahman, M.; Sanders, P.W.; Textor, S.C.; Weir, M.R.; Townsend, R.R. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am. J. Kidney Dis. 2013, 62, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Franco, D.; Sorriento, D.; Strisciuglio, T.; Barbato, E.; Morisco, C. Modulation of Insulin Sensitivity by Exercise Training: Implications for Cardiovascular Prevention. J. Cardiovasc. Transl. Res. 2021, 14, 256–270. [Google Scholar] [CrossRef]
- Monno, I.; Ogura, Y.; Xu, J.; Koya, D.; Kitada, M. Exercise Ameliorates Diabetic Kidney Disease in Type 2 Diabetic Fatty Rats. Antioxidants 2021, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Ruderman, N.; Campaigne, B.N.; Devlin, J.T.; Schneider, S.H. Physical activity/exercise and diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 1), S73–S77. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Jia, Y.; Zou, M.; Zhen, Z.; Xue, Y. Effect of a sodium restriction diet on albuminuria and blood pressure in diabetic kidney disease patients: A meta-analysis. Int. Urol. Nephrol. 2022, 54, 1249–1260. [Google Scholar] [CrossRef]
- Pfeiffer, A.F.H.; Pedersen, E.; Schwab, U.; Risérus, U.; Aas, A.M.; Uusitupa, M.; Thanopoulou, A.; Kendall, C.; Sievenpiper, J.L.; Kahleová, H.; et al. The Effects of Different Quantities and Qualities of Protein Intake in People with Diabetes Mellitus. Nutrients 2020, 12, 365. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.P.; Tsuprykov, O.; Hasan, A.A.; Reichetzeder, C.; Tian, M.; Zhang, X.L.; Zhang, Q.; Sun, G.Y.; Guo, J.; et al. Folate treatment of pregnant rat dams abolishes metabolic effects in female offspring induced by a paternal pre-conception unhealthy diet. Diabetologia 2018, 61, 1862–1876. [Google Scholar] [CrossRef]
- Kataoka, S.; Norikura, T.; Sato, S. Maternal green tea polyphenol intake during lactation attenuates kidney injury in high-fat-diet-fed male offspring programmed by maternal protein restriction in rats. J. Nutr. Biochem. 2018, 56, 99–108. [Google Scholar] [CrossRef]
- Wood-Bradley, R.J.; Barrand, S.; Giot, A.; Armitage, J.A. Understanding the role of maternal diet on kidney development; an opportunity to improve cardiovascular and renal health for future generations. Nutrients 2015, 7, 1881–1905. [Google Scholar] [CrossRef] [Green Version]
- Haseler, E.; Melhem, N.; Sinha, M.D. Renal disease in pregnancy: Fetal, neonatal and long-term outcomes. Best Pract. Research. Clin. Obstet. Gynaecol. 2019, 57, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.S.; Lecomte, V.; Erlich, J.H.; Maloney, C.A.; Morris, M.J. Paternal High Fat Diet in Rats Leads to Renal Accumulation of Lipid and Tubular Changes in Adult Offspring. Nutrients 2016, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hasan, A.A.; Wu, H.; Gaballa, M.M.S.; Zeng, S.; Liu, L.; Xie, L.; Jung, T.; Grune, T.; Krämer, B.K.; et al. High-fat, sucrose and salt-rich diet during rat spermatogenesis lead to the development of chronic kidney disease in the female offspring of the F2 generation. FASEB J. 2022, 36, e22259. [Google Scholar] [CrossRef]
- Hur, S.S.J.; Cropley, J.E.; Suter, C.M. Paternal epigenetic programming: Evolving metabolic disease risk. J. Mol. Endocrinol. 2017, 58, R159–R168. [Google Scholar] [CrossRef] [PubMed]
- Larkin, B.P.; Glastras, S.J.; Chen, H.; Pollock, C.A.; Saad, S. DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J. 2018, 32, 5215–5226. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- De Boer, I.H. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 24–30. [Google Scholar] [CrossRef]
- Bebu, I.; Braffett, B.H.; Schade, D.; Sivitz, W.; Malone, J.I.; Pop-Busui, R.; Lorenzi, G.M.; Lee, P.; Trapani, V.R.; Wallia, A.; et al. An Observational Study of the Equivalence of Age and Duration of Diabetes to Glycemic Control Relative to the Risk of Complications in the Combined Cohorts of the DCCT/EDIC Study. Diabetes Care 2020, 43, 2478–2484. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- FOR THE DIABETES, The Writing Team; Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003, 290, 2159–2167. [Google Scholar] [CrossRef] [Green Version]
- Adler, A.I.; Stevens, R.J.; Manley, S.E.; Bilous, R.W.; Cull, C.A.; Holman, R.R. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003, 63, 225–232. [Google Scholar] [CrossRef]
- Wong, M.G.; Perkovic, V.; Chalmers, J.; Woodward, M.; Li, Q.; Cooper, M.E.; Hamet, P.; Harrap, S.; Heller, S.; MacMahon, S.; et al. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care 2016, 39, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Kornelius, E.; Lo, S.C.; Huang, C.N.; Wang, Y.H.; Yang, Y.S. Association of blood glucose and renal end points in advanced diabetic kidney disease. Diabetes Res. Clin. Pract. 2020, 161, 108011. [Google Scholar] [CrossRef] [PubMed]
- Papademetriou, V.; Lovato, L.; Doumas, M.; Nylen, E.; Mottl, A.; Cohen, R.M.; Applegate, W.B.; Puntakee, Z.; Yale, J.F.; Cushman, W.C. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015, 87, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [CrossRef]
- Griffin, S.J.; Borch-Johnsen, K.; Davies, M.J.; Khunti, K.; Rutten, G.E.; Sandbæk, A.; Sharp, S.J.; Simmons, R.K.; van den Donk, M.; Wareham, N.J.; et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): A cluster-randomised trial. Lancet 2011, 378, 156–167. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Anzalone, D.A.; Cain, V.A.; Cressman, M.D.; Heerspink, H.J.; Molitoris, B.A.; Monyak, J.T.; Parving, H.H.; Remuzzi, G.; Sowers, J.R.; et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): A randomised clinical trial. Lancet Diabetes Endocrinol. 2015, 3, 181–190. [Google Scholar] [CrossRef]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- Patel, D.M.; Bose, M.; Cooper, M.E. Glucose and Blood Pressure-Dependent Pathways-The Progression of Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 2218. [Google Scholar] [CrossRef] [Green Version]
- Zatz, R.; Dunn, B.R.; Meyer, T.W.; Anderson, S.; Rennke, H.G.; Brenner, B.M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J. Clin. Investig. 1986, 77, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.; Arima, H.; Rothwell, P.M.; Woodward, M.; Zoungas, S.; Anderson, C.; Patel, A.; Neal, B.; Glasziou, P.; Hamet, P.; et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: The ADVANCE trial. Circulation 2013, 128, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Viazzi, F.; Bonino, B.; Mirijello, A.; Fioretto, P.; Giorda, C.; Ceriello, A.; Guida, P.; Russo, G.T.; De Cosmo, S.; Pontremoli, R. Long-term blood pressure variability and development of chronic kidney disease in type 2 diabetes. J. Hypertens. 2019, 37, 805–813. [Google Scholar] [CrossRef]
- Viazzi, F.; Russo, E.; Mirijello, A.; Fioretto, P.; Giorda, C.; Ceriello, A.; Copetti, M.; Russo, G.T.; Di Bartolo, P.; Manicardi, V.; et al. Long-term blood pressure variability, incidence of hypertension and changes in renal function in type 2 diabetes. J. Hypertens. 2020, 38, 2279–2286. [Google Scholar] [CrossRef]
- Sun, H.J. Current Opinion for Hypertension in Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kliewe, F.; Kaling, S.; Lötzsch, H.; Artelt, N.; Schindler, M.; Rogge, H.; Schröder, S.; Scharf, C.; Amann, K.; Daniel, C.; et al. Fibronectin is up-regulated in podocytes by mechanical stress. FASEB J. 2019, 33, 14450–14460. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R. Back to the Future: Glomerular Hyperfiltration and the Diabetic Kidney. Diabetes 2017, 66, 14–16. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Ryder, J.R.; Gaesser, G.A.; Shaibi, G.Q. Achievement of goals in U.S. diabetes care, 1999-2010. N. Engl. J. Med. 2013, 369, 287. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Koszegi, S.; Molnar, A.; Lenart, L.; Hodrea, J.; Balogh, D.B.; Lakat, T.; Szkibinszkij, E.; Hosszu, A.; Sparding, N.; Genovese, F.; et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J. Physiol. 2019, 597, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Garg, P. A Review of Podocyte Biology. Am. J. Nephrol. 2018, 47 (Suppl. 1), 3–13. [Google Scholar] [CrossRef]
- An, J.; Niu, F.; Sim, J.J. Cardiovascular and kidney outcomes of spironolactone or eplerenone in combination with ACEI/ARBs in patients with diabetic kidney disease. Pharmacotherapy 2021, 41, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Girerd, S.; Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: Pathophysiological basis. Kidney Int. 2019, 96, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Bjornstad, P.; van Raalte, D.H.; Heerspink, H.L.; Cherney, D.Z.I. The New Biology of Diabetic Kidney Disease-Mechanisms and Therapeutic Implications. Endocr. Rev. 2020, 41, 202–231. [Google Scholar] [CrossRef]
- Fujisawa, G.; Okada, K.; Muto, S.; Fujita, N.; Itabashi, N.; Kusano, E.; Ishibashi, S. Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int. 2004, 66, 1493–1502. [Google Scholar] [CrossRef]
- Guo, C.; Martinez-Vasquez, D.; Mendez, G.P.; Toniolo, M.F.; Yao, T.M.; Oestreicher, E.M.; Kikuchi, T.; Lapointe, N.; Pojoga, L.; Williams, G.H.; et al. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 2006, 147, 5363–5373. [Google Scholar] [CrossRef]
- Kang, Y.S.; Ko, G.J.; Lee, M.H.; Song, H.K.; Han, S.Y.; Han, K.H.; Kim, H.K.; Han, J.Y.; Cha, D.R. Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol. Dial. Transplant. 2009, 24, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Xiong, W.; Cao, L.; Wen, X.; Li, A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin. Ther. 2015, 37, 2086–2103.e2010. [Google Scholar] [CrossRef] [PubMed]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Piko, N.; Petreski, T.; Hojs, R. Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease. Pharmaceuticals 2021, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Abreu, P.; McMurray, J.J.V.; van Veldhuisen, D.J.; Swedberg, K.; Pocock, S.J.; Vincent, J.; Lins, K.; Rossignol, P.; Pitt, B.; et al. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS-HF trial. Eur. J. Heart Fail. 2019, 21, 345–351. [Google Scholar] [CrossRef]
- Rico-Mesa, J.S.; White, A.; Ahmadian-Tehrani, A.; Anderson, A.S. Mineralocorticoid Receptor Antagonists: A Comprehensive Review of Finerenone. Curr. Cardiol. Rep. 2020, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Yamada, D.; Nakayama, M.; Yamada, T.; Myoishi, M.; Kato, M.; Nowack, C.; Kolkhof, P.; Yamasaki, Y. A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 758–765. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Zannad, F.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Jamal, W.; Steubl, D.; Schueler, E.; et al. Interplay of Mineralocorticoid Receptor Antagonists and Empagliflozin in Heart Failure: EMPEROR-Reduced. J. Am. Coll. Cardiol. 2021, 77, 1397–1407. [Google Scholar] [CrossRef]
- Lachaux, M.; Barrera-Chimal, J.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes. Metab. 2018, 20, 2399–2407. [Google Scholar] [CrossRef]
- Tokuyama, H.; Wakino, S.; Hara, Y.; Washida, N.; Fujimura, K.; Hosoya, K.; Yoshioka, K.; Hasegawa, K.; Minakuchi, H.; Homma, K.; et al. Role of mineralocorticoid receptor/Rho/Rho-kinase pathway in obesity-related renal injury. Int. J. Obes. 2012, 36, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, K.; Tsuruya, K.; Nakano, T.; Taniguchi, M.; Higashi, H.; Katafuchi, R.; Kanai, H.; Nakayama, M.; Hirakata, H.; Kitazono, T. Impact of combined losartan/hydrochlorothiazide on proteinuria in patients with chronic kidney disease and hypertension. Hypertens. Res. 2014, 37, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Pappas, M.K.; Ammous, F. Chlorthalidone for poorly controlled hypertension in chronic kidney disease: An interventional pilot study. Am. J. Nephrol. 2014, 39, 171–182. [Google Scholar] [CrossRef]
- Trujillo, H.; Caravaca-Fontán, F.; Caro, J.; Morales, E.; Praga, M. The Forgotten Antiproteinuric Properties of Diuretics. Am. J. Nephrol. 2021, 52, 435–449. [Google Scholar] [CrossRef]
- Kwakernaak, A.J.; Krikken, J.A.; Binnenmars, S.H.; Visser, F.W.; Hemmelder, M.H.; Woittiez, A.J.; Groen, H.; Laverman, G.D.; Navis, G. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: A randomised clinical trial. Lancet Diabetes Endocrinol. 2014, 2, 385–395. [Google Scholar] [CrossRef]
- Vogt, L.; Waanders, F.; Boomsma, F.; de Zeeuw, D.; Navis, G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J. Am. Soc. Nephrol. 2008, 19, 999–1007. [Google Scholar] [CrossRef]
- Esnault, V.L.; Ekhlas, A.; Nguyen, J.M.; Moranne, O. Diuretic uptitration with half dose combined ACEI + ARB better decreases proteinuria than combined ACEI + ARB uptitration. Nephrol. Dial. Transplant. 2010, 25, 2218–2224. [Google Scholar] [CrossRef]
- Hoshino, T.; Ookawara, S.; Miyazawa, H.; Ito, K.; Ueda, Y.; Kaku, Y.; Hirai, K.; Mori, H.; Yoshida, I.; Tabei, K. Renoprotective effects of thiazides combined with loop diuretics in patients with type 2 diabetic kidney disease. Clin. Exp. Nephrol. 2015, 19, 247–253. [Google Scholar] [CrossRef]
- Svenningsen, P.; Bistrup, C.; Friis, U.G.; Bertog, M.; Haerteis, S.; Krueger, B.; Stubbe, J.; Jensen, O.N.; Thiesson, H.C.; Uhrenholt, T.R.; et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J. Am. Soc. Nephrol. 2009, 20, 299–310. [Google Scholar] [CrossRef]
- Unruh, M.L.; Pankratz, V.S.; Demko, J.E.; Ray, E.C.; Hughey, R.P.; Kleyman, T.R. Trial of Amiloride in Type 2 Diabetes with Proteinuria. Kidney Int. Rep. 2017, 2, 893–904. [Google Scholar] [CrossRef] [Green Version]
- León Jiménez, D.; Gómez Huelgas, R.; Fernández Romero, A.J.; López Chozas, J.M.; Pérez de Isla, L.; Miramontes González, J.P. Diuretic treatment of the patient with diabetes and heart failure. Role of SGLT2 inhibitors and similarities with carbonic anhydrase inhibitors. Rev. Clin. Esp. 2019, 219, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bradwell, A.R.; Wright, A.D.; Winterborn, M.; Imray, C. Acetazolamide and high altitude diseases. Int. J. Sports Med. 1992, 13 (Suppl. 1), S63–S64. [Google Scholar] [CrossRef] [PubMed]
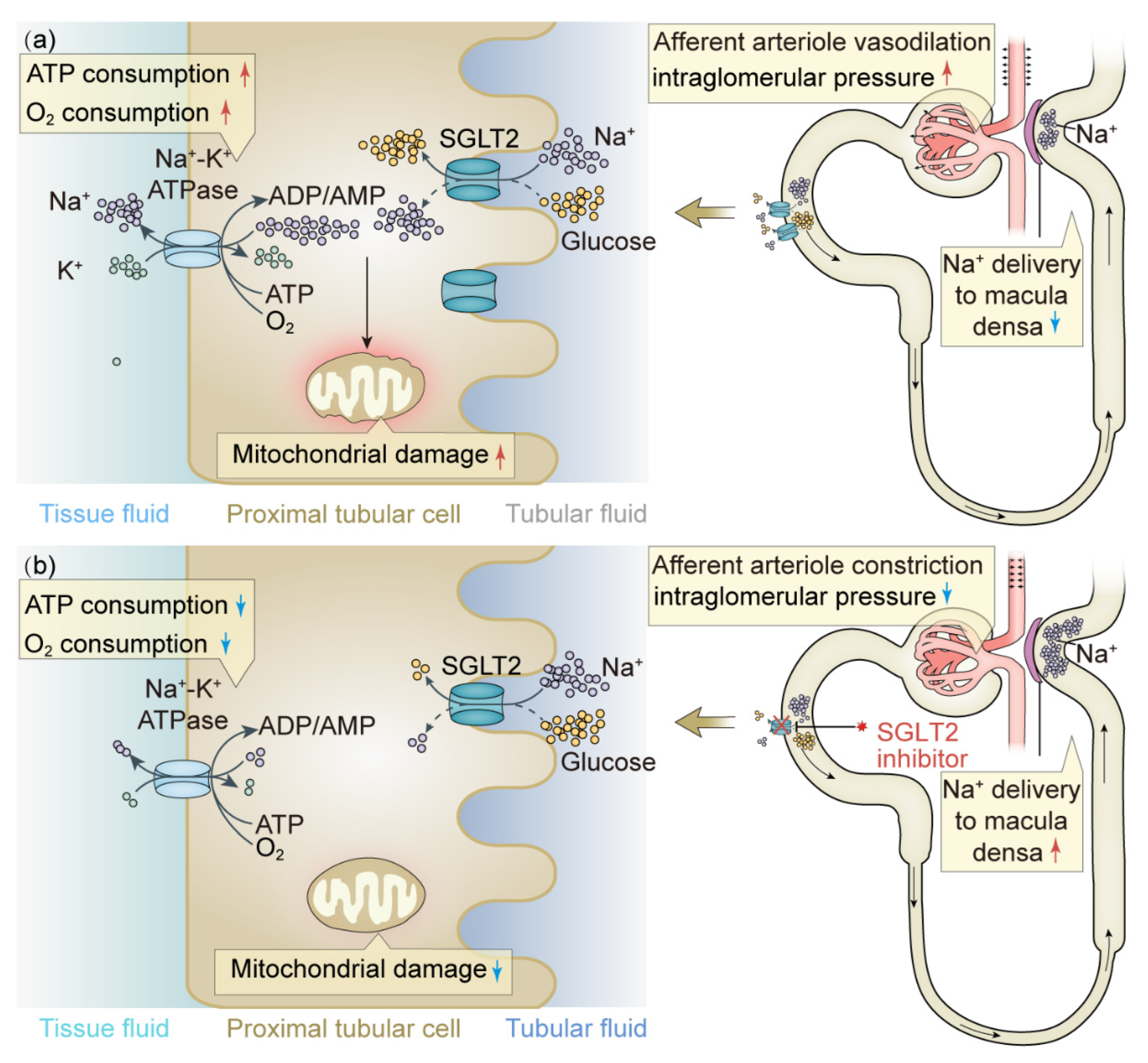
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Ren. Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Chu, C.; Lu, Y.P.; Yin, L.; Hocher, B. The SGLT2 Inhibitor Empagliflozin Might Be a New Approach for the Prevention of Acute Kidney Injury. Kidney Blood Press. Res. 2019, 44, 149–157. [Google Scholar] [CrossRef]
- Chu, C.; Delić, D.; Alber, J.; Feger, M.; Xiong, Y.; Luo, T.; Hasan, A.A.; Zeng, S.; Gaballa, M.M.S.; Chen, X.; et al. Head-to-head comparison of two SGLT-2 inhibitors on AKI outcomes in a rat ischemia-reperfusion model. Biomed. Pharmacother. 2022, 153, 113357. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Sen, T.; Heerspink, H.J.L. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab. 2021, 33, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Eklöf, A.C.; Celsi, G.; Aperia, A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 1994, 43, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Li, J.; Takagaki, Y.; Kitada, M.; Nitta, K.; Takasu, T.; Kanasaki, K.; Koya, D. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J. Diabetes Investig. 2018, 9, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. An update on the safety of SGLT2 inhibitors. Expert Opin. Drug Saf. 2019, 18, 295–311. [Google Scholar] [CrossRef]
- Zeng, S.; Delic, D.; Chu, C.; Xiong, Y.; Luo, T.; Chen, X.; Gaballa, M.M.S.; Xue, Y.; Chen, X.; Cao, Y.; et al. Antifibrotic effects of low dose SGLT2 Inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet. Biomed. Pharmacother. 2022, 146, 112606. [Google Scholar] [CrossRef]
- Greco, E.V.; Russo, G.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; De Cosmo, S. GLP-1 Receptor Agonists and Kidney Protection. Medicina 2019, 55, 233. [Google Scholar] [CrossRef]
- Mikov, M.; Pavlović, N.; Stanimirov, B.; Đanić, M.; Goločorbin-Kon, S.; Stankov, K.; Al-Salami, H. DPP-4 Inhibitors: Renoprotective Potential and Pharmacokinetics in Type 2 Diabetes Mellitus Patients with Renal Impairment. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 1–14. [Google Scholar] [CrossRef]
- Dahiya, L.; Kaur, R.; Kumar, R.; Kumar, M.; Palta, K. GLP-1 Receptor Agonists in Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 16, 279–292. [Google Scholar] [CrossRef]
- Thomas, M.C. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017, 43 (Suppl. 1), 2S20–2S27. [Google Scholar] [CrossRef]
- Puglisi, S.; Rossini, A.; Poli, R.; Dughera, F.; Pia, A.; Terzolo, M.; Reimondo, G. Effects of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Renin-Angiotensin-Aldosterone System. Front. Endocrinol. 2021, 12, 738848. [Google Scholar] [CrossRef] [PubMed]
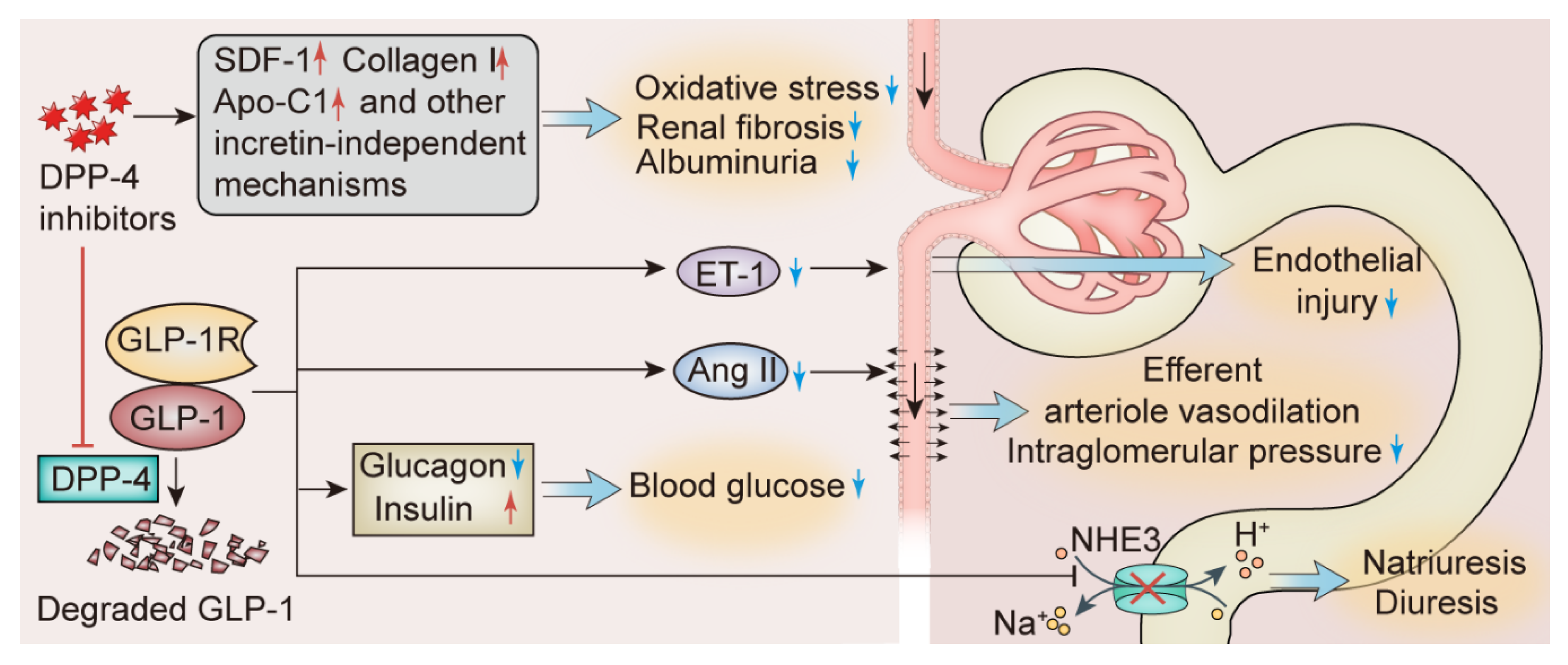
- Dai, Y.; Mehta, J.L.; Chen, M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc. Drugs Ther. 2013, 27, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.; Taher, J.; Adeli, K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, S.; Wang, Y.; Parlevliet, E.T.; Boon, M.R.; Edelschaap, D.; Snaterse, G.; Pijl, H.; Romijn, J.A.; Rensen, P.C. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 2015, 58, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Hocher, B.; Tsuprykov, O. Diabetic nephropathy: Renoprotective effects of GLP1R agonists and SGLT2 inhibitors. Nat. Rev. Nephrol. 2017, 13, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Cherney, D.Z.I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018, 61, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Rieg, T.; Gerasimova, M.; Murray, F.; Masuda, T.; Tang, T.; Rose, M.; Drucker, D.J.; Vallon, V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Ren. Physiol. 2012, 303, F963–F971. [Google Scholar] [CrossRef]
- Takashima, S.; Fujita, H.; Fujishima, H.; Shimizu, T.; Sato, T.; Morii, T.; Tsukiyama, K.; Narita, T.; Takahashi, T.; Drucker, D.J.; et al. Stromal cell-derived factor-1 is upregulated by dipeptidyl peptidase-4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int. 2016, 90, 783–796. [Google Scholar] [CrossRef]
- Kanasaki, K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin. Sci. 2018, 132, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Tsuprykov, O.; Ando, R.; Reichetzeder, C.; von Websky, K.; Antonenko, V.; Sharkovska, Y.; Chaykovska, L.; Rahnenführer, J.; Hasan, A.A.; Tammen, H.; et al. The dipeptidyl peptidase inhibitor linagliptin and the angiotensin II receptor blocker telmisartan show renal benefit by different pathways in rats with 5/6 nephrectomy. Kidney Int. 2016, 89, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; von Websky, K.; Reichetzeder, C.; Tsuprykov, O.; Gaballa, M.M.S.; Guo, J.; Zeng, S.; Delić, D.; Tammen, H.; Klein, T.; et al. Mechanisms of GLP-1 receptor-independent renoprotective effects of the dipeptidyl peptidase type 4 inhibitor linagliptin in GLP-1 receptor knockout mice with 5/6 nephrectomy. Kidney Int. 2019, 95, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Penno, G.; Natali, A.; Barutta, F.; Di Paolo, S.; Reboldi, G.; Gesualdo, L.; De Nicola, L. Diabetic kidney disease: New clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1127–1150. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef]
- Matthaei, S.; Catrinoiu, D.; Celiński, A.; Ekholm, E.; Cook, W.; Hirshberg, B.; Chen, H.; Iqbal, N.; Hansen, L. Randomized, Double-Blind Trial of Triple Therapy With Saxagliptin Add-on to Dapagliflozin Plus Metformin in Patients With Type 2 Diabetes. Diabetes Care 2015, 38, 2018–2024. [Google Scholar] [CrossRef]
- Frías, J.P.; Guja, C.; Hardy, E.; Ahmed, A.; Dong, F.; Öhman, P.; Jabbour, S.A. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): A 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 1004–1016. [Google Scholar] [CrossRef]
- Jabbour, S.A.; Frías, J.P.; Ahmed, A.; Hardy, E.; Choi, J.; Sjöström, C.D.; Guja, C. Efficacy and Safety Over 2 Years of Exenatide Plus Dapagliflozin in the DURATION-8 Study: A Multicenter, Double-Blind, Phase 3, Randomized Controlled Trial. Diabetes Care 2020, 43, 2528–2536. [Google Scholar] [CrossRef]
- Naing, S.; Ramesh, G.; Garcha, J.; Poliyedath, A.; Khandelwal, S.; Mills, P.K. Is the stepping-down approach a better option than multiple daily injections in obese patients with poorly controlled Type 2 diabetes on advanced insulin therapy? Endocrinol. Diabetes Metab. 2021, 4, e00204. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years From Discovery to Therapy. Hypertension 2019, 74, 1232–1265. [Google Scholar] [CrossRef] [PubMed]
- Kasztan, M.; Pollock, D.M. Impact of ET-1 and sex in glomerular hyperfiltration in humanized sickle cell mice. Clin. Sci. 2019, 133, 1475–1486. [Google Scholar] [CrossRef]
- Mann, J.F.; Green, D.; Jamerson, K.; Ruilope, L.M.; Kuranoff, S.J.; Littke, T.; Viberti, G. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Parving, H.H.; Andress, D.L.; Bakris, G.; Correa-Rotter, R.; Hou, F.F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947. [Google Scholar] [CrossRef]
- Miyauchi, T.; Sakai, S. Endothelin and the heart in health and diseases. Peptides 2019, 111, 77–88. [Google Scholar] [CrossRef]
- Raina, R.; Chauvin, A.; Chakraborty, R.; Nair, N.; Shah, H.; Krishnappa, V.; Kusumi, K. The Role of Endothelin and Endothelin Antagonists in Chronic Kidney Disease. Kidney Dis. 2020, 6, 22–34. [Google Scholar] [CrossRef]
- Smeijer, J.D.; Kohan, D.E.; Webb, D.J.; Dhaun, N.; Heerspink, H.J.L. Endothelin receptor antagonists for the treatment of diabetic and nondiabetic chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2021, 30, 456–465. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, S.; Hou, J.; Chen, G.; Xu, Z.G. Endothelin receptor antagonists for the treatment of diabetic nephropathy: A meta-analysis and systematic review. World J. Diabetes 2020, 11, 553–566. [Google Scholar] [CrossRef]
- Cohen, R.V.; Pereira, T.V.; Aboud, C.M.; Caravatto, P.P.; Petry, T.B.; Correa, J.L.; Schiavon, C.A.; Correa, M.; Pompílio, C.E.; Pechy, F.N.; et al. Microvascular Outcomes after Metabolic Surgery (MOMS) in patients with type 2 diabetes mellitus and class I obesity: Rationale and design for a randomised controlled trial. BMJ Open 2017, 7, e013574. [Google Scholar] [CrossRef]
- Shulman, A.; Peltonen, M.; Sjöström, C.D.; Andersson-Assarsson, J.C.; Taube, M.; Sjöholm, K.; le Roux, C.W.; Carlsson, L.M.S.; Svensson, P.A. Incidence of end-stage renal disease following bariatric surgery in the Swedish Obese Subjects Study. Int. J. Obes. 2018, 42, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Canney, A.L.; Cohen, R.V.; Elliott, J.A.; Aboud, C.M.; Martin, W.P.; Docherty, N.G.; le Roux, C.W. Improvements in diabetic albuminuria and podocyte differentiation following Roux-en-Y gastric bypass surgery. Diabetes Vasc. Dis. Res. 2020, 17, 1479164119879039. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.V.; Pereira, T.V.; Aboud, C.M.; Petry, T.B.Z.; Lopes Correa, J.L.; Schiavon, C.A.; Pompílio, C.E.; Pechy, F.N.Q.; da Costa Silva, A.C.C.; de Melo, F.L.G.; et al. Effect of Gastric Bypass vs Best Medical Treatment on Early-Stage Chronic Kidney Disease in Patients With Type 2 Diabetes and Obesity: A Randomized Clinical Trial. JAMA Surg. 2020, 155, e200420. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Martin, W.P.; Zhernovkov, V.; Elliott, J.A.; Fearon, N.; Eckhardt, H.; McCormack, J.; Godson, C.; Brennan, E.P.; Fandriks, L.; et al. Characterization of the renal cortical transcriptome following Roux-en-Y gastric bypass surgery in experimental diabetic kidney disease. BMJ Open Diabetes Res. Care 2020, 8, e001113. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, W.; Xu, Q.; Ruze, R.; Yan, Z.; Li, J.; Hu, S.; Zhong, M.; Cheng, Y.; Zhang, G. Sleeve Gastrectomy Attenuates Diabetic Nephropathy by Upregulating Nephrin Expressions in Diabetic Obese Rats. Obes. Surg. 2020, 30, 2893–2904. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, Y.G.; Huang, X.; Zhong, M.W.; Liu, S.Z.; Hu, S.Y. Downregulation of lncRNA MALAT1 contributes to renal functional improvement after duodenal-jejunal bypass in a diabetic rat model. J. Physiol. Biochem. 2018, 74, 431–439. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef]
- Menne, J.; Park, J.K.; Boehne, M.; Elger, M.; Lindschau, C.; Kirsch, T.; Meier, M.; Gueler, F.; Fiebeler, A.; Bahlmann, F.H.; et al. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-alpha-deficient diabetic mice. Diabetes 2004, 53, 2101–2109. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Chao, J.; Chen, C.; Lv, L.L.; Han, Y.C.; Liu, B.C. The PKCβ-p66shc-NADPH oxidase pathway plays a crucial role in diabetic nephropathy. J. Pharm. Pharmacol. 2019, 71, 338–347. [Google Scholar] [CrossRef]
- Al-Onazi, A.S.; Al-Rasheed, N.M.; Attia, H.A.; Al-Rasheed, N.M.; Ahmed, R.M.; Al-Amin, M.A.; Poizat, C. Ruboxistaurin attenuates diabetic nephropathy via modulation of TGF-β1/Smad and GRAP pathways. J. Pharm. Pharmacol. 2016, 68, 219–232. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 2005, 28, 2686–2690. [Google Scholar] [CrossRef]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol. Sin. 2018, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Ramachandrarao, S.; Qiu, G.; Usui, H.K.; Zhu, Y.; Dunn, S.R.; Ouedraogo, R.; Hough, K.; McCue, P.; Chan, L.; et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Investig. 2008, 118, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Bae, E.H.; Hu, A.; Liu, G.C.; Zhou, X.; Williams, V.; Maksimowski, N.; Lu, C.; Konvalinka, A.; John, R.; et al. Deletion of the gene for adiponectin accelerates diabetic nephropathy in the Ins2 (+/C96Y) mouse. Diabetologia 2015, 58, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yang, J.W.; Han, B.G.; Choi, S.O.; Kim, J.S. Adiponectin for the treatment of diabetic nephropathy. Korean J. Intern. Med. 2019, 34, 480–491. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Yoon, H.E.; Shin, S.J.; Choi, B.S.; Kim, Y.S.; Chang, Y.S.; Park, C.W. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J. Am. Soc. Nephrol. 2018, 29, 1108–1127. [Google Scholar] [CrossRef]
- Woroniecka, K.I.; Park, A.S.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome analysis of human diabetic kidney disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef]
- Wilson, P.C.; Wu, H.; Kirita, Y.; Uchimura, K.; Ledru, N.; Rennke, H.G.; Welling, P.A.; Waikar, S.S.; Humphreys, B.D. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2019, 116, 19619–19625. [Google Scholar] [CrossRef]
- Guiteras, R.; Sola, A.; Flaquer, M.; Manonelles, A.; Hotter, G.; Cruzado, J.M. Exploring macrophage cell therapy on Diabetic Kidney Disease. J. Cell. Mol. Med. 2019, 23, 841–851. [Google Scholar] [CrossRef]
- Shang, J.; Wang, L.; Zhang, Y.; Zhang, S.; Ning, L.; Zhao, J.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. Chemerin/ChemR23 axis promotes inflammation of glomerular endothelial cells in diabetic nephropathy. J. Cell. Mol. Med. 2019, 23, 3417–3428. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.S.; Kinsey, G.R.; Khutsishvili, K.; Gao, T.; Bolton, W.K.; Okusa, M.D. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am. J. Physiol. Ren. Physiol. 2011, 301, F1358–F1366. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Hsu, Y.H. The role of IL-20 in chronic kidney disease and diabetic nephropathy: Pathogenic and therapeutic implications. J. Leukoc. Biol. 2018, 104, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Šenolt, L.; Leszczynski, P.; Dokoupilová, E.; Göthberg, M.; Valencia, X.; Hansen, B.B.; Cañete, J.D. Efficacy and Safety of Anti-Interleukin-20 Monoclonal Antibody in Patients With Rheumatoid Arthritis: A Randomized Phase IIa Trial. Arthritis Rheumatol. 2015, 67, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Koenig, W.; Libby, P.; Everett, B.M.; Lefkowitz, M.; Thuren, T.; Cornel, J.H. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients With Chronic Kidney Disease. J. Am. Coll. Cardiol. 2018, 71, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Giunti, S.; Barutta, F.; Perin, P.C.; Gruden, G. Targeting the MCP-1/CCR2 System in diabetic kidney disease. Curr. Vasc. Pharmacol. 2010, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Purohit, S.; Wang, H.; Bode, B.; Reed, J.C.; Steed, R.D.; Anderson, S.W.; Steed, L.; Hopkins, D.; Xia, C.; et al. Chemokine (C-C motif) ligand 2 (CCL2) in sera of patients with type 1 diabetes and diabetic complications. PLoS ONE 2011, 6, e17822. [Google Scholar] [CrossRef]
- Menne, J.; Eulberg, D.; Beyer, D.; Baumann, M.; Saudek, F.; Valkusz, Z.; Więcek, A.; Haller, H. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dial. Transplant. 2017, 32, 307–315. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Bekker, P.; Henkel, E.; Hasslacher, C.; Gouni-Berthold, I.; Mehling, H.; Potarca, A.; Tesar, V.; Heerspink, H.J.; Schall, T.J. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: A randomised trial. Lancet Diabetes Endocrinol. 2015, 3, 687–696. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Brosius, F.C.; Tuttle, K.R.; Kretzler, M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia 2016, 59, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Brosius, F.C., 3rd; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M.E.; et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant. 2018, 33, 1950–1959. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Liles, J.T.; Corkey, B.K.; Notte, G.T.; Budas, G.R.; Lansdon, E.B.; Hinojosa-Kirschenbaum, F.; Badal, S.S.; Lee, M.; Schultz, B.E.; Wise, S.; et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Investig. 2018, 128, 4485–4500. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Pergola, P.E.; Chen, F.; Kirby, B.J.; Sundy, J.S.; Patel, U.D. Effects of Selonsertib in Patients with Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2019, 30, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free. Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, T.; Nangaku, M. Nuclear factor erythroid 2-related factor 2 as a treatment target of kidney diseases. Curr. Opin. Nephrol. Hypertens. 2020, 29, 128–135. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Kanda, H.; Takama, H.; Ichikawa, T.; Hase, H.; Akizawa, T. Randomized Clinical Trial on the Effect of Bardoxolone Methyl on GFR in Diabetic Kidney Disease Patients (TSUBAKI Study). Kidney Int. Rep. 2020, 5, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Takama, H.; Ichikawa, T.; Mukai, K.; Kojima, M.; Suzuki, Y.; Watada, H.; Wada, T.; Ueki, K.; Narita, I.; et al. Randomized, double-blind, placebo-controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: Design and baseline characteristics of AYAME study. Nephrol. Dial. Transplant. 2022, gfac242. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, F.R.; Wang, J.N.; Gao, L.; Jiang, L.; Li, H.D.; Ma, Q.; Liu, X.Q.; Wei, B.; Zhou, L.; et al. Nox4 in renal diseases: An update. Free. Radic. Biol. Med. 2018, 124, 466–472. [Google Scholar] [CrossRef]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef]
- Reutens, A.T.; Jandeleit-Dahm, K.; Thomas, M.; Salim, A.; De Livera, A.M.; Bach, L.A.; Colman, P.G.; Davis, T.M.E.; Ekinci, E.I.; Fulcher, G.; et al. A physician-initiated double-blind, randomised, placebo-controlled, phase 2 study evaluating the efficacy and safety of inhibition of NADPH oxidase with the first-in-class Nox-1/4 inhibitor, GKT137831, in adults with type 1 diabetes and persistently elevated urinary albumin excretion: Protocol and statistical considerations. Contemp. Clin. Trials 2020, 90, 105892. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Okabe, J.; Gray, S.P.; Hettige, T.; Chow, B.S.M.; Thallas-Bonke, V.; De Vos, L.; Holterman, C.E.; Coughlan, M.T.; et al. NADPH Oxidase Nox5 Accelerates Renal Injury in Diabetic Nephropathy. Diabetes 2017, 66, 2691–2703. [Google Scholar] [CrossRef]
- Boonthongkaew, C.; Tong-Un, T.; Kanpetta, Y.; Chaungchot, N.; Leelayuwat, C.; Leelayuwat, N. Vitamin C supplementation improves blood pressure and oxidative stress after acute exercise in patients with poorly controlled type 2 diabetes mellitus: A randomized, placebo-controlled, cross-over study. Chin. J. Physiol. 2021, 64, 16–23. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, M.Y.; Hong, S.W.; Chung, C.H.; Hong, S.Y. Blockade of oxidative stress by vitamin C ameliorates albuminuria and renal sclerosis in experimental diabetic rats. Yonsei Med. J. 2007, 48, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free. Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Arun, N.; Nalini, N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum. Nutr. 2002, 57, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Mito, S.; Harima, M.; Thandavarayan, R.A.; Suzuki, K.; Nagata, M.; Takagi, R.; et al. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: Possible involvement of PKC-MAPK signaling pathway. Eur. J. Pharm. Sci. 2012, 47, 604–614. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.S.; Kim, D.H.; Kim, S.J.; Kim, S.H.; Cho, N.C.; Na, H.K.; Surh, Y.J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Qiao, Y.; Gao, K.; Wang, Y.; Wang, X.; Cui, B. Resveratrol ameliorates diabetic nephropathy in rats through negative regulation of the p38 MAPK/TGF-β1 pathway. Exp. Ther. Med. 2017, 13, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Chi, Y.; Kang, Y.; Lu, H.; Niu, H.; Liu, W.; Li, Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019, 234, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Y.; Ma, X.; Zhang, N.; Qin, G. The effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy rats. Mol. Biol. Rep. 2012, 39, 9085–9093. [Google Scholar] [CrossRef] [PubMed]
- RamachandraRao, S.P.; Zhu, Y.; Ravasi, T.; McGowan, T.A.; Toh, I.; Dunn, S.R.; Okada, S.; Shaw, M.A.; Sharma, K. Pirfenidone is renoprotective in diabetic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1765–1775. [Google Scholar] [CrossRef]
- Sharma, K.; Ix, J.H.; Mathew, A.V.; Cho, M.; Pflueger, A.; Dunn, S.R.; Francos, B.; Sharma, S.; Falkner, B.; McGowan, T.A.; et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Chen, R.H.; Chen, Y.M.; Chiang, W.C.; Lai, C.F.; Wu, K.D.; Tsai, T.J. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J. Am. Soc. Nephrol. 2005, 16, 2702–2713. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; Chahin, J.; Méndez, M.L.; Gallego, E.; Macía, M.; del Castillo, N.; Rivero, A.; Getino, M.A.; et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J. Am. Soc. Nephrol. 2015, 26, 220–229. [Google Scholar] [CrossRef]
- Isaka, Y. Targeting TGF-β Signaling in Kidney Fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef]
- Soma, J.; Sato, K.; Saito, H.; Tsuchiya, Y. Effect of tranilast in early-stage diabetic nephropathy. Nephrol. Dial. Transplant. 2006, 21, 2795–2799. [Google Scholar] [CrossRef]
- Soma, J.; Sugawara, T.; Huang, Y.D.; Nakajima, J.; Kawamura, M. Tranilast slows the progression of advanced diabetic nephropathy. Nephron 2002, 92, 693–698. [Google Scholar] [CrossRef]
- Gilbert, R.E.; Zhang, Y.; Williams, S.J.; Zammit, S.C.; Stapleton, D.I.; Cox, A.J.; Krum, H.; Langham, R.; Kelly, D.J. A purpose-synthesised anti-fibrotic agent attenuates experimental kidney diseases in the rat. PLoS ONE 2012, 7, e47160. [Google Scholar] [CrossRef] [Green Version]
- Voelker, J.; Berg, P.H.; Sheetz, M.; Duffin, K.; Shen, T.; Moser, B.; Greene, T.; Blumenthal, S.S.; Rychlik, I.; Yagil, Y.; et al. Anti-TGF-β1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, J.; Wang, D. Promotion of chemerin in rat diabetic kidney disease through enhancement of TGF-β1/Smads/CTGF pathway. Am. J. Transl. Res. 2021, 13, 10206–10217. [Google Scholar]
- Adler, S.G.; Schwartz, S.; Williams, M.E.; Arauz-Pacheco, C.; Bolton, W.K.; Lee, T.; Li, D.; Neff, T.B.; Urquilla, P.R.; Sewell, K.L. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010, 5, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, J.; Hu, B.; Liu, C.; Wang, H. The Role of Cell Division Autoantigen 1 (CDA1) in Renal Fibrosis of Diabetic Nephropathy. BioMed Res. Int. 2021, 2021, 6651075. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Wu, T.; Dai, A.; Huynh, P.; Koentgen, F.; Krippner, G.; Ren, S.; Cooper, M.E. Targeting the CDA1/CDA1BP1 Axis Retards Renal Fibrosis in Experimental Diabetic Nephropathy. Diabetes 2019, 68, 395–408. [Google Scholar] [CrossRef]
- Ito, K.; Chen, J.; Seshan, S.V.; Khodadadian, J.J.; Gallagher, R.; El Chaar, M.; Vaughan, E.D., Jr.; Poppas, D.P.; Felsen, D. Dietary arginine supplementation attenuates renal damage after relief of unilateral ureteral obstruction in rats. Kidney Int. 2005, 68, 515–528. [Google Scholar] [CrossRef]
- Tesfamariam, B. Targeting heme-oxidized soluble guanylate cyclase to promote osteoblast function. Drug Discov. Today 2020, 25, 422–429. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Sandner, P.; Follmann, M.; Becker-Pelster, E.; Hahn, M.G.; Meier, C.; Freitas, C.; Roessig, L.; Stasch, J.P. Soluble GC stimulators and activators: Past, present and future. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Schinner, E.; Schramm, A.; Kees, F.; Hofmann, F.; Schlossmann, J. The cyclic GMP-dependent protein kinase Iα suppresses kidney fibrosis. Kidney Int. 2013, 84, 1198–1206. [Google Scholar] [CrossRef]
- Peters, H.; Wang, Y.; Loof, T.; Martini, S.; Kron, S.; Krämer, S.; Neumayer, H.H. Expression and activity of soluble guanylate cyclase in injury and repair of anti-thy1 glomerulonephritis. Kidney Int. 2004, 66, 2224–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasch, J.P.; Dembowsky, K.; Perzborn, E.; Stahl, E.; Schramm, M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: In vivo studies. Br. J. Pharmacol. 2002, 135, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Sharkovska, Y.; Kalk, P.; Lawrenz, B.; Godes, M.; Hoffmann, L.S.; Wellkisch, K.; Geschka, S.; Relle, K.; Hocher, B.; Stasch, J.P. Nitric oxide-independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J. Hypertens. 2010, 28, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.M.; Alter, M.L.; von Websky, K.; Kretschmer, A.; Tsuprykov, O.; Sharkovska, Y.; Krause-Relle, K.; Raila, J.; Henze, A.; Stasch, J.P.; et al. Effects of stimulation of soluble guanylate cyclase on diabetic nephropathy in diabetic eNOS knockout mice on top of angiotensin II receptor blockade. PLoS ONE 2012, 7, e42623. [Google Scholar] [CrossRef] [PubMed]
- Czirok, S.; Fang, L.; Radovits, T.; Szabó, G.; Szénási, G.; Rosivall, L.; Merkely, B.; Kökény, G. Cinaciguat ameliorates glomerular damage by reducing ERK1/2 activity and TGF-ß expression in type-1 diabetic rats. Sci. Rep. 2017, 7, 11218. [Google Scholar] [CrossRef]
- Harloff, M.; Prüschenk, S.; Seifert, R.; Schlossmann, J. Activation of soluble guanylyl cyclase signalling with cinaciguat improves impaired kidney function in diabetic mice. Br. J. Pharmacol. 2022, 179, 2460–2475. [Google Scholar] [CrossRef]
- Boustany-Kari, C.M.; Harrison, P.C.; Chen, H.; Lincoln, K.A.; Qian, H.S.; Clifford, H.; Wang, H.; Zhang, X.; Gueneva-Boucheva, K.; Bosanac, T.; et al. A Soluble Guanylate Cyclase Activator Inhibits the Progression of Diabetic Nephropathy in the ZSF1 Rat. J. Pharmacol. Exp. Ther. 2016, 356, 712–719. [Google Scholar] [CrossRef]
- Catrina, S.B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Gunton, J.E. Hypoxia-inducible factors and diabetes. J. Clin. Investig. 2020, 130, 5063–5073. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Q.; Guo, C.; Dong, G.; Liu, Y.; Tang, C.; Dong, Z. Hypoxia, HIF, and Associated Signaling Networks in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 950. [Google Scholar] [CrossRef]
- Ohtomo, S.; Nangaku, M.; Izuhara, Y.; Takizawa, S.; Strihou, C.; Miyata, T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol. Dial. Transplant. 2008, 23, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, M.; Tanaka, S.; Tanaka, T.; Saito, H.; Ishimoto, Y.; Wakashima, T.; Ueda, M.; Fukui, K.; Shimizu, A.; Inagi, R.; et al. Prolyl Hydroxylase Domain Inhibitor Protects against Metabolic Disorders and Associated Kidney Disease in Obese Type 2 Diabetic Mice. J. Am. Soc. Nephrol. 2020, 31, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front. Physiol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.M.; Mahmoud, M.M.; Shoukry, H.S.; Rashed, L.; Kamar, S.S.; Ahmed, M.M. Interactive effects of apelin, renin-angiotensin system and nitric oxide in treatment of obesity-induced type 2 diabetes mellitus in male albino rats. Arch. Physiol. Biochem. 2019, 125, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhong, X.; Tan, Y.X.; Liu, D. Apelin-13 alleviates diabetic nephropathy by enhancing nitric oxide production and suppressing kidney tissue fibrosis. Int. J. Mol. Med. 2021, 48, 175. [Google Scholar] [CrossRef]
- Yamada, Y.; Nishii, K.; Kuwata, K.; Nakamichi, M.; Nakanishi, K.; Sugimoto, A.; Ikemoto, K. Effects of pyrroloquinoline quinone and imidazole pyrroloquinoline on biological activities and neural functions. Heliyon 2020, 6, e03240. [Google Scholar] [CrossRef]
- Qu, X.; Zhai, B.; Liu, Y.; Chen, Y.; Xie, Z.; Wang, Q.; Wu, Y.; Liu, Z.; Chen, J.; Mei, S.; et al. Pyrroloquinoline quinone ameliorates renal fibrosis in diabetic nephropathy by inhibiting the pyroptosis pathway in C57BL/6 mice and human kidney 2 cells. Biomed. Pharmacother. 2022, 150, 112998. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Hu, L.; Yan, F.; Chen, J. miR-494 induces EndMT and promotes the development of HCC (Hepatocellular Carcinoma) by targeting SIRT3/TGF-β/SMAD signaling pathway. Sci. Rep. 2019, 9, 7213. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Li, J.; Kitada, M.; Fujita, H.; Yamada, Y.; Goodwin, J.E.; Kanasaki, K.; Koya, D. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis. 2018, 9, 997. [Google Scholar] [CrossRef]
- Yang, D.; Livingston, M.J.; Liu, Z.; Dong, G.; Zhang, M.; Chen, J.K.; Dong, Z. Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell. Mol. Life Sci. 2018, 75, 669–688. [Google Scholar] [CrossRef]
- Ding, Y.; Choi, M.E. Autophagy in diabetic nephropathy. J. Endocrinol. 2015, 224, R15–R30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Wang, Y.; Li, H.; Fan, J.; Shen, J.; Yu, X.; Zhou, Y.; Mao, H. ATG5-mediated autophagy suppresses NF-κB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 2019, 10, 253. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Qin, L.; Shou, Z.; Zhao, J.; Wang, H.; Chen, Y.; Chen, J. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am. J. Nephrol. 2007, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Vautier, M.; Allenbach, Y.; Zahr, N.; Benveniste, O.; Funck-Brentano, C.; Salem, J.E. Sirolimus and mTOR Inhibitors: A Review of Side Effects and Specific Management in Solid Organ Transplantation. Drug Saf. 2019, 42, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Jiang, C.H.; Liu, Y.; Lou, D.X.; Huang, Y.P.; Gao, M.; Zhang, J.; Yin, Z.Q.; Pan, K. Cyclocarya paliurus triterpenic acids fraction attenuates kidney injury via AMPK-mTOR-regulated autophagy pathway in diabetic rats. Phytomedicine 2019, 64, 153060. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, F.; Yu, M.; Zhao, Y.; Yan, G.; Xue, J.; Sun, Y.; Zhao, D.; Li, X.; Qi, W.; et al. Jiedu Tongluo Baoshen formula enhances podocyte autophagy and reduces proteinuria in diabetic kidney disease by inhibiting PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2022, 293, 115246. [Google Scholar] [CrossRef]
- Ma, Z.; Li, L.; Livingston, M.J.; Zhang, D.; Mi, Q.; Zhang, M.; Ding, H.F.; Huo, Y.; Mei, C.; Dong, Z. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. J. Clin. Investig. 2020, 130, 5011–5026. [Google Scholar] [CrossRef]
- Hung, P.H.; Hsu, Y.C.; Chen, T.H.; Lin, C.L. Recent Advances in Diabetic Kidney Diseases: From Kidney Injury to Kidney Fibrosis. Int. J. Mol. Sci. 2021, 22, 11857. [Google Scholar] [CrossRef]
- Porrini, E.; Ruggenenti, P.; Mogensen, C.E.; Barlovic, D.P.; Praga, M.; Cruzado, J.M.; Hojs, R.; Abbate, M.; de Vries, A.P. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet. Diabetes Endocrinol. 2015, 3, 382–391. [Google Scholar] [CrossRef]
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of kidney function in diabetes: A clinicopathological update. Nat. Rev. Nephrol. 2021, 17, 740–750. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hanai, K.; Mori, T.; Yokoyama, Y.; Yoshida, N.; Murata, H.; Shinozaki, T.; Babazono, T. Kidney outcomes and all-cause mortality in people with type 2 diabetes exhibiting non-albuminuric kidney insufficiency. Diabetologia 2022, 65, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou-Marketou, N.; Kanaka-Gantenbein, C.; Marketos, N.; Chrousos, G.P.; Papassotiriou, I. Biomarkers of diabetic nephropathy: A 2017 update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Donato, V.; Pintaudi, B.; Di Vieste, G.; Chirico, V.; Buemi, A.; Di Benedetto, A.; Arena, A.; Buemi, M. “Normoalbuminuric” diabetic nephropathy: Tubular damage and NGAL. Acta Diabetol. 2013, 50, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Kamijo-Ikemori, A.; Sugaya, T.; Yasuda, T.; Kawata, T.; Ota, A.; Tatsunami, S.; Kaise, R.; Ishimitsu, T.; Tanaka, Y.; Kimura, K. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011, 34, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Makino, H.; Seiki, K.; Urade, Y. Urinary excretions of lipocalin-type prostaglandin D synthase predict renal injury in type-2 diabetes: A cross-sectional and prospective multicentre study. Nephrol. Dial. Transplant. 2009, 24, 475–482. [Google Scholar] [CrossRef]
- Chen, C.; Wang, C.; Hu, C.; Han, Y.; Zhao, L.; Zhu, X.; Xiao, L.; Sun, L. Normoalbuminuric diabetic kidney disease. Front. Med. 2017, 11, 310–318. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar] [CrossRef]
- Thomas, M.C.; Macisaac, R.J.; Jerums, G.; Weekes, A.; Moran, J.; Shaw, J.E.; Atkins, R.C. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009, 32, 1497–1502. [Google Scholar] [CrossRef]
- Boronat, M.; García-Cantón, C.; Quevedo, V.; Lorenzo, D.L.; López-Ríos, L.; Batista, F.; Riaño, M.; Saavedra, P.; Checa, M.D. Non-albuminuric renal disease among subjects with advanced stages of chronic kidney failure related to type 2 diabetes mellitus. Ren. Fail. 2014, 36, 166–170. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Dagogo-Jack, S.; Cosentino, F.; Pratley, R.E.; Frederich, R.; Maldonado, M.; Liu, C.C.; Cannon, C.P. Heart and Kidney Outcomes With Ertugliflozin in People with Non-albuminuric Diabetic Kidney Disease: A post hoc Analysis from the Randomized VERTIS CV Trial. Kidney Int. Rep. 2022, 7, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Clinical Trials | Study Design | Number of Patients | Primary Outcomes | Secondary Outcomes | Safety Signal | Reference |
|---|---|---|---|---|---|---|---|
| Eplerenone |
Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) study | Multicenter, randomized, double-blind, placebo-controlled trial of eplerenone 25–50 mg qd in patients who aged at least 55 years with NYHA functional class II symptoms and an ejection fraction ≤ 35% (median 21 months). | 2737 | 37% reduction in MACE (death from CV causes or hospitalization for HF) | 24% reduction in death from CV causes; 42% reduction in hospitalization for HF; 31% reduction in hospitalization for CV causes; no effect on hospitalization for worsening renal function; no effect on renal failure. | Increased risk of hyperkalemia (11.8% vs. 7.2%, p < 0.001) in group with eplerenone compared with placebo. | [63] |
| Finerenone | Chronic Kidney Disease Outcomes in Type 2 Diabetes (FIDELIO) study | Multicenter, randomized, double-blind, placebo-controlled trial of finerenone 10–20 mg qd in patients with T2DM who had persistent, moderately elevated albuminuria with an eGFR of 25 to < 60 mL/minute/1.73 m2 or persistent, severely elevated albuminuria with an eGFR of 25 to <75 mL/minute/1.73 m2 (median 2.6 years). | 5734 | 18% reduction in composite renal outcome (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes); 13% reduction in kidney failure (ESRD or sustained decrease in eGFR to <15 mL/min/1.73 m2); 19% reduction in sustained decrease of ≥40% in eGFR from baseline. | 14% reduction in key secondary composite outcome (the death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for HF). | The rate of hyperkalemia and hyperkalemia-related discontinuation for patients with finerenone vs. placebo were 18.3% vs. 9.0% and 2.3% vs. 0.9%. | [67] |
| Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes (FIGARO) study | Multicenter, randomized, double-blind, placebo-controlled trial of finerenone 10–20 mg qd in patients with T2DM who had persistent, moderately elevated albuminuria with an eGFR of 25 to 90 mL/minute/1.73 m2 or persistent, severely elevated albuminuria and an eGFR of at least 60 mL/minute/1.73 m2 (median 3.4 years). | 7437 | 13% reduction in MACE (the death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for HF). | 18% reduction in composite renal outcome (kidney failure, a sustained decrease from baseline of at least 40% in the eGFR, or death from renal causes); 29% reduction in kidney failure (ESRD or sustained decrease in eGFR to <15 mL/min/1.73 m2); 13% reduction in sustained decrease of ≥40% in eGFR from baseline. | The rate of hyperkalemia, hyperkalemia-related discontinuation and hospitalization for patients with finerenone vs. placebo were 10.8% vs. 5.3%, 1.2% vs. 0.4% and 0.6% vs. 0.1%. | [68] |
| Drugs | Clinical Trials | Study Design | Number of Patients | Primary Outcomes | Secondary Outcomes | Safety Signals | Reference |
|---|---|---|---|---|---|---|---|
| Empagliflozin | Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG) Outcome Trial | Multicenter, randomized, double-blind, placebo-controlled trial of empagliflozin 10 mg or 25 mg qw in patients with T2DM who had a BMI of 45 or less and an eGFR of at least 30 mL/min/1.73 m2 (median 3.1 years). | 7020 | 14% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | 11% reduction in key secondary composite outcome (MACE or hospitalization for UA); 39% reduction in composite renal outcome (onset of macroalbuminuria, doubling of the SCr and an eGFR of ≤45 mL/min/1.73 m2, the need for renal replacement therapy, or death from renal disease). | Increased risk of genital infection in group with 10 mg empagliflozin (6.5% vs. 1.8%, p < 0.001) and group with 25 mg empagliflozin (6.3% vs. 1.8%, p < 0.001) compared with placebo. | [85] |
| Canagliflozin | Canagliflozin Cardiovascular Assessment Study (CANVAS) | Multicenter, randomized, double-blind, placebo-controlled trial of canagliflozin 100 mg or 300 mg qd in patients with T2DM who had a history of symptomatic atherosclerotic CV disease or were 50 years of age or older with at least 2 risk factors for CV disease (median 188.2 weeks). | 10,142 | 14% reduction in the death from CV causes, nonfatal MI, or nonfatal stroke. | 70% increase in regression of albuminuria; 40% reduction in composite renal outcome (40% reduction in eGFR, the need for renal replacement therapy, or death from renal disease). | Increased risk of genital infection (0.35% vs. 0.11%, p < 0.001) and increased risk of amputation of toes, feet, or legs (0.63% vs. 0.34%, p < 0.001) in group with canagliflozin compared with placebo. | [86] |
| Dapagliflozin | Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial | Multicenter, randomized, double-blind, placebo-controlled trial of dapagliflozin 10 mg qd patients with T2DM who had or were at risk for atherosclerotic CV disease and had a HbA1c ≥ 6.5% but <12.0%, and a Scr clearance ≥ 60 mL/min (median 4.2 years). | 17,160 | No effect on MACE (the death from CV causes, nonfatal MI, or nonfatal stroke); 17% reduction in CV death or hospitalization for heart failure. | 24% reduction in composite renal outcome (new ESRD, ≥40% decrease in eGFR to <60, death from renal or CV causes); No effect on secondary efficacy outcomes (the death from any cause). | Increased risk of genital infection (0.9% vs. 0.1%, p < 0.001) in group with dapagliflozin compared with placebo. | [87] |
| Canagliflozin | Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial | Multicenter, randomized, double-blind, placebo-controlled trial of canagliflozin 100 mg qd in patients with T2DM who had an eGFR of 30 to <90 mL/min/1.73 m2 and albuminuria and were treated with RAAS inhibitors (median 2.62 years). | 4401 | 32% reduction in ESRD (the need for renal replacement therapy or sustained eGFR of <15 mL/min/1.73 m2); 34% reduction in composite renal outcome (ESRD, doubling of SCr, or death from renal disease). | 20% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | No significant difference in rates of adverse events (amputation, fracture, and diabetic ketoacidosis) between two groups. | [88] |
| Drugs | Clinical Trials | Study Design | Number of Patients | Primary Outcomes | Secondary Outcomes | Safety Signals | Reference |
|---|---|---|---|---|---|---|---|
| Lixisenatide | Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome (ELIXA) trial | Multicenter, randomized, double-blind, placebo-controlled trial of lixisenatide 10–20 μg qd in patients with T2DM who had a MI or had been hospitalized for UA within the previous 180 days (median 25 months). | 6068 | No effect on MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | 19.2% reduction in new onset macroalbuminuria; No significant effect on eGFR | Increased risk of gastrointestinal event (4.9% vs. 1.2%, p < 0.001) in group with lixisenatide compared with placebo. | [106] |
| Liraglutide | Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER) study | Multicenter, randomized, double-blind, placebo-controlled trial of liraglutide 1.8 mg qd in patients with T2DM who had a HbA1c ≥ 7.0% and aged at least 50 years old with at least one CV coexisting condition or aged at least 60 years with at least one CV risk factor (median 3.8 years). | 9340 | 13% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | 22% reduction in composite renal outcome (onset of macroalbuminuria, doubling of the SCr and an eGFR of ≤45 mL/minute/1.73 m2, the need for continuous renal replacement therapy, or death from renal disease); 26% reduction in progression of albuminuria | Acute gallstone disease is the main severe adverse event. Nausea, vomiting and diarrhea are the most common causes leading to the discontinuation. | [107] |
| Semaglutide | Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes (SUSTAIN-6) trial | Multicenter, randomized, double-blind, placebo-controlled trial of semaglutide 0.5 or 1.0 mg qw in patients with T2DM who had a HbA1c level ≥ 7% and aged at least 50 years or with established CV disease, chronic heart failure or CKD of stage 3, or aged at least with one CV risk factor (median 2.1 years). | 3297 | 26% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | 36% reduction in new or worsening nephropathy | Increased risk of gastrointestinal disease and reduced risk of severe adverse events (including serious cardiac disorders) in group with semaglutide compared with placebo. | [108] |
| Exenatide | Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes (EXSCEL) trial | Multicenter, randomized, double-blind, placebo-controlled trial of exenatide 2 mg qw in T2DM patients who had HbA1c of 6.5–10.0% and with or without previous CV disease (median 3.2 years). | 14,752 | 9% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | 12% reduction in composite renal outcome (onset of macroalbuminuria, doubling of the Scr and an eGFR of ≤45 mL/minute/1.73 m2, the need for continuous renal replacement therapy, or death from renal disease); 26% reduction in progression of albuminuria) | No significant difference in risk of adverse events (acute pancreatitis, pancreatic cancer, severe hypoglycemia and medullary thyroid carcinoma) between two groups. | [109] |
| Albiglutide | Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (HARMONY) trial | Multicenter, randomized, double-blind, placebo-controlled trial of albiglutide 1.5 mg qw in patients aged at least 40 years with T2DM who had HbA1c ≥7.0% and established disease of the coronary, cerebrovascular, or peripheral arterial circulation (median 1.5 years). | 9463 | 22% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | Renal outcomes are not available | No significant difference in risk of adverse events (acute pancreatitis, pancreatic cancer, severe hypoglycemia and medullary thyroid carcinoma) between two groups. | [110] |
| Dulaglutide | Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND) trial | Multicenter, randomized, double-blind, placebo-controlled trial of dulaglutide 1.5 mg qw in patients with T2DM who aged at least 50 years with CV or renal risk factors (median 5.4 years). | 9901 | 22% reduction in MACE (the death from CV causes, nonfatal MI, or nonfatal stroke) | 15% reduction in composite renal outcome (development of UACR >33·9 mg/mmol in those with lower baseline concentration, sustained ≥30% decline in eGFR, or the need of renal replacement therapy) | No significant difference in risk of severe adverse events and increased risk in gastrointestinal event (47.4% vs. 37.1%, p < 0.0001) in group with dulaglutide compared with placebo. | [111] |
| Drugs | Clinical Trials | Study Design | Number of Patients | Primary Outcomes | Secondary Outcomes | Safety Signals | Reference |
|---|---|---|---|---|---|---|---|
| Saxagliptin | Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus (SAVOR- TIMI 53) trial | Multicenter, randomized, double-blind, placebo-controlled trial of saxagliptin 5 mg qd in patients with T2DM who had a HbA1c of 6.5–12.0%, and either a history of established CV disease or multiple risk factors for vascular disease (median 2.1 years). | 16,492 | No effect on MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | No effect on secondary efficacy end point (MACE plus hospitalization for heart failure, coronary revascularization, or UA); No effect on eGFR. | Increased risk of hypoglycemia (15.3% vs. 13.4%, p < 0.001) in group with saxagliptin compared with placebo. | [115] |
| Alogliptin | Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes (EXAMINE) | Multicenter, randomized, double-blind, placebo-controlled trial of alogliptin 25 mg/12.5 mg/6.25 mg qd in patients with T2DM and either an acute MI or UA requiring hospitalization within the previous 15 to 90 days (median 18 months). | 5380 | No effect on MACE (the death from CV causes, nonfatal MI, or nonfatal stroke). | No effect on principal secondary end point (MACE with the addition of urgent revascularization due to UA within 24 h after hospital admission); No effect on eGFR renal replacement therapy | No significant difference in risk of adverse events (severe hypoglycemia, cancer, dialysis, acute pancreatitis, pancreatic cancer and angioedema) between two groups. | [116] |
| Sitagliptin | Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes (TECOS) study | Multicenter, randomized, double-blind, placebo-controlled trial of sitagliptin 100 mg qd in patients with T2DM who had established atherosclerotic CV disease, and HbA1c of 6.5–8.0% and were receiving stable-dose monotherapy or dual-combination therapy of hypoglycemic agent (median 3.0 years). | 14,671 | No effect on MACE (time to CV death, nonfatal MI, nonfatal stroke, or hospitalization for UA). | no effect on eGFR | No significant difference in risk of adverse events (infections, cancer, renal failure, severe hypoglycemia, acute pancreatitis and pancreatic cancer) between two groups. | [117] |
| Linagliptin | The Cardiovascular and Renal Microvascular Outcome Study With Linagliptin (CARMELINA) | Multicenter, randomized, double-blind, placebo-controlled trial of linagliptin 5 mg qd in patients with T2DM who had HbA1c of 6.5–10.0%, history of vascular disease and UACR > 200 mg/g, and reduced eGFR and micro- or macroalbuminuria (median 2.2 years). | 6991 | No effect on MACE (time to first occurrence of the composite of CV death, nonfatal MI, or nonfatal stroke). | No effect on secondary outcome (time to first occurrence of adjudicated death due to renal failure, ESRD, or sustained ≥40% decrease in eGFR from baseline). | The rate of adverse events, hypoglycemia, and acute pancreatitis for patients with linagliptin vs. placebo were 77.2% vs. 78.1%, 29.7% vs. 29.4%, and 0.3% vs. 0.1%. | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Chen, Y.-Y.; Li, Z.-Q.; He, F.-F.; Zhang, C. Recent Advances in the Emerging Therapeutic Strategies for Diabetic Kidney Diseases. Int. J. Mol. Sci. 2022, 23, 10882. https://doi.org/10.3390/ijms231810882
Huang W, Chen Y-Y, Li Z-Q, He F-F, Zhang C. Recent Advances in the Emerging Therapeutic Strategies for Diabetic Kidney Diseases. International Journal of Molecular Sciences. 2022; 23(18):10882. https://doi.org/10.3390/ijms231810882
Chicago/Turabian StyleHuang, Wei, Yi-Yuan Chen, Zi-Qi Li, Fang-Fang He, and Chun Zhang. 2022. "Recent Advances in the Emerging Therapeutic Strategies for Diabetic Kidney Diseases" International Journal of Molecular Sciences 23, no. 18: 10882. https://doi.org/10.3390/ijms231810882
APA StyleHuang, W., Chen, Y.-Y., Li, Z.-Q., He, F.-F., & Zhang, C. (2022). Recent Advances in the Emerging Therapeutic Strategies for Diabetic Kidney Diseases. International Journal of Molecular Sciences, 23(18), 10882. https://doi.org/10.3390/ijms231810882






