CpMAX1a, a Cytochrome P450 Monooxygenase Gene of Chimonanthus praecox Regulates Shoot Branching in Arabidopsis
Abstract
:1. Introduction
2. Results
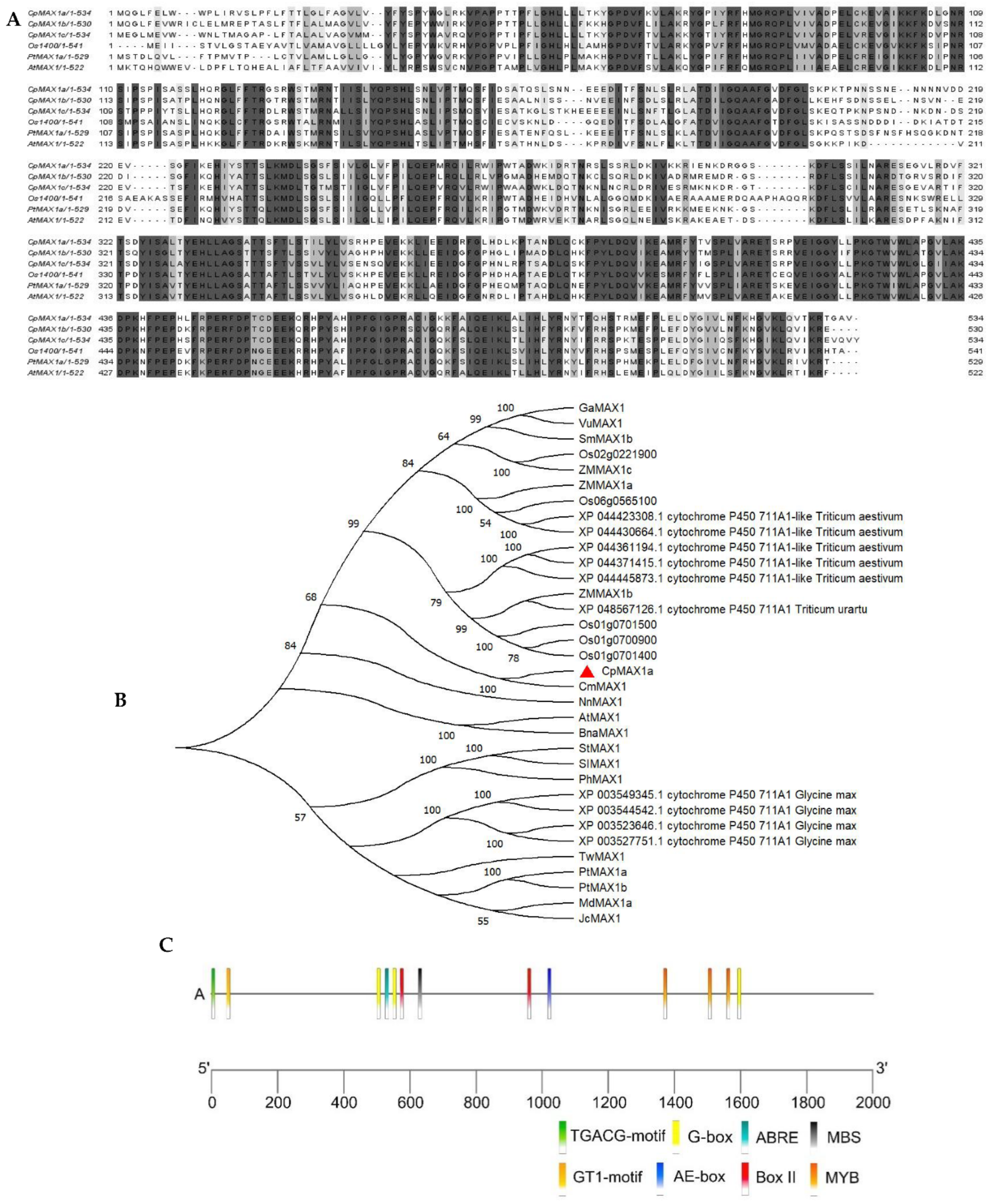
2.1. CpMAX1a Cloning and Phylogenetic Analysis
2.2. Expression Pattern of CpMAX1a
2.3. Subcellular Localization of CpMAX1a
2.4. Effect of Environmental Factors on the Expression of CpMAX1a
2.5. Effects of Overexpression of the CpMAX1a Gene on Branching in Arabidopsis
2.6. Overexpression of the CpMAX1a Gene Restores the Branching Phenotype of Arabidopsis max1 Mutants
3. Discussion
4. Materials and Methods
4.1. Plants
4.2. Cloning of CpMAX1a Gene
4.3. Bioinformatics Analysis
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Construction of Expression Vectors
4.6. Generation and Screening of Transgenic Arabidopsis
4.7. Subcellular Localization of CpMAX1a
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shimizu-Sato, S.; Mori, H. Control of Outgrowth and Dormancy in Axillary Buds. Plant Physiol. 2001, 127, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Theres, K. Shoot and inflorescence branching. Curr. Opin. Plant Biol. 2005, 8, 506–511. [Google Scholar] [CrossRef]
- Mcsteen, P.; Leyser, O. Shoot Branching. Annu. Rev. Plant Biol. 2005, 56, 353. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J. Branching in rice. Curr. Opin. Plant Biol. 2011, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone Acts Downstream of Auxin to Regulate Bud Outgrowth in Pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic Action of Strigolactone and Cytokinin in Bud Outgrowth Control. Plant Physiol. 2011, 158, 487–498. [Google Scholar] [CrossRef]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef]
- Barnett, H.L. Annual Review of Phytopathology. Mycologia 1965, 57, 677. [Google Scholar] [CrossRef]
- Xie, X.N.; Yoneyama, K.; Yoneyama, K. The Strigolactone Story. In Annual Review of Phytopathology; VanAlfen, N.K., Bruening, G., Leach, J.E., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 48, pp. 93–117. [Google Scholar]
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Van Ha, C.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Van Dong, N.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Koltai, H. Strigolactone Involvement in Root Development, Response to Abiotic Stress, and Interactions with the Biotic Soil Environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A. Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul. 2000, 32, 193–203. [Google Scholar] [CrossRef]
- Foo, E.; Turnbull, C.G.N.; Beveridge, C.A. Long-Distance Signaling and the Control of Branching in the rms1 Mutant of Pea. Plant Physiol. 2001, 126, 203–209. [Google Scholar] [CrossRef]
- Morris, S.E.; Turnbull, C.G.N.; Murfet, I.C.; Beveridge, C.A. Mutational Analysis of Branching in Pea. Evidence That Rms1 and Rms5 Regulate the Same Novel Signal. Plant Physiol. 2001, 126, 1205–1213. [Google Scholar] [CrossRef]
- Stirnberg, P.; van de Sande, K.; Leyser, H.M.O. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar] [CrossRef]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 Is a Carotenoid Cleavage Dioxygenase Required for the Synthesis of a Novel Plant Signaling Molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef]
- Sorefan, K.; Booker, J.; Haurogné, K.; Goussot, M.; Bainbridge, K.; Foo, E.; Chatfield, S.; Ward, S.; Beveridge, C.; Rameau, C.; et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003, 17, 1469–1474. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, C.G.N.; Booker, J.P.; Leyser, H.M.O. Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 2002, 32, 255–262. [Google Scholar] [CrossRef]
- Napoli, C. Highly Branched Phenotype of the Petunia dad1-1 Mutant Is Reversed by Grafting. Plant Physiol. 1996, 111, 27–37. [Google Scholar] [CrossRef]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an Iron-Containing Protein Required for the Biosynthesis of Strigolactones, Regulates Rice Tiller Bud Outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef]
- Waters, M.T.; Brewer, P.B.; Bussell, J.D.; Smith, S.M.; Beveridge, C.A. The Arabidopsis Ortholog of Rice DWARF27 Acts Upstream of MAX1 in the Control of Plant Development by Strigolactones. Plant Physiol. 2012, 159, 1073–1085. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Loewen, M.C. The Biochemical Characterization of Two Carotenoid Cleavage Enzymes from Arabidopsis Indicates That a Carotenoid-derived Compound Inhibits Lateral Branching. J. Biol. Chem. 2004, 279, 46940–46945. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Holdermann, I.; Beyer, P.; Al-Babili, S. Carotenoid oxygenases involved in plant branching catalyse a highly specific conserved apocarotenoid cleavage reaction. Biochem. J. 2008, 416, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-Carotene to Carlactone, a Strigolactone-Like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Sado, A.; Tanaka, K.; Kisugi, T.; Asami, K.; Ota, S.; Kim, H.I.; Yoneyama, K.; Xie, X.; Ohnishi, T.; et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 18084–18089. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Wang, M.; Liu, Y.; Yuan, S.; Gao, Y.; Yin, L.; Sun, W.; Peng, L.; Zhang, W.; et al. DWARF3 Participates in an SCF Complex and Associates with DWARF14 to Suppress Rice Shoot Branching. Plant Cell Physiol. 2014, 55, 1096–1109. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, C.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; Furner, I.J.; Leyser, H.M.O. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007, 50, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14–SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 2013, 504, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G.; et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 583, 277–281. [Google Scholar] [CrossRef]
- Cardoso, C.; Zhang, Y.; Jamil, M.; Hepworth, J.; Charnikhova, T.; Dimkpa, S.O.N.; Meharg, C.; Wright, M.H.; Liu, J.; Meng, X.; et al. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc. Natl. Acad. Sci. USA 2014, 111, 2379–2384. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Wang, Y.; Díez-Simón, C.; Flokova, K.; Bimbo, A.; Bouwmeester, H.J.; Ruyter-Spira, C. The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone. New Phytol. 2018, 219, 297–309. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 Acts as an Integrator of Branching Signals within Axillary Buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Lin, J.; Zhu, T.; Liu, N.; Yang, X.; Ma, J.; Sui, S. Carotenoid Cleavage Dioxygenase Genes of Chimonanthus praecox, CpCCD7 and CpCCD8, Regulate Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 8750. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Ma, M.; Zhou, Q.; Zhao, Y.; Zhao, B.; Wang, B.; Wei, H.; Wang, H. Arabidopsis FHY3 and FAR1 integrate light and strigolactone signaling to regulate branching. Nat. Commun. 2020, 11, 1955. [Google Scholar] [CrossRef]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; Al-Babili, S.; et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xu, J.; Wang, C.; Pang, Y.; Li, L.; Tang, X.; Li, B.; Sun, Q. Genome-wide analysis of the strigolactone biosynthetic and signaling genes in grapevine and their response to salt and drought stresses. PeerJ 2022, 10, e13551. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, X.; Gu, P.; Yang, G.; Deng, H.; Zhang, J.; Zheng, Z. Transcriptomic analysis reveals phytohormone and photosynthetic molecular mechanisms of a submerged macrophyte in response to microcystin-LR stress. Aquat. Toxicol. 2022, 245, 106119. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Xu, X.; Wang, M.; Zhang, H.; Fang, P.; Zhou, J.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Strigolactones positively regulate abscisic acid-dependent heat and cold tolerance in tomato. Hortic. Res. 2021, 8, 237. [Google Scholar] [CrossRef]
- Lantzouni, O.; Klermund, C.; Schwechheimer, C. Largely additive effects of gibberellin and strigolactone on gene expression in Arabidopsis thaliana seedlings. Plant J. 2017, 92, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, E.S.; Gürel, S.; Aflaki, F.; Pazuki, A.; Şahin, G.; Gürel, E. Identification and expressional profiling of putative MAX1 gene in sugar beet (Beta vulgaris L.). Turk. J. Bot. 2020, 44, 377–387. [Google Scholar] [CrossRef]
- Xiu-Mei, W.; Yue-Yang, L.; Ling, L.; Chang-Wei, G.; Hai-Peng, W.; Xiao-Xi, H.; Shuang-Cheng, L.; Qi-Ming, D.; Jun, Z.; Ai-Ping, Z.; et al. Identification and Cloning of Tillering-Related Genes OsMAX1 in Rice. Rice Sci. 2015, 22, 255–263. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, L.; Tang, M.; Liu, J.; Liu, H.; Yang, H.; Fan, S.; Terzaghi, W.; Wang, H.; Hua, W. Knockout of two BnaMAX1 homologs by CRISPR/Cas9-targeted mutagenesis improves plant architecture and increases yield in rapeseed (Brassica napus L.). Plant Biotechnol. J. 2020, 18, 644–654. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Q.; Xiong, F.; Liu, N.; Zhang, S. Isolation and Functional Analysis of CmMAX1 from Chrysanthemum. J. Am. Soc. Hortic. Sci. 2018, 143, 430–435. [Google Scholar] [CrossRef]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 Encodes a Cytochrome P450 Family Member that Acts Downstream of MAX3/4 to Produce a Carotenoid-Derived Branch-Inhibiting Hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Hervé, C. TCP Transcription Factors Predate the Emergence of Land Plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef]
- Wang, M.; Le Moigne, M.-A.; Bertheloot, J.; Crespel, L.; Perez-Garcia, M.-D.; Ogé, L.; Demotes-Mainard, S.; Hamama, L.; Davière, J.-M.; Sakr, S. BRANCHED1: A Key Hub of Shoot Branching. Front. Plant Sci. 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Li, Z.; Chen, L.; Zhang, Y.; Liu, M.; Yan, X.; Fang, Y. Transcriptome analysis revealed hormone signaling response of grapevine buds to strigolactones. Sci. Hortic. 2021, 283, 109936. [Google Scholar] [CrossRef]
- Muhr, M.; Prüfer, N.; Paulat, M.; Teichmann, T. Knockdown of strigolactone biosynthesis genes in Populus affects BRANCHED1 expression and shoot architecture. New Phytol. 2016, 212, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Vidaurre, D.; Toh, S.; Hanada, A.; Nambara, E.; Kamiya, Y.; Yamaguchi, S.; McCourt, P. A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat. Chem. Biol. 2010, 6, 741–749. [Google Scholar] [CrossRef]
- Huang, R.; Liu, D.; Huang, M.; Ma, J.; Li, Z.; Ma, J.; Li, M.; Sui, S. CpWRKY71, a WRKY Transcription Factor Gene of Wintersweet (Chimonanthus praecox), Promotes Flowering and Leaf Senescence in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 5325. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Hua, R.; Wang, X.; Wu, H.; Ou, H.; Lu, X.; Huang, Y.; Liu, D.; Sui, S. CpMAX1a, a Cytochrome P450 Monooxygenase Gene of Chimonanthus praecox Regulates Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 10888. https://doi.org/10.3390/ijms231810888
Zhang H, Hua R, Wang X, Wu H, Ou H, Lu X, Huang Y, Liu D, Sui S. CpMAX1a, a Cytochrome P450 Monooxygenase Gene of Chimonanthus praecox Regulates Shoot Branching in Arabidopsis. International Journal of Molecular Sciences. 2022; 23(18):10888. https://doi.org/10.3390/ijms231810888
Chicago/Turabian StyleZhang, Haiyuan, Run Hua, Xia Wang, Huafeng Wu, Hua Ou, Xin Lu, Yan Huang, Daofeng Liu, and Shunzhao Sui. 2022. "CpMAX1a, a Cytochrome P450 Monooxygenase Gene of Chimonanthus praecox Regulates Shoot Branching in Arabidopsis" International Journal of Molecular Sciences 23, no. 18: 10888. https://doi.org/10.3390/ijms231810888




