The Controversial Role of LPS in Platelet Activation In Vitro
Abstract
1. Introduction
2. The Importance of CD14 in Mediating LPS Binding to Platelet TLR4
3. Platelet Preparation Methods: Different Protocols Give Different Results
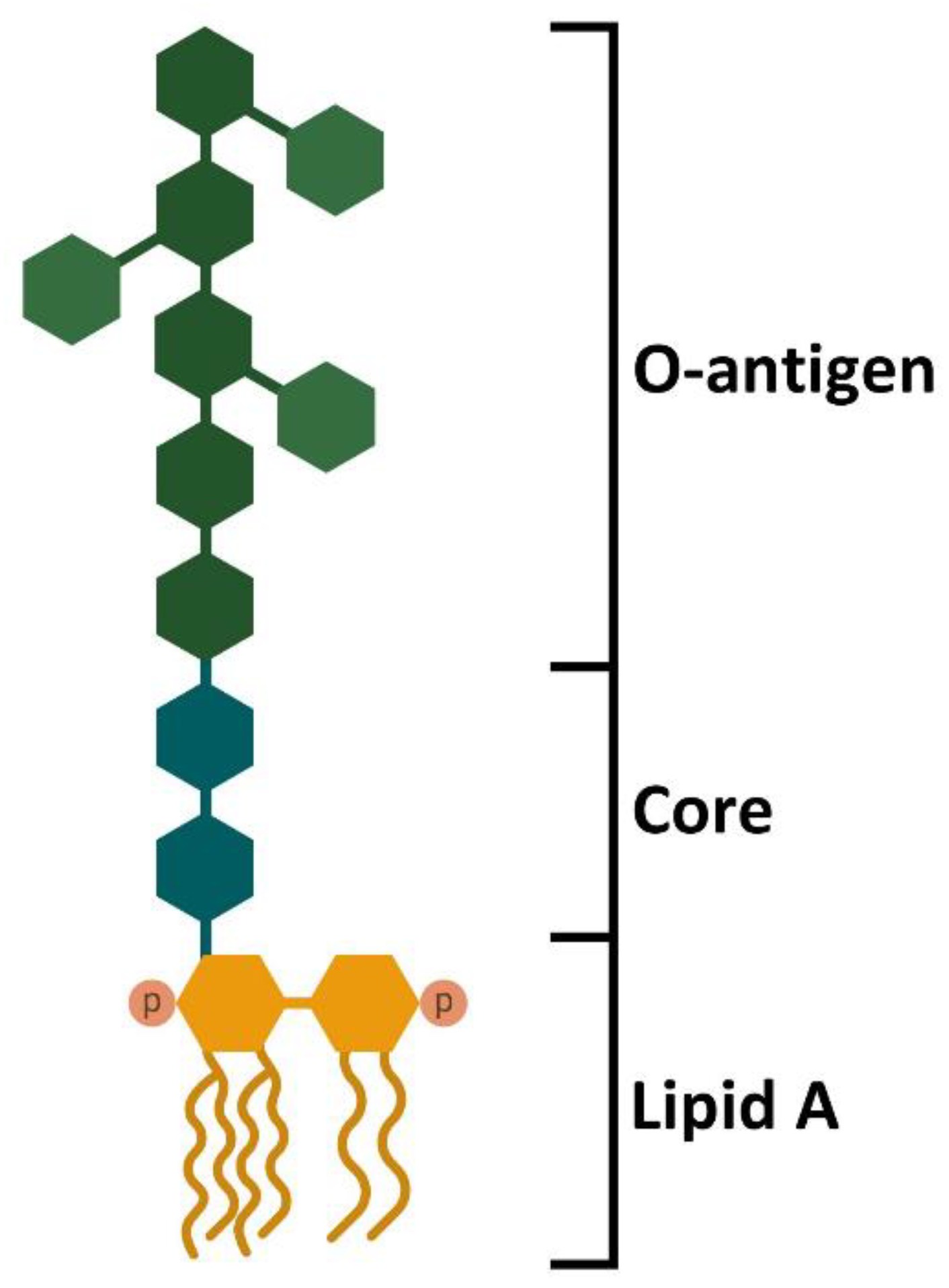
4. Sources of LPS, Doses, and Time of Stimulation
5. Effect of LPS on Platelet Activation
5.1. LPS Promotes Maturation and Release of IL-1β
5.2. LPS in Platelet–Leukocyte Aggregate Formation and NETosis
5.3. Role of LPS in Platelet Granule Secretion
5.4. LPS Promotes ROS Generation
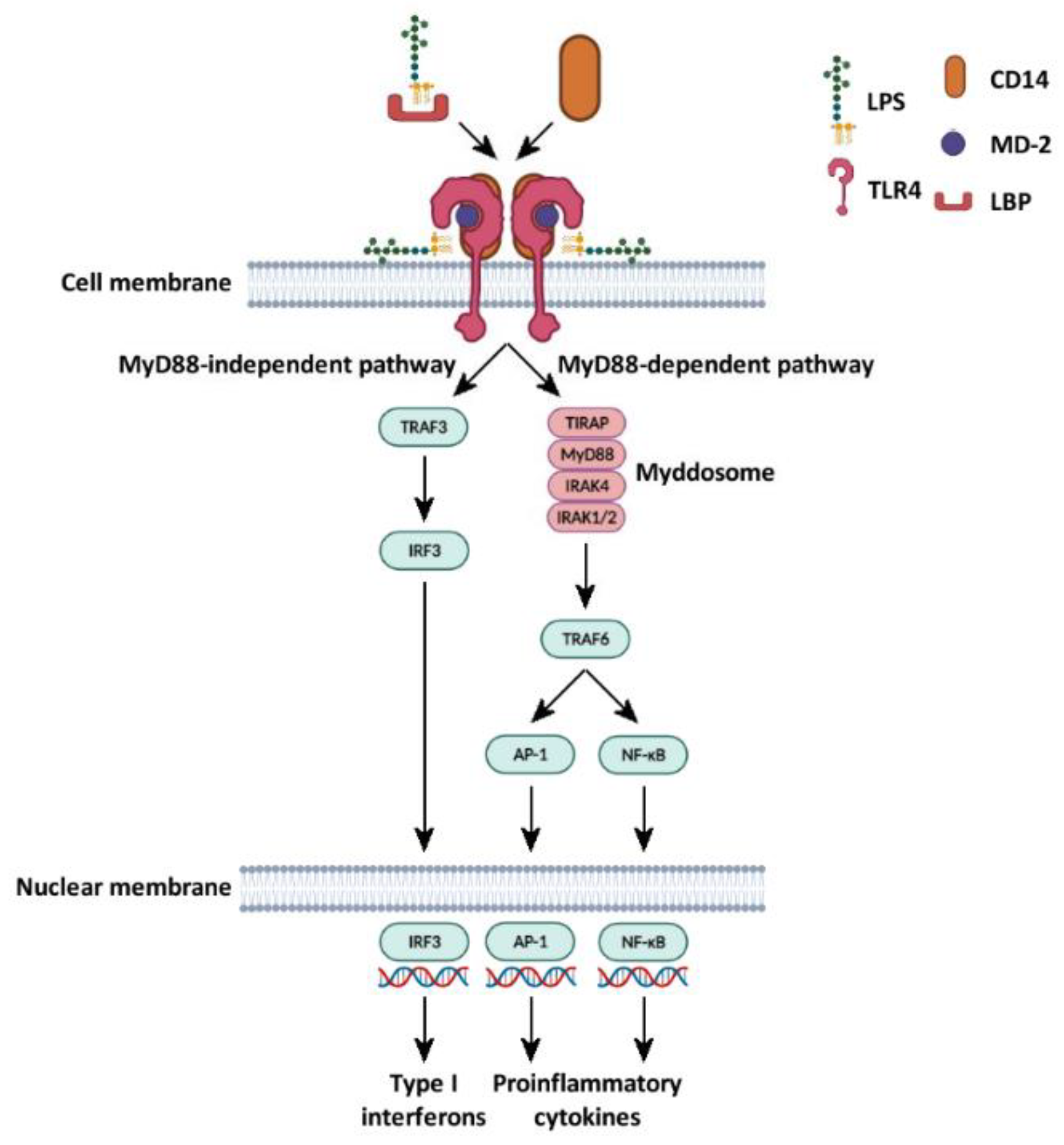
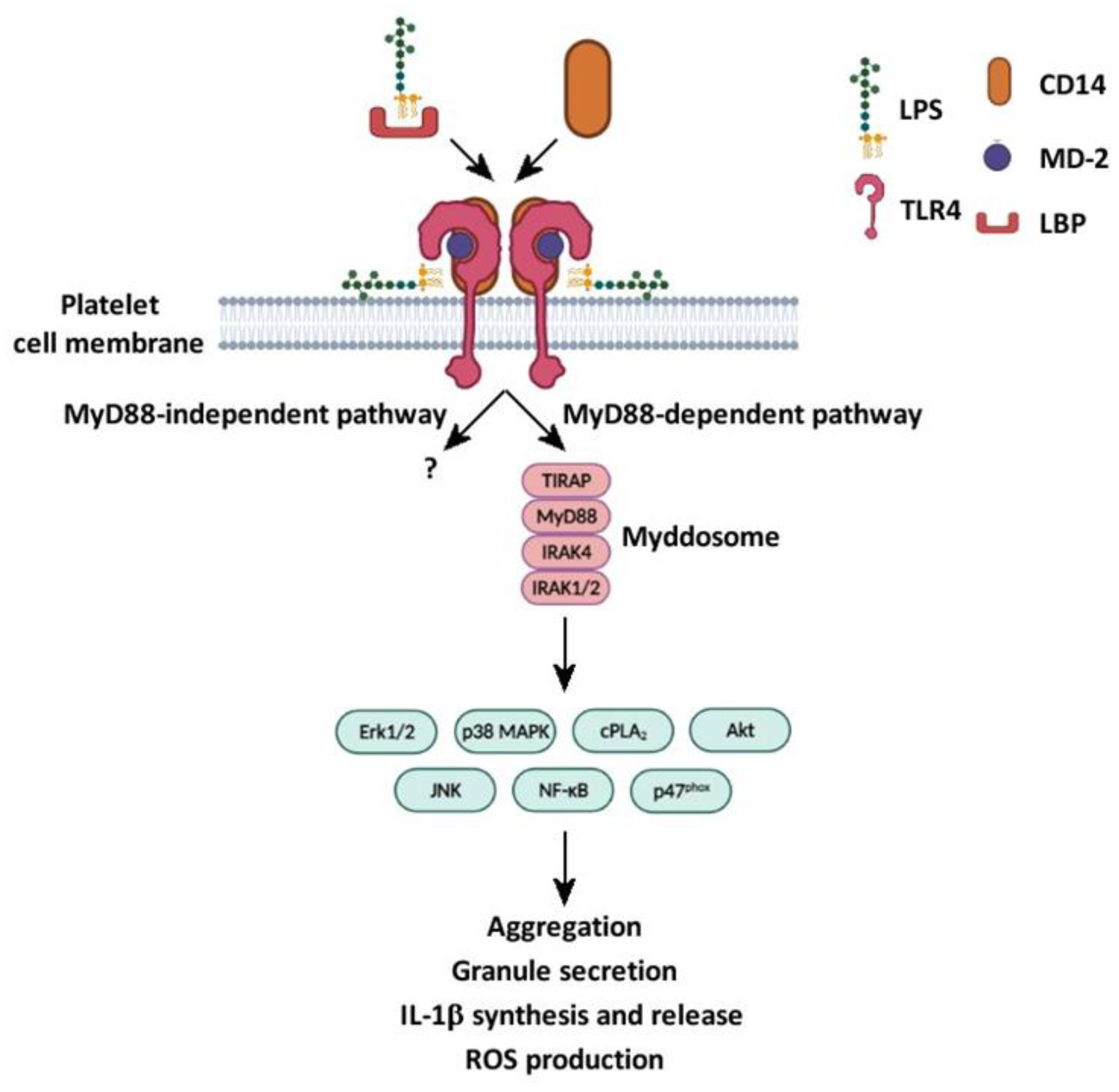
5.5. LPS Is Involved in the Activation of Canonical and Non-Canonical Signaling Pathways
5.6. LPS’ Effects on Integrin αIIbβ3 Activation
5.7. LPS and Platelet Aggregation
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thon, J.N.; Italiano, J.E. Platelets: Production, Morphology and Ultrastructure. In Antiplatelet Agents; Gresele, P., Born, G., Patrono, C., Page, C., Eds.; Handbook of Experimental Pharmacology Series No. 210; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. [Google Scholar] [CrossRef]
- Krishnegowda, M.; Rajashekaraiah, V. Platelet disorders: An overview. Blood Coagul. Fibrinolysis 2015, 26, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Platelets in inflammation and infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Kolappa, K.P.; Becker, R.C. Beyond thrombosis: The versatile platelet in critical illness. Chest 2011, 139, 658–668. [Google Scholar] [CrossRef]
- Shannon, O. Platelet interaction with bacterial toxins and secreted products. Platelets 2015, 26, 302–308. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Andonegui, G.; Kerfoot, S.M.; McNagny, K.; Ebbert, K.V.; Patel, K.D.; Kubes, P. Platelets express functional Toll-like receptor-4. Blood 2005, 106, 2417–2423. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Koessler, J.; Niklaus, M.; Weber, K.; Koessler, A.; Kuhn, S.; Boeck, M.; Kobsar, A. The Role of Human Platelet Preparation for Toll-Like Receptors 2 and 4 Related Platelet Responsiveness. TH Open 2019, 3, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Lopes Pires, M.E.; Clarke, S.R.; Marcondes, S.; Gibbins, J.M. Lipopolysaccharide potentiates platelet responses via toll-like receptor 4-stimulated Akt-Erk-PLA2 signalling. PLoS ONE 2017, 12, e0186981. [Google Scholar] [CrossRef] [PubMed]
- Shashkin, P.N.; Brown, G.T.; Ghosh, A.; Marathe, G.K.; McIntyre, T.M. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J. Immunol. 2008, 181, 3495–3502. [Google Scholar] [CrossRef]
- Zhang, G.; Han, J.; Welch, E.J.; Ye, R.D.; Voyno-Yasenetskaya, T.A.; Malik, A.B.; Du, X.; Li, Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 2009, 182, 7997–8004. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, L.; Carestia, A.; Etulain, J.; Pozner, R.G.; Fondevila, C.; Negrotto, S.; Schattner, M. Regulation of platelet responses triggered by Toll-like receptor 2 and 4 ligands is another non-genomic role of nuclear factor-kappaB. Thromb. Res. 2014, 133, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Carnevale, R.; Bartimoccia, S.; Novo, M.; Cangemi, R.; Pastori, D.; Calvieri, C.; Pignatelli, P.; Violi, F. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb. Haemost. 2017, 117, 1558–1570. [Google Scholar] [CrossRef]
- Martyanov, A.A.; Maiorov, A.S.; Filkova, A.A.; Ryabykh, A.A.; Svidelskaya, G.S.; Artemenko, E.O.; Gambaryan, S.P.; Panteleev, M.A.; Sveshnikova, A.N. Effects of bacterial lipopolysaccharides on platelet function: Inhibition of weak platelet activation. Sci. Rep. 2020, 10, 12296. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Delezay, O.; Pozzetto, B.; McNicol, A.; Garraud, O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br. J. Haematol. 2008, 141, 84–91. [Google Scholar] [CrossRef]
- Berthet, J.; Damien, P.; Hamzeh-Cognasse, H.; Arthaud, C.A.; Eyraud, M.A.; Zéni, F.; Pozzetto, B.; McNicol, A.; Garraud, O.; Cognasse, F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 2012, 145, 189–200. [Google Scholar] [CrossRef]
- Vallance, T.M.; Zeuner, M.T.; Williams, H.F.; Widera, D.; Vaiyapuri, S. Toll-Like Receptor 4 Signalling and Its Impact on Platelet Function, Thrombosis, and Haemostasis. Mediat. Inflamm. 2017, 2017, 9605894. [Google Scholar] [CrossRef]
- Domínguez-Medina, C.C.; Pérez-Toledo, M.; Schager, A.E.; Marshall, J.L.; Cook, C.N.; Bobat, S.; Hwang, H.; Chun, B.J.; Logan, E.; Bryant, J.A.; et al. Outer membrane protein size and LPS O-antigen define protective antibody targeting to the Salmonella surface. Nat. Commun. 2020, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Gonzalez, V.; Tomás, J.M. The first sugar of the repeat units is essential for the Wzy polymerase activity and elongation of the O-antigen lipopolysaccharide. Future Microbiol. 2016, 11, 903–918. [Google Scholar] [CrossRef]
- Zweigner, J.; Gramm, H.J.; Singer, O.C.; Wegscheider, K.; Schumann, R.R. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 2001, 98, 3800–3808. [Google Scholar] [CrossRef]
- Akira, S. Toll-like receptors and innate immunity. Adv. Immunol. 2001, 78, 1–56. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; Van Der Veen, A.I.P.; Horn, J.; Schultz, M.J.; Houtkooper, R.H.; Van ’t Veer, C.; Van Der Poll, T. Platelet Toll-like receptor expression and activation induced by lipopolysaccharide and sepsis. Platelets 2019, 30, 296–304. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.L.; Hommel, C.; Nguyen, N. Lipopolysaccharide-Activated Canine Platelets Upregulate High Mobility Group Box-1 via Toll-Like Receptor 4. Front. Vet. Sci. 2021, 8, 674678. [Google Scholar] [CrossRef] [PubMed]
- Cazenave, J.P.; Ohlmann, P.; Cassel, D.; Eckly, A.; Hechler, B.; Gachet, C. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol. Biol. 2004, 272, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthetic Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Berens, C.; Oldenburg, J.; Pötzsch, B.; Müller, J. Glycophorin A-based exclusion of red blood cells for flow cytometric analysis of platelet glycoprotein expression in citrated whole blood. Clin. Chem. Lab. Med. 2020, 58, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, L.; Pennings, G.J.; Connor, D.; Campbell, H.; Kritharides, L.; Chen, V.M. Flow Cytometry Protocols for Assessment of Platelet Function in Whole Blood. Methods Mol. Biol. 2017, 1646, 369–389. [Google Scholar] [CrossRef]
- Bergmeier, W.; Schulte, V.; Brockhoff, G.; Bier, U.; Zirngibl, H.; Nieswandt, B. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry 2002, 48, 80–86. [Google Scholar] [CrossRef]
- Schoenfeld, H.; Muhm, M.; Doepfmer, U.; Exadaktylos, A.; Radtke, H. Platelet activity in washed platelet concentrates. Anesth. Analg. 2004, 99, 17–20. [Google Scholar] [CrossRef]
- Hechler, B.; Dupuis, A.; Mangin, P.H.; Gachet, C. Platelet preparation for function testing in the laboratory and clinic: Historical and practical aspects. Res. Pract. Thromb. Haemost. 2019, 3, 615–625. [Google Scholar] [CrossRef]
- Burzynski, L.C.; Pugh, N.; Clarke, M.C.H. Platelet Isolation and Activation Assays. Bio-Protocol 2019, 9, e3405. [Google Scholar] [CrossRef] [PubMed]
- Aburima, A.; Walladbegi, K.; Wake, J.D.; Naseem, K.M. cGMP signaling inhibits platelet shape change through regulation of the RhoA-Rho Kinase-MLC phosphatase signaling pathway. J. Thromb. Haemost. 2017, 15, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Damien, P.; Cognasse, F.; Eyraud, M.A.; Arthaud, C.A.; Pozzetto, B.; Garraud, O.; Hamzeh-Cognasse, H. PS stimulation of purified human platelets is partly dependent on plasma soluble CD14 to secrete their main secreted product, soluble-CD40-Ligand. BMC Immunol. 2015, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; McIntyre, T.M. Lipopolysaccharide signaling without a nucleus: Kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. J. Immunol. 2011, 186, 5489–5496. [Google Scholar] [CrossRef] [PubMed]
- Vallance, T.M.; Ravishankar, D.; Albadawi, D.A.I.; Layfield, H.; Sheard, J.; Vaiyapuri, R.; Dash, P.; Patel, K.; Widera, D.; Vaiyapuri, S. Effect of ultrapure lipopolysaccharides derived from diverse bacterial species on the modulation of platelet activation. Sci. Rep. 2019, 9, 18258. [Google Scholar] [CrossRef] [PubMed]
- De Stoppelaar, S.F.; Claushuis, T.A.; Schaap, M.C.; Hou, B.; van der Poll, T.; Nieuwland, R.; van ’t Veer, C. Toll-Like Receptor Signalling Is Not Involved in Platelet Response to Streptococcus pneumoniae In Vitro or In Vivo. PLoS ONE 2016, 11, e0156977. [Google Scholar] [CrossRef]
- McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; Keys, E.M.; Allen-Vercoe, E.; Devinney, R.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Ward, J.R.; Bingle, L.; Judge, H.M.; Brown, S.B.; Storey, R.F.; Whyte, M.K.; Dower, S.K.; Buttle, D.J.; Sabroe, I. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb. Haemost. 2005, 94, 831–838. [Google Scholar] [CrossRef]
- Feng, G.; Yang, X.; Li, Y.; Wang, X.; Tan, S.; Chen, F. LPS enhances platelets aggregation via TLR4, which is related to mitochondria damage caused by intracellular ROS, but not extracellular ROS. Cell. Immunol. 2018, 328, 86–92. [Google Scholar] [CrossRef]
- Kappelmayer, J.; Beke Debreceni, I.; Vida, A.; Antal-Szalmás, P.; Clemetson, K.J.; Nagy, B., Jr. Distinct effects of Re- and S-forms of LPS on modulating platelet activation. J. Thromb. Haemost. 2013, 11, 775–778. [Google Scholar] [CrossRef]
- Zeuner, M.; Bieback, K.; Widera, D. Controversial Role of Toll-like Receptor 4 in Adult Stem Cells. Stem Cell Rev. Rep. 2015, 11, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Brown, G.T.; Narayanan, P.; Li, W.; Silverstein, R.L.; McIntyre, T.M. Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop. J. Immunol. 2013, 191, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- Finsterbusch, M.; Schrottmaier, W.C.; Kral-Pointner, J.B.; Salzmann, M.; Assinger, A. Measuring and interpreting platelet-leukocyte aggregates. Platelets 2018, 29, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, E.; Shao, K.; Shen, W.; Gu, Y.; Li, M. Circulating platelet-neutrophil aggregates as risk factor for deep venous thrombosis. Clin. Chem. Lab. Med. 2019, 57, 707–715. [Google Scholar] [CrossRef]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pão, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Ma, A.C.; Kubes, P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J. Thromb. Haemost. 2008, 6, 415–420. [Google Scholar] [CrossRef]
- Li, S.; Pettersson, U.S.; Hoorelbeke, B.; Kolaczkowska, E.; Schelfhout, K.; Martens, E.; Kubes, P.; Van Damme, J.; Phillipson, M.; Opdenakker, G. nterference with glycosaminoglycan-chemokine interactions with a probe to alter leukocyte recruitment and inflammation in vivo. PLoS ONE 2014, 9, e104107. [Google Scholar] [CrossRef]
- Tanaka, K.; Koike, Y.; Shimura, T.; Okigami, M.; Ide, S.; Toiyama, Y.; Okugawa, Y.; Inoue, Y.; Araki, T.; Uchida, K.; et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS ONE 2014, 9, e111888. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, H.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016, 6, 37252. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Miyashita, T.; Okazaki, M.; Yamaguchi, T.; Ohbatake, Y.; Nakanuma, S.; Okamoto, K.; Sakai, S.; Kinoshita, J.; Makino, I.; et al. Role for Neutrophil Extracellular Traps (NETs) and Platelet Aggregation in Early Sepsis-induced Hepatic Dysfunction. In Vivo 2017, 31, 1051–1058. [Google Scholar] [CrossRef]
- Denning, N.L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Qu, M.; Nan, K.; Cao, H.; Cata, J.P.; Chen, W.; Miao, C. Review: The Emerging Role of Neutrophil Extracellular Traps in Sepsis and Sepsis-Associated Thrombosis. Front. Cell. Infect. Microbiol. 2021, 11, 653228. [Google Scholar] [CrossRef]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Bakdash, N.; Williams, M.S. Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic. Biol. Med. 2008, 45, 158–166. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.; Hosseini, E. Platelet granule release is associated with reactive oxygen species generation during platelet storage: A direct link between platelet pro-inflammatory and oxidation states. Thromb. Res. 2017, 156, 101–104. [Google Scholar] [CrossRef]
- Bennett, J.S. Structure and function of the platelet integrin alphaIIbbeta3. J. Clin. Investig. 2005, 115, 3363–3369. [Google Scholar] [CrossRef]
- Dewitte, A.; Lepreux, S.; Villeneuve, J.; Rigothier, C.; Combe, C.; Ouattara, A.; Ripoche, J. Blood platelets and sepsis pathophysiology: A new therapeutic prospect in critically [corrected] ill patients? Ann. Intensive Care 2017, 7, 115. [Google Scholar] [CrossRef]
- Tsai, J.C.; Lin, Y.W.; Huang, C.Y.; Lin, F.Y.; Tsai, C.S. Calpain activity and Toll-like receptor 4 expression in platelet regulate haemostatic situation in patients undergoing cardiac surgery and coagulation in mice. Mediat. Inflamm. 2014, 2014, 484510. [Google Scholar] [CrossRef] [PubMed]
- Zarà, M.; Vismara, M.; Dona, G.; Trivigno, S.M.G.; Amadio, P.; Sandrini, L.; Guidetti, G.F.; Barbieri, S.S. The Impact of Platelet Isolation Protocol on the Release of Extracellular Vesicles. Front. Biosci. 2022, 27, 161. [Google Scholar] [CrossRef] [PubMed]
- Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int. J. Mol. Sci. 2014, 15, 22342–22364. [Google Scholar] [CrossRef] [PubMed]
- Gerskowitch, V.P.; Hodge, J.; Hull, R.A.; Shankley, N.P.; Kalindjian, S.B.; McEwen, J.; Black, J.W. Unexpected relationship between plasma protein binding and the pharmacodynamics of 2-NAP, a CCK1-receptor antagonist. Br. J. Clin. Pharmacol. 2007, 63, 618–622. [Google Scholar] [CrossRef]
- D’Atri, L.P.; Schattner, M. Platelet toll-like receptors in thromboinflammation. Front. Biosci. 2017, 22, 1867–1883. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Gonzalez, D.A.; Kumar, R.; Asif, S.; Bali, A.; Dang, A.K. Sepsis and Thrombocytopenia: A Nowadays Problem. Cureus 2022, 14, e25421. [Google Scholar] [CrossRef]
- Good, D.W.; George, T.; Watts, B.A., III. Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. J. Biol. Chem. 2012, 287, 20208–20220. [Google Scholar] [CrossRef]
- Francisco, S.; Billod, J.M.; Merino, J.; Punzón, C.; Gallego, A.; Arranz, A.; Martin-Santamaria, S.; Fresno, M. Induction of TLR4/TLR2 Interaction and Heterodimer Formation by Low Endotoxic Atypical LPS. Front. Immunol. 2021, 12, 748303. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Devant, P.; Cao, A.; Kagan, J.C. Evolution-inspired redesign of the LPS receptor caspase-4 into an interleukin-1β converting enzyme. Sci. Immunol. 2021, 6, eabh3567. [Google Scholar] [CrossRef] [PubMed]
| LPS Source/Strain | Platelet Preparation | LPS (µg/mL) | CD14/LBP (µg/mL) | Time (min) | Results | Ref. |
|---|---|---|---|---|---|---|
| E. coli/ O111:B4 | WP | 5 | Serum | 30 | LPS promotes platelet binding to fibrinogen but not α-granule secretion | [10] |
| E. coli/ O111:B4 S. minnesota/ R595 | WB PRP WP | 0.125–2 | No | 20–25 | LPS does not increase α-granule secretion and ROS production both per se and by CRP. LPS does not increase ADP/collagen/TRAP6-induced aggregation | [46] |
| E. coli K. pneumoniae P. aeruginosa | WB PRP WP | 0.1–5 | No | 10–90 | LPS does not increase α- and δ-granule secretion, PS exposition, and PNA and PMA formation per se and by ADP/CRP | [32] |
| E. coli | WB | 5 | No | 30 | LPS does not induce α- and δ-granule secretion and PLA formation | [47] |
| E. coli/ O111:B4 | WP | 5 | No | 10 | LPS induces PNA but not NET formation, α-granule secretion, and aggregation per se | [48] |
| E. coli/ O111:B4 | PRP | 1 | No | 60 | LPS does not promote α-granule secretion and aggregation per se | [49] |
| E. coli/ O111:B4 | PRP WP | 10 | No | 30–120 | LPS does not promote granule secretion, fibrinogen binding, and PS exposure. LPS decreases ADP-induced aggregation in hirudinated PRP | [18] |
| E. coli/ O111:B4; K12 | PRP WP | 0.5–7.5 | No | 5–10 | LPS promotes α-granule secretion and integrin activation and potentiates U46619/CRP activity. LPS does not induce ROS production and aggregation per se, but both are potentiated by U46619 and CRP | [13] |
| E. coli/ O111:B4 | WP | 0.5–10 | No | 15 | LPS induces vWF release. LPS does not promote ADP release and aggregation per se, but both are potentiated by thrombin | [16] |
| E. coli/ O111:B4 | PRP WP | 15 | No | 30 | LPS induces RANTES, PDGF, and PF4 release in PRP and WP, but NAP-2 and sCD40L release only in WP. LPS potentiates thrombin-induced aggregation in WP | [12] |
| PRP WP | 10 | No | 30 | LPS promotes α-granule secretion, ROS production, and aggregation per se | [50] | |
| E. coli/ O111:B4 | PRP WP | 1–10 | 0.25/No | 30 | LPS promotes α- and δ-granule secretion | [44] |
| E. coli/ O111:B4 S. minnesota | PRP WP | 3 | 1/No | 30 | LPS induces δ- but not α-granule secretion. LPS promotes sCD40L and sCD14, and inhibits RANTES and PDGF-AB | [20] |
| E. coli | WP | 0.1 | 0.1/ 0.1 | 180 | LPS promotes IL-1β maturation and release | [45] |
| E. coli/ O111:B4; O127:B8; O55:B5 | WP | 1 | No | 10–30 | LPS promotes α-granule and ADP secretion. LPS does not induce aggregation per se but potentiates thrombin/collagen-induced aggregation | [15] |
| E. coli | WP | 0.1 | 0.15/ 0.1 Serum | 60–180 | LPS promotes IL-1β maturation and release, PNA formation, and α-granule secretion. LPS does not induce aggregation per se but potentiates ADP-induced aggregation | [14] |
| E. coli | PRP | 0.5–2 | No | 15 | LPS promotes δ-granule secretion. LPS induces sCD40L and PAF4, and inhibits RANTES, PDGF-AB, and angiogenin | [19] |
| E. coli/ O111:B4 | WP | <0.1 | No | 15 | LPS does not induce aggregation per se but potentiates ADP/collagen-induced aggregation TxA2 and ROS production | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, L.; Guidetti, G.F.; Torti, M.; Canobbio, I. The Controversial Role of LPS in Platelet Activation In Vitro. Int. J. Mol. Sci. 2022, 23, 10900. https://doi.org/10.3390/ijms231810900
Galgano L, Guidetti GF, Torti M, Canobbio I. The Controversial Role of LPS in Platelet Activation In Vitro. International Journal of Molecular Sciences. 2022; 23(18):10900. https://doi.org/10.3390/ijms231810900
Chicago/Turabian StyleGalgano, Luca, Gianni Francesco Guidetti, Mauro Torti, and Ilaria Canobbio. 2022. "The Controversial Role of LPS in Platelet Activation In Vitro" International Journal of Molecular Sciences 23, no. 18: 10900. https://doi.org/10.3390/ijms231810900
APA StyleGalgano, L., Guidetti, G. F., Torti, M., & Canobbio, I. (2022). The Controversial Role of LPS in Platelet Activation In Vitro. International Journal of Molecular Sciences, 23(18), 10900. https://doi.org/10.3390/ijms231810900








