From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants
Abstract
1. Introduction
2. Molecular Regulation of Flowering Phase Transition
2.1. The Role of PEPB Gene Family in the Flowering Phase Transition
2.2. MicroRNAs in Flowering Phase Transition
3. Regulation Mechanism of Floral Organogenesis
4. DAM/SVP Genes Associated with Dormancy and “Indirect Flowering”
5. Epigenetic Modification in Flowering Regulation
6. Final Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosas, U.; Mei, Y.; Xie, Q.G.; Banta, J.A.; Zhou, R.W.; Seufferheld, G.; Gerard, S.; Chou, L.; Bhambhra, N.; Parks, J.D.; et al. Variation in Arabidopsis flowering time associated with cis-regulatory variation in CONSTANS. Nat. Commun. 2014, 5, 3651. [Google Scholar] [CrossRef] [PubMed]
- Mouradov, A.; Cremer, F.; Coupland, G. Control of fowering time: Interacting pathways as a basis for diversity. Plant Cell 2002, 14, S111–S130. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, A.; Schmid, M. Regulation of fowering time: All roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Teotia, S.; Tang, G. To bloom or not to bloom: Role of microRNAs in plant flowering. Mol. Plant 2015, 8, 359–377. [Google Scholar] [CrossRef]
- Albani, M.C.; Coupland, G. Comparative analysis of flowering in annual and perennial plants. Curr. Top. Dev. Biol. 2010, 91, 323–348. [Google Scholar]
- Wang, D.L.; Gao, Z.Z.; Du, P.Y.; Xiao, W.; Tan, Q.P.; Chen, X.D.; Li, L.; Gao, D.S. Expression of ABA metabolism-related genes suggests similarities and differences between seed dormancy and bud dormancy of Peach (Prunus persica). Front. Plant Sci. 2016, 6, 1248. [Google Scholar] [CrossRef]
- Wu, R.M.; Tomes, S.; Karunairetnam, S.; Tustin, S.D.; Hellens, R.P.; Allan, A.C.; Macknight, R.C.; Varkonyi-Gasic, E. SVP-like MADS box genes control dormancy and budbreak in apple. Front. Plant Sci. 2017, 8, 477. [Google Scholar] [CrossRef]
- Ito, A.; Tuan, P.A.; Saito, T.; Bai, S.L.; Kita, M.; Moriguchi, T. Changes in phytohormone content and associated gene expression throughout the stages of pear (Pyrus pyrifolia Nakai) dormancy. Tree Physiol. 2019, 41, 529–543. [Google Scholar] [CrossRef]
- Meir, M.; Ransbotyn, V.; Raveh, E.; Barak, S.; Tel-Zur, N.; Zaccai, M. Dormancy release and flowering time in Ziziphus jujuba Mill., a “direct flowering” fruit tree, has a facultative requirement for chilling. J. Plant Physiol. 2016, 192, 118–127. [Google Scholar] [CrossRef]
- Meng, X.W.; Li, Y.; Yuan, Y.; Zhang, Y.; Li, H.T.; Zhao, J.; Liu, M.J. The regulatory pathways of distinct flowering characteristics in Chinese jujube. Hortic. Res. 2020, 7, 123. [Google Scholar] [CrossRef]
- Remay, A.; Lalanne, D.; Thouroude, T.; Le Couviour, F.; Oyant, L.H.-S.; Foucher, F. A survey of flowering genes reveals the role of gibberellins in floral control in rose. Theor. Appl. Genet. 2009, 119, 767–781. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, L.Y.; Wei, Q.; Ju, Y.; Zou, X.; Wan, X.X.; Liu, X.; Yin, Z.F. A new insight into flowering regulation: Molecular basis of flowering initiation in Magnolia × soulangeana ‘Changchun’. Genes 2020, 11, 15. [Google Scholar] [CrossRef]
- Jones, R.C.; Valérie, F.G.H.; Potts, B.M.; Vaillancourt, R.E.; Weller, J.L. Expression of a FLOWERING LOCUS T homologue is temporally associated with annual flower bud initiation in Eucalyptus globulus subsp. globulus (Myrtaceae). Aust. J. Bot. 2011, 59, 756–769. [Google Scholar] [CrossRef]
- Su, M.Y.; Wang, N.; Jiang, S.H.; Fang, H.C.; Xu, H.F.; Wang, Y.C.; Zhang, Z.; Zhang, J.; Xu, L.; Zhang, Z.Y.; et al. Molecular characterization and expression analysis of the critical floral gene MdAGL24-like in red-fleshed apple. Plant Sci. 2018, 276, 189–198. [Google Scholar] [CrossRef]
- Wei, X.J.; Ma, J.L.; Wang, K.; Liang, X.J.; Lan, J.X.; Li, Y.J.; Li, K.X.; Liang, H.Y. Early flowering induction in golden Camellia seedlings and effects of paclobutrazol. HortScience 2018, 53, 1849–1854. [Google Scholar] [CrossRef]
- Velázquez, K.; Agüero, J.; Vives, M.C.; Aleza, P.; Pina, J.A.; Moreno, P.; Navarro, L.; Guerri, J. Precocious flowering of juvenile citrus induced by a viral vector based on citrus leaf blotch virus: A new tool for genetics and breeding. Plant Biotechnol. J. 2016, 14, 1976–1985. [Google Scholar] [CrossRef]
- Jordan, G.J.; Potts, B.M.; Chalmers, P.; Wiltshire, R.J.E. Quantitativegenetic evidence that the timing of vegetative phase change in Eucalyptus globulus ssp. globulusis an adaptive trait. Aust. J. Bot. 2000, 48, 561–567. [Google Scholar] [CrossRef]
- Tang, M.Y.; Bai, X.; Niu, L.J.; Chai, X.; Chen, M.S.; Xu, Z.F. miR172 regulates both vegetative and reproductive development in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2018, 59, 2549–2563. [Google Scholar] [CrossRef]
- Sheng, Y.; Hao, Z.D.; Peng, Y.; Liu, S.Q.; Hu, L.F.; Shen, Y.B.; Shi, J.S.; Chen, J.H. Morphological, phenological, and transcriptional analyses provide insight into the diverse flowering traits of a mutant of the relic woody plant Liriodendron chinense. Hortic. Res. 2021, 8, 174. [Google Scholar] [CrossRef]
- Yuceer, C.; Kubiske, M.E.; Harkess, R.L.; Land, S.B. Effects of induction treatments on flowering in Populus deltoides. Tree Physiol. 2003, 23, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.L.; Chen, Y.; Su, X.H.; Zhang, S.G.; Pan, H.X.; Huang, M.R. Two FT orthologs from Populus simonii Carrière induce early flowering in Arabidopsis and poplar trees. Plant Cell Tiss. Organ Cult. 2012, 108, 371–379. [Google Scholar] [CrossRef]
- Xing, W.; Wang, Z.; Wang, X.Q.; Bao, M.Z.; Ning, G.G. Over-expression of an FT homolog from Prunus mume reduces juvenile phase and induces early flowering in rugosa rose. Sci. Hortic. 2014, 172, 68–72. [Google Scholar] [CrossRef]
- Karlgren, A.; Gyllenstrand, N.; Kallman, T.; Sundstrom, J.F.; Moore, D.; Lascoux, M.; Lagercrantz, U. Evolution of the PEBP gene family in plants: Functional diversification in seed plant evolution. Plant Physiol. 2011, 156, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Mo, R.L.; Chen, W.X.; Zhang, Q.L.; Sheng, F.; Wu, C.Y.; Zhang, R.; Luo, Z.R. Identification and comparative analysis of genes and microRNAs involved in the floral transition of the Xinjiang early-flowering walnut (Juglans regia L.). Horticulturae 2022, 8, 136. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Liu, Y.X.; Luthe, D.S.; Yuceer, C. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 2006, 18, 1846–1861. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ke, J.P.; Huang, P.; Fatima, I.; Cheng, T.; Tang, M.Y. Promotion of natural flowers by JcFT depends on JcLFY in the perennial woody species Jatropha curcas. Plant Sci. 2022, 318, 111236. [Google Scholar] [CrossRef]
- Tränkner, C.; Lehmann, S.; Hoenicka, H.; Hanke, M.-V.; Fladung, M.; Lenhardt, D.; Dunemann, F.; Gau, A.; Schlangen, K.; Malnoy, M.; et al. Over-expression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta 2010, 232, 1309–1324. [Google Scholar] [CrossRef]
- Xia, W.; Liu, R.; Zhang, J.; Mason, A.S.; Li, Z.Y.; Gong, S.F.; Zhong, Y.Z.; Dou, Y.J.; Sun, X.W.; Fan, H.K.; et al. Alternative splicing of flowering time gene FT is associated with halving of time to flowering in coconut. Sci. Rep. 2020, 10, 11640. [Google Scholar] [CrossRef]
- Kotoda, N.; Iwanami, H.; Takahashi, S.; Abe, K. Antisense expression of MdTFL1, a TFL1-like gene, reduces the juvenile phase in apple. J. Amer. Soc. Hort. Sci. 2006, 131, 74–81. [Google Scholar] [CrossRef]
- Flachowsky, H.; Szankowski, I.; Waidmann, S.; Peil, A.; Tränkner, C.; Hanke, M.V. The MdTFL1 gene of apple (Malus × domestica Borkh.) reduces vegetative growth and generation time. Tree Physiol. 2012, 32, 1288–1301. [Google Scholar] [CrossRef]
- Freiman, A.; Shlizerman, L.; Golobovitch, S.; Yablovitz, Z.; Korchinsky, R.; Cohen, Y.; Samach, A.; Chevreau, E.; Le Roux, P.M.; Patocchi, A.; et al. Development of a transgenic early flowering pear (Pyrus communis L.) genotype by RNAi silencing of PcTFL1-1 and PcTFL1-2. Planta 2012, 235, 1239–1251. [Google Scholar] [CrossRef]
- Sasaki, S.; Yamagishi, N.; Yoshikawa, N. Efficient virus-induced gene silencing in apple, pear and Japanese pear using Apple latent spherical virus vectors. Plant Methods 2011, 7, 15. [Google Scholar] [CrossRef]
- Mohamed, R.; Wang, C.T.; Ma, C.; Shevchenko, O.; Dye, S.J.; Puzey, J.R.; Etherington, E.; Sheng, X.Y.; Meilan, R.; Strauss, S.H.; et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 2010, 62, 674–688. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wang, T.C.; Voogd, C.; Jeon, S.; Drummond, R.S.M.; Gleave, A.P.; Allan, A.C. Mutagenesis of kiwifruit CENTRORADIALIS-like genes transforms a climbing woody perennial with long juvenility and axillary flowering into a compact plant with rapid terminal flowering. Plant Biotechnol. J. 2019, 17, 869–880. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Abuahmad, A.; Franks, R.G.; Xie, D.Y.; Xiang, Q.Y. Analysis of two TFL1 homologs of dogwood species (Cornus L.) indicates functional conservation in control of transition to flowering. Planta 2016, 243, 1129–1141. [Google Scholar] [CrossRef]
- Iwata, H.; Gaston, A.; Remay, A.; Thouroude, T.; Jeauffre, J.; Kawamura, K.; Oyant, L.H.-S.; Araki, T.; Denoyes, B.; Foucher, F. The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J. 2016, 69, 116–125. [Google Scholar] [CrossRef]
- Nishikawa, F.; Endo, T.; Shimada, T.; Fujii, H.; Shimizu, T.; Omura, M.; Ikoma, Y. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). J. Exp. Bot. 2007, 58, 3915–3927. [Google Scholar] [CrossRef]
- Tang, L.; Lovatt, C.J. Effects of low temperature and gibberellic acid on floral gene expression and floral determinacy in ‘Washington’ navel orange (Citrus sinensis L. Osbeck). Sci. Hortic. 2019, 243, 92–100. [Google Scholar] [CrossRef]
- Ito, A.; Saito, T.; Nishijima, T.; Moriguchi, T. Effect of extending the photoperiod with low-intensity red or far-red light on the timing of shoot elongation and flower-bud formation of 1-year-old Japanese pear (Pyrus pyrifolia). Tree Physiol. 2014, 34, 534–546. [Google Scholar] [CrossRef]
- Zou, X.Y.; Xiang, W.; Zhang, L.Z.; Gao, C.; An, N.; Xing, L.B.; Ma, J.J.; Zhao, C.P.; Zhang, D. Identification of apple TFL1-interacting proteins uncovers an expanded flowering network. Plant Cell Rep. 2021, 40, 2325–2340. [Google Scholar]
- Song, G.Q.; Walworth, A.; Zhao, D.; Ning, J.; Hancock, J.F. The Vaccinium corymbosum FLOWERING LOCUS T-like gene (VcFT): A flowering activator reverses photoperiodic and chilling requirements in blueberry. Plant Cell Rep. 2013, 32, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Gao, J.; Xue, J.Q.; Xue, Y.Q.; Li, D.D.; Guan, Y.R.; Zhang, X.X. De novo sequencing of tree peony (Paeonia suffruticosa) transcriptome to identify critical genes involved in flowering and floral organ development. BMC Genom. 2019, 20, 572. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.G.; Jackson, S. Floral induction and flower formation—The role and potential applications of miRNAs. Plant Biotechnol. J. 2015, 13, 282–292. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Fang, L.S.; Wang, Y.M. MicroRNAs in woody plants. Front. Plant Sci. 2021, 12, 686831. [Google Scholar] [CrossRef]
- Wang, J.W.; Park, M.Y.; Wang, L.J.; Koo, Y.; Chen, X.Y.; Weigel, D.; Poethig, R.S. MiRNA control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Jia, X.L.; Chen, Y.K.; Xu, X.Z.; Shen, F.; Zheng, Q.B.; Du, Z.; Wang, Y.; Wu, T.; Xu, X.F.; Han, Z.H.; et al. miR156 switches on vegetative phase change under the regulation of redox signals in apple seedlings. Sci. Rep. 2017, 7, 14223. [Google Scholar] [CrossRef]
- Xing, L.B.; Zhang, D.; Li, Y.M.; Zhao, C.P.; Zhang, S.W.; Shen, Y.W.; An, N.; Han, M.Y. Genome-wide identification of vegetative phase transition-associated microRNAs and target predictions using degradome sequencing in Malus hupehensis. BMC Genom. 2014, 15, 1125. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Lough, R.H.; Moss, S.M.A.; Wu, R.M.; Hellens, R.P. Kiwifruit floral gene APETALA2 is alternatively spliced and accumulates in aberrant indeterminate flowers in the absence of miR172. Plant Mol. Biol. 2012, 78, 417–429. [Google Scholar] [CrossRef]
- Zhang, X.N.; Li, X.; Liu, J.H. Identification of conserved and novel cold-responsive microRNAs in trifoliate orange (Poncirus trifoliata (L.) Raf.) using high-throughput sequencing. Plant Mol. Biol. Rep. 2014, 32, 328–341. [Google Scholar] [CrossRef]
- Ahsan, M.U.; Hayward, A.; Irihimovitch, V.; Fletcher, S.; Tanurdzic, M.; Pocock, A.; Beveridge, C.A.; Mitter, N. Juvenility and vegetative phase transition in tropical/subtropical tree crops. Front. Plant Sci. 2019, 10, 729. [Google Scholar] [CrossRef]
- Wang, T.; Pan, H.; Wang, J.; Yang, W.R.; Cheng, T.R.; Zhang, Q.X. Identification and profiling of novel and conserved microRNAs during the flower opening process in Prunus mume via deep sequencing. Mol. Genet. Genom. 2014, 289, 169–183. [Google Scholar] [CrossRef]
- Choudhary, S.; Thakur, S.; Najar, R.A.; Majeed, A.; Singh, A.; Bhardwaj, P. Transcriptome characterization and screening of molecular markers in ecologically important Himalayan species (Rhododendron arboreum). Genome 2018, 61, 417–428. [Google Scholar] [CrossRef]
- Rao, S.P.; Li, Y.; Chen, J.H. Combined analysis of microRNAs and target Genes revealed miR156-SPLs and miR172-AP2 are involved in a delayed flowering phenomenon after chromosome doubling in black Goji (Lycium ruthencium). Front. Genet. 2021, 12, 706930. [Google Scholar] [CrossRef]
- Sun, L.Y.; Jiang, Z.; Ju, Y.; Zou, X.; Wan, X.X.; Chen, Y.; Yin, Z.F. A potential endogenous gibberellin-mediated signaling cascade regulated foral transition in Magnolia × soulangeana ‘Changchun’. Mol. Genet. Genom. 2021, 296, 207–222. [Google Scholar] [CrossRef]
- Guo, X.W.; Ma, Z.Y.; Zhang, Z.H.; Cheng, L.L.; Zhang, X.R.; Li, T.H. Small RNA-sequencing links physiological changes and RdDM process to vegetative-to-floral transition in apple. Front. Plant Sci. 2017, 8, 873. [Google Scholar] [CrossRef]
- Xu, W.Y.; Bao, W.Q.; Liu, H.M.; Chen, C.; Bai, H.K.; Huang, M.Z.; Zhu, G.P.; Zhao, H.; Gou, N.N.; Chen, Y.X.; et al. Insights into the molecular mechanisms of late flowering in Prunus sibirica by whole-genome and transcriptome analyses. Front. Plant Sci. 2022, 12, 802827. [Google Scholar] [CrossRef]
- Wellmer, F.; Riechmann, J.L. Gene networks controlling theinitiation of flower development. Trends Genet. 2010, 26, 519–527. [Google Scholar] [CrossRef]
- Weigel, D.; Alvarez, J.; Smyth, D.R.; Yanofsky, M.F.; Meyerowitz, E.M. LEAFY controls floral meristem identity in Arabidopsis. Cell 1992, 69, 843–859. [Google Scholar] [CrossRef]
- Rottmann, W.H.; Meilan, R.; Sheppard, L.A.; Brunner, A.M.; Skinner, J.S.; Ma, C.P.; Cheng, S.P.; Jouanin, L.; Pilate, G.; Strauss, S.H. Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant J. 2000, 22, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Huang, J.Q.; Huang, Y.J.; Chen, F.F.; Zheng, B.S. Cloning and characterization of a homologue of the FLORICAULA/LEAFY gene in hickory (Carya cathayensis Sarg). Plant Mol. Biol. Rep. 2012, 30, 794–805. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Li, R.; Mauk, P.; Santiago, L.; Lovatt, C.J. Effects of temperature, soil moisture and light intensity on the temporal pattern of floral gene expression and flowering of avocado buds (Persea americana cv. Hass). Sci. Hortic. 2021, 280, 109940. [Google Scholar] [CrossRef]
- An, L.J.; Lei, H.J.; Shen, X.J.; Li, T.H. Identification and characterization of PpLFL, a homolog of FLORICAULA/LEAFY in peach (Prunus persica). Plant Mol. Biol. Rep. 2012, 30, 1488–1495. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, H.X.; He, X.H.; Lu, T.T.; Huang, X.; Luo, C. Isolation and functional characterization of a LEAFY gene in mango (Mangifera indica L.). Int. J. Mol. Sci. 2022, 23, 3974. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, M.C.; Rodriguez, A.P.M. The tropical cedar tree (Cedrela fissilis Vell., Meliaceae) homolog of the Arabidopsis LEAFY gene is expressed in reproductive tissues and can complement Arabidopsis leafy mutants. Planta 2006, 223, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; An, Y.Y.; Li, J.; Wang, L.J. Cloning and characterization of a homologue of the FLORICAULA/LEAFY gene in Ficus carica L., FcLFY, and its role in flower bud differentiation. Sci. Hortic. 2020, 261, 109014. [Google Scholar] [CrossRef]
- Klocko, A.L.; Brunner, A.M.; Huang, J.; Meilan, R.; Lu, H.W.; Ma, C.; Morel, A.; Zhao, D.Z.; Ault, K.; Dow, M.; et al. Containment of transgenic trees by suppression of LEAFY. Nat. Biotechnol. 2016, 34, 918–922. [Google Scholar] [CrossRef]
- Klocko, A.L.; Goddard, A.L.; Jacobson, J.R.; Magnuson, A.C.; Strauss, S.H. RNAi suppression of LEAFY gives stable floral sterility, and reduced growth rate and leaf size, in field-grown poplars. Plants 2021, 10, 1594. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef]
- Favaro, R.; Pinyopich, A.; Battaglia, R.; Kooiker, M.; Borghi, L.; Ditta, G.; Yanofsky, M.F.; Kater, M.M.; Colombo, L. MADS-Box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell 2003, 15, 2603–2611. [Google Scholar] [CrossRef]
- Lemmetyinen, J.; Hassinen, M.; Elo, A.; Porali, I.; Sopanen, T. Functional characterization of SEPALLATA3 and AGAMOUS orthologues in silver birch. Physiol. Plant. 2010, 121, 149–162. [Google Scholar] [CrossRef]
- Jing, D.L.; Xia, Y.; Chen, F.J.; Wang, Z.; Zhang, S.G.; Wang, J.H. Ectopic expression of a Catalpa bungei (Bignoniaceae) PISTILLATA homologue rescues the petal and stamen identities in Arabidopsis pi-1 mutant. Plant Sci. 2015, 231, 40–51. [Google Scholar] [CrossRef]
- Louati, M.; Salazar-Sarasua, B.; Roque, E.; Beltrán, J.P.; Hannachi, A.S.; Gómez-Mena, C. Isolation and functional analysis of a PISTILLATA-like MADS-box gene from argan tree (Argania spinosa). Plants 2021, 10, 1665. [Google Scholar] [CrossRef]
- Klocko, A.L.; Brunner, A.M.; Ma, C.; Etherington, E.; Rosenstiel, K.; Magnuson, A.; Taylor, B.J.; JLockwood, T.C.; Covarrubias, N.; Bao, M.Z.; et al. RNAi of AGAMOUS genes in sweetgum alters reproductive organ identity and decreases fruit persistence. Plant Direct 2020, 4, e00225. [Google Scholar] [CrossRef]
- Lu, H.W.; Klocko, A.L.; Brunner, A.M.; Ma, C.; Magnuson, A.C.; Howe, G.T.; An, X.M.; Strauss, S.H. RNAi suppression of AGAMOUS and SEEDSTICK alters floral organ identity and impairs floral organ determinacy, ovule differentiation, and seed-hair development in Populus. New Phytol. 2018, 222, 923–937. [Google Scholar] [CrossRef]
- Buzgo, M.; Soltis, P.S.; Soltis, D.E. Floral developmental morphology of Amborella trichopoda (Amborellaceae). Int. J. Plant Sci. 2004, 165, 925–947. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; Albert, V.A.; Leebens-Mack, J.; Altman, N.S.; Soltis, D.E.; Soltis, P.S. Transcriptional signatures of ancient floral developmental genetics in avocado (Persea americana; Lauraceae). Proc. Natl. Acad. Sci. USA 2009, 106, 8929–8934. [Google Scholar] [CrossRef]
- Luo, H.L.; Chen, S.M.; Jiang, J.F.; Chen, Y.; Chen, F.D.; Teng, N.J.; Yin, D.M.; Huang, C.B. The expression of floral organ identity genes in contrasting water lily cultivars. Plant Cell Rep. 2011, 30, 1909–1918. [Google Scholar] [CrossRef]
- Soltis, D.E.; Chanderbali, A.S.; Kim, S.; Buzgo, M.; Soltis, P.S. The ABC model and its applicability to basal angiosperms. Ann. Bot. 2007, 100, 155–163. [Google Scholar] [CrossRef]
- Soltis, D.E.; Ma, H.; Frohlich, M.W.; Soltis, P.S.; Albert, V.A.; Oppenheimer, D.G.; Altman, N.S.; dePamphilis, C.; Leebens-Mack, J. The floral genome: An evolutionary history of gene duplication and shifting patterns of gene expression. Trends Plant Sci. 2007, 12, 358–367. [Google Scholar] [CrossRef]
- Wróblewska, M.; Dołzbłasz, A.; Zagórska-Marek, B. The role of ABC genes in shaping perianth phenotype in the basal angiosperm Magnolia. Plant Biol. 2016, 18, 230–238. [Google Scholar] [CrossRef]
- Kim, S.; Koh, J.; Yoo, M.-J.; Kong, H.Z.; Hu, Y.; Ma, H.; Soltis, P.S.; Soltis, D.E. Expression of floral MADS-box genes in basal angiosperms: Implications for the evolution of floral regulators. Plant J. 2005, 43, 724–744. [Google Scholar] [CrossRef]
- Wu, W.T.; Chen, F.J.; Jing, D.L.; Liu, Z.X.; Ma, L.Y. Isolation and characterization of an AGAMOUS-Like gene from Magnolia wufengensis (Magnoliaceae). Plant Mol. Biol. Rep. 2012, 30, 690–698. [Google Scholar] [CrossRef]
- Jing, D.L.; Liu, Z.X.; Zhang, B.; Ma, J.; Han, Y.Y.; Chen, F.J. Two ancestral APETALA3 homologs from the basal angiosperm Magnolia wufengensis (Magnoliaceae) can affect flower development of Arabidopsis. Gene 2014, 537, 100–107. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.X.; Ma, J.; Song, Y.; Chen, F.J. Alternative splicing of the AGAMOUS orthologous gene in double flower of Magnolia stellata (Magnoliaceae). Plant Sci. 2015, 241, 277–285. [Google Scholar] [CrossRef]
- Liu, W.; Shen, X.L.; Liang, H.W.; Wang, Y.B.; He, Z.Q.; Zhang, D.C.; Chen, F.J. Isolation and functional analysis of PISTILLATA homolog from Magnolia wufengensis. Front. Plant Sci. 2018, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Chen, L.Y.; Fan, X.N.; Qi, W.J.; Ma, J.; Tian, T.; Zhou, T.; Ma, L.Y.; Chen, F.J. MawuAP1 promotes flowering and fruit development in the basal angiosperm Magnolia wufengensis (Magnoliaceae). Tree Physiol. 2020, 40, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Dreni, L.; Zhang, D.B. Flower development: The evolutionary history and functions of the AGL6 subfamily MADS-box genes. J. Exp. Bot. 2016, 67, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Pabón-Mora, N.; Suárez-Baron, H.; Ambrose, B.A.; González, F. Flower development and perianth identity candidate genes in the basal angiosperm Aristolochia fimbriata (Piperales: Aristolochiaceae). Front. Plant Sci. 2015, 6, 1095. [Google Scholar] [CrossRef]
- Mouradov, A.; Glassick, T.V.; Hamdorf, B.A.; Murphy, L.C.; Marla, S.S.; Yang, Y.M.; Teasdale, R.D. Family of MADS-box genes expressed early in male and female reproductive structures of Monterey pine. Plant Physiol. 1998, 117, 55–61. [Google Scholar] [CrossRef][Green Version]
- Wang, B.G.; Zhang, Q.; Wang, L.G.; Duan, K.; Pan, A.H.; Tang, X.M.; Sui, S.Z.; Li, M.Y. The AGL6-like gene CpAGL6, a potential regulator of floral time and organ identity in wintersweet (Chimonanthus praecox). J. Plant Growth Regul. 2011, 30, 343–352. [Google Scholar] [CrossRef]
- Ma, J.; Deng, S.X.; Chen, L.Y.; Jia, Z.K.; Sang, Z.Y.; Zhu, Z.L.; Ma, L.Y.; Chen, F.J.; Plomion, C. Gene duplication led to divergence of expression patterns, protein–protein interaction patterns and floral development functions of AGL6-like genes in the basal angiosperm Magnolia wufengensis (Magnoliaceae). Tree Physiol. 2019, 39, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Gattolin, S.; Cirilli, M.; Pacheco, I.; Ciacciulli, A.; da Silva Linge, C.; Mauroux, J.-B.; Lambert, P.; Cammarata, E.; Bassi, D.; Pascal, T.; et al. Deletion of the miR172 target site in a TOE-type gene is a strong candidate variant for dominant double-flower trait in Rosaceae. Plant J. 2018, 96, 358–371. [Google Scholar] [CrossRef] [PubMed]
- François, L.; Verdenaud, M.; Fu, X.P.; Ruleman, D.; Dubois, A.; Vandenbussche, M.; Bendahmane, A.; Raymond, O.; Just, J.; Bendahmane, M. A miR172 target-deficient AP2-like gene correlates with the double flower phenotype in roses. Sci. Rep. 2018, 8, 12912. [Google Scholar] [CrossRef] [PubMed]
- Fadón, E.; Rodrigo, J. Unveiling winter dormancy through empirical experiments. Environ. Exp. Bot. 2017, 152, 28–36. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Guillamón, J.G.; Dicenta, F.; Sánchez-Pérez, R. Advancing endodormancy release in temperate fruit trees using agrochemical treatments. Front. Plant Sci. 2022, 12, 812621. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, S.J.; Park, S.H.; Hwang, I.; Lee, J.S.; Ahn, J.H. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007, 21, 397–402. [Google Scholar] [CrossRef]
- Goralogia, G.S.; Howe, G.T.; Brunner, A.M.; Helliwell, E.; Nagle, M.F.; Ma, C.; Lu, H.W.; Goddard, A.L.; Magnuson, A.C.; Klocko, A.L.; et al. Overexpression of SHORT VEGETATIVE PHASE-LIKE (SVL) in Populus delays onset and reduces abundance of flowering in field-grown trees. Hortic. Res. 2021, 8, 167. [Google Scholar] [CrossRef]
- Singh, R.K.; Maurya, J.P.; Azeez, A.; Miskolczi, P.; Tylewicz, S.; Stojkovič, K.; Delhomme, N.; Busov, V.; Bhalerao, R.P. A genetic network mediating the control of bud break in hybrid aspen. Nat. Commun. 2018, 9, 4173. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jiu, S.T.; Xu, Y.; Sabir, I.A.; Wang, L.; Ma, C.; Xu, W.P.; Wang, S.P.; Zhang, C.X. SVP-like gene PavSVP potentially suppressing flowering with PavSEP, PavAP1, and PavJONITLESS in sweet cherries (Prunus avium L.). Plant Physiol. Bioch. 2021, 159, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.M.; Wang, T.C.; Warren, B.A.W.; Allan, A.C.; Macknight, R.C.; Varkonyi-Gasic, E. Kiwifruit SVP2 gene prevents premature budbreak during dormancy. J. Exp. Bot. 2017, 68, 1071–1082. [Google Scholar] [CrossRef]
- Yang, Q.S.; Gao, Y.H.; Wu, X.Y.; Moriguchi, T.; Bai, S.L.; Teng, Y.W. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hortic. Res. 2021, 8, 139. [Google Scholar] [CrossRef]
- Bielenberg, D.G.; Wang, Y.; Fan, S.; Reighard, G.L.; Scorza, R.; Abbott, A.G. A deletion affecting several gene candidates is present in the evergrowing peach mutant. J. Hered. 2004, 95, 436–444. [Google Scholar] [CrossRef]
- Bielenberg, D.G.; Wang, Y.; Li, Z.G.; Zhebentyayeva, T.; Fan, S.H.; Reighard, G.L.; Scorza, R.; Abbott, A.G. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet. Genomes 2008, 4, 495–507. [Google Scholar] [CrossRef]
- Falavigna, V.S.; Guitton, B.; Costes, E.; Andrés, F. I want to (bud) break free: The potential role of DAM and SVP-like genes in regulating dormancy cycle in temperate fruit trees. Front. Plant Sci. 2019, 9, 1990. [Google Scholar] [CrossRef]
- Li, Z.G.; Reighard, G.L.; Abbott, A.G.; Bielenberg, D.G. Dormancy-associated MADS genes from the EVG locus of peach [Prunus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. J. Exp. Bot. 2009, 60, 3521–3530. [Google Scholar] [CrossRef]
- Yamane, H.; Ooka, T.; Jotatsu, H.; Hosaka, Y.; Sasaki, R.; Tao, R. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. J. Exp. Bot. 2011, 62, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, A.S.; Hoeberichts, F.A.; Dicenta, F.; Martínez-Gómez, P.; Sánchez-Pérez, R. Identification of early and late flowering time candidate genes inendodormant and ecodormant almond flower buds. Tree Physiol. 2021, 41, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.M.; Cooney, J.; Tomes, S.; Rebstock, R.; Karunairetnam, S.; Allan, A.C.; Macknight, R.C.; Varkonyi-Gasic, E. RNAi-mediated repression of dormancy-related genes results in evergrowing apple trees. Tree Physiol. 2021, 41, 1510–1523. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.F.; Li, J.Z.; Cai, D.Y.; Qian, M.J.; Jia, H.M.; Bai, S.L.; Hussain, S.; Liu, G.Q.; Teng, Y.W.; Zheng, X.Y. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J. Exp. Bot. 2015, 67, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Vergara, R.; Noriega, X.; Pérez, F.J. VvDAM-SVPs genes are regulated by FLOWERING LOCUS T (VvFT) and not by ABA/low temperature-induced VvCBFs transcription factors in grapevine buds. Planta 2021, 253, 31. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, Y.Z.; Ahmad, S.; Yong, X.; Xie, X.H.; Han, Y.; Li, Y.S.; Sun, L.D.; Zhang, Q.X. PmCBFs synthetically affect PmDAM6 by alternative promoter binding and protein complexes towards the dormancy of bud for Prunus mume. Sci. Rep. 2018, 8, 4527. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.N.; Fan, S.H.; Chandra, A.; Bielenberg, D.G.; Reighard, G.L.; Okie, W.R.; Abbott, A.G. Dissection of chilling requirement and bloom date QTLs in peach using a whole genome sequencing of sibling trees from an F2 mapping population. Tree Genet. Genomes 2014, 10, 35–51. [Google Scholar] [CrossRef]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Kumar, G.; Rattan, U.K.; Singh, A.K. Chilling-mediated DNA methylation changes during dormancy and its release reveal the importance of epigenetic regulation during winter dormancy in apple (Malus × domestica Borkh.). PLoS ONE 2016, 11, e0149934. [Google Scholar] [CrossRef]
- Xin, H.; Zhang, Y.X.; Wang, X.T.; Liu, C.Y.; Feng, W.R.; Gai, S.P. Morphological, anatomical and DNA methylation changes of tree peony buds during chilling induced dormancy release. Plant Physiol. Biochem. 2019, 144, 64–72. [Google Scholar] [CrossRef]
- Xing, L.B.; Li, Y.M.; Qi, S.Y.; Zhang, C.G.; Ma, W.C.; Zou, X.Y.; Liang, J.Y.; Gao, C.; Jia, P.; Shah, K.; et al. Comparative RNA-sequencing and DNA methylation analyses of apple (Malus domestica Borkh.) buds with diverse flowering capabilities reveal novel insights into the regulatory mechanisms of flower bud formation. Plant Cell Physiol. 2019, 60, 1702–1721. [Google Scholar] [CrossRef]
- Fan, S.; Gao, X.H.; Gao, C.; Yang, Y.; Zhu, X.Z.; Feng, W.; Li, R.M.; Tahir, M.M.; Zhang, D.; Han, M.Y.; et al. Dynamic cytosine DNA methylation patterns associated with mRNA and siRNA expression profiles in alternate bearing apple trees. J. Agric. Food Chem. 2019, 67, 5250–5264. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Peng, X.Y.; Yu, Y.C.; Sun, Z.Y.; Han, L. The effects of dna methylation inhibition on flower development in the dioecious plant Salix Viminalis. Forests 2019, 10, 173. [Google Scholar] [CrossRef]
- Sun, K.R.; Xue, Y.Q.; Prijic, Z.; Wang, S.L.; Markovic, T.; Tian, C.H.; Wang, Y.Y.; Xue, J.Q.; Zhang, X.X. DNA demethylation induces tree peony flowering with a low deformity rate compared to gibberellin by inducing PsFT expression under forcing culture conditions. Int. J. Mol. Sci. 2022, 23, 6632. [Google Scholar] [CrossRef]
- Rothkegel, K.; Sánchez, E.; Montes, C.; Greve, M.; Tapia, S.; Bravo, S.; Prieto, H.; Almeida, A.M. DNA methylation and small interference RNAs participate in the regulation of MADS-box genes involved in dormancy in sweet cherry (Prunus avium L.). Tree Physiol. 2017, 37, 1739–1751. [Google Scholar] [CrossRef]
- Rothkegel, K.; Sandoval, P.; Soto, E.; Ulloa, L.; Riveros, A.; Lillo-Carmona, V.; Cáceres-Molina, J.; Almeida, A.M.; Meneses, C. Dormant but active: Chilling accumulation modulates the epigenome and transcriptome of Prunus avium during bud dormancy. Front. Plant Sci. 2020, 11, 1115. [Google Scholar] [CrossRef]
- Prudencio, Á.S.; Werner, O.; Martínez-García, P.J.; Dicenta, F.; Ros, R.M.; Martínez-Gómez, P. DNA methylation analysis of dormancy release in almond (Prunus dulcis) flower buds using pi-genotyping by sequencing. Int. J. Mol. Sci. 2018, 19, 3542. [Google Scholar] [CrossRef]
- Santamaría, M.E.; Hasbún, R.; Valera, M.J.; Meijón, M.; Valledor, L.; Rodríguez, J.L.; Toorop, P.E.; Cañal, M.J.; Rodríguez, R. Acetylated H4 histone and genomic DNA methylation patterns during bud set and bud burst in Castanea sativa. J. Plant Physiol. 2009, 166, 1360–1369. [Google Scholar] [CrossRef]
- Santamaría, M.E.; Rodríguez, R.; Cañal, M.J.; Toorop, P.E. Transcriptome analysis of chestnut (Castanea sativa) tree buds suggests a putative role for epigenetic control of bud dormancy. Ann. Bot. 2011, 108, 485–498. [Google Scholar] [CrossRef]
- Meijón, M.; Valledor, L.; Santamaría, M.E.; Testillano, P.S.; Risueño, C.; Rodríguez, R.; Feito, I.; Cañal, M.J. Epigenetic characterization of the vegetative and floral stages of azalea buds: Dynamics of DNA methylation and histone H4 acetylation. J. Plant Physiol. 2009, 166, 1624–1636. [Google Scholar] [CrossRef]
- Meijón, M.; Feito, I.; Valledor, L.; Rodríguez, R.; Cañal, M.J. Dynamics of DNA methylation and Histone H4 acetylation during floral bud differentiation in azalea. BMC Plant Biol. 2010, 10, 10. [Google Scholar] [CrossRef]
- Meijón, M.; Cañal, M.J.; Valledor, L.; Rodríguez, R.; Feito, I. Epigenetic and physiological effects of gibberellin inhibitors and chemical pruners on the floral transition of azalea. Physiol. Plant. 2011, 141, 276–288. [Google Scholar] [CrossRef]
- Fan, S.; Wang, J.; Lei, C.; Gao, C.; Yang, Y.; Li, Y.M.; An, N.; Zhang, D.; Han, M.Y. Identification and characterization of histone modification gene family reveal their critical responses to flower induction in apple. BMC Plant Biol. 2018, 18, 173. [Google Scholar] [CrossRef]
- Vimont, N.; Quah, F.X.; Schöepfer, D.G.; Roudier, F.; Dirlewanger, E.; Wigge, P.A.; Wenden, B.; Cortijo, S. ChIP-seq and RNA-seq for complex and low-abundance tree buds reveal chromatin and expression co-dynamics during sweet cherry bud dormancy. Tree Genet. Genomes 2020, 16, 9. [Google Scholar] [CrossRef]
- Saito, T.; Bai, S.L.; Imai, T.; Ito, A.; Nakajima, I.; Moriguchi, T. Histone modification and signalling cascade of the dormancy-associated MADS-box gene, PpMADS13-1, in Japanese pear (Pyrus pyrifolia) during endodormancy. Plant Cell Environ. 2015, 38, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Leida, C.; Conesa, A.; Llácer, G.; Badenes, M.L.; Ríos, G. Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytol. 2012, 193, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, P.Y.; Zhong, S.L.; Dardick, C.; Callahan, A.; An, Y.Q.; van Knocker, S.; Yang, Y.Z.; Zhong, G.Y.; Abbott, A.; et al. Thermal-responsive genetic and epigenetic regulation of DAM cluster controlling dormancy and chilling requirement in peach floral buds. Hortic. Res. 2020, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, L.; Conesa, A.; Lloret, A.; Badenes, M.L.; Ríos, G. Genome-wide changes in histone H3 lysine 27 trimethylation associated with bud dormancy release in peach. Tree Genet. Genomes 2015, 11, 45. [Google Scholar] [CrossRef]
- Horvath, D.P.; Sung, S.; Kim, D.; Chao, W.; Anderson, J. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Mol. Biol. 2010, 73, 169–179. [Google Scholar] [CrossRef]
- Wu, R.M.; Wang, T.C.; Richardson, A.C.; Allan, A.C.; Macknight, R.C.; Varkonyi-Gasic, E. Histone modification and activation by SOC1-like and drought stress-related transcription factors may regulate AcSVP2 expression during kiwifruit winter dormancy. Plant Sci. 2019, 281, 242–250. [Google Scholar] [CrossRef]
- Yordanov, Y.S.; Ma, C.; Strauss, S.H.; Busov, V.B. EARLY BUD-BREAK 1 (EBB1) is a regulator of release from seasonal dormancy in poplar trees. Proc. Natl. Acad. Sci. USA 2014, 111, 10001–10006. [Google Scholar] [CrossRef]
- Tuan, P.A.; Bai, S.L.; Saito, T.; Imai, T.; Ito, A.; Moriguchi, T. Involvement of EARLY BUD-BREAK, an AP2/ERF transcription factor gene, in bud break in Japanese pear (Pyrus pyrifolia Nakai) lateral flower buds: Expression, histone modifications and possible target genes. Plant Cell Physiol. 2016, 57, 1038–1047. [Google Scholar] [CrossRef]
- Agustí, A.; Mesejo, C.; Muñoz-Fambuena, N.; Vera-Sirera, F.; de Lucas, M.; Martínez-Fuentes, A.; Reig, C.; Iglesias, D.J.; Primo-Millo, E.; Blázquez, M.A. Fruit-dependent epigenetic regulation of flowering in Citrus. New Phytol. 2020, 225, 376–384. [Google Scholar] [CrossRef]
- Fu, X.L.; Xiao, W.; Wang, D.L.; Chen, M.; Tan, Q.P.; Li, L.; Chen, X.D.; Gao, D.S. Roles of endoplasmic reticulum stress and unfolded protein response associated genes in seed stratification and bud endodormancy during chilling accumulation in Prunus persica. PLoS ONE 2014, 9, e101808. [Google Scholar] [CrossRef]
- Song, Z.T.; Sun, L.; Lu, S.J.; Tian, Y.K.; Ding, Y.; Liu, J.X. Transcription factor interaction with COMPASS-like complex regulates histone H3K4 trimethylation for specific gene expression in plants. Proc. Natl. Acad. Sci. USA 2015, 112, 2900–2905. [Google Scholar] [CrossRef]
- Liao, X.L.; Li, Y.; Hu, Z.Z.; Lin, Y.; Zheng, B.; Ding, J.H. Poplar acetylome profiling reveals lysine acetylation dynamics in seasonal bud dormancy release. Plant Cell Environ. 2021, 44, 1830–1845. [Google Scholar] [CrossRef]
- Aregawi, K.; Shen, J.Q.; Pierroz, G.; Sharma, M.K.; Dahlberg, J.; Owiti, J.; Lemaux, P.G. Morphogene-assisted transformation of Sorghum bicolor allows more efficient genome editing. Plant Biotechnol. J. 2021, 20, 748–760. [Google Scholar] [CrossRef]
- Ellison, E.E.; Nagalakshmi, U.; Gamo, M.E.; Huang, P.J.; Dinesh-Kumar, S.; Voytas, D.F. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants 2020, 6, 620–624. [Google Scholar] [CrossRef]
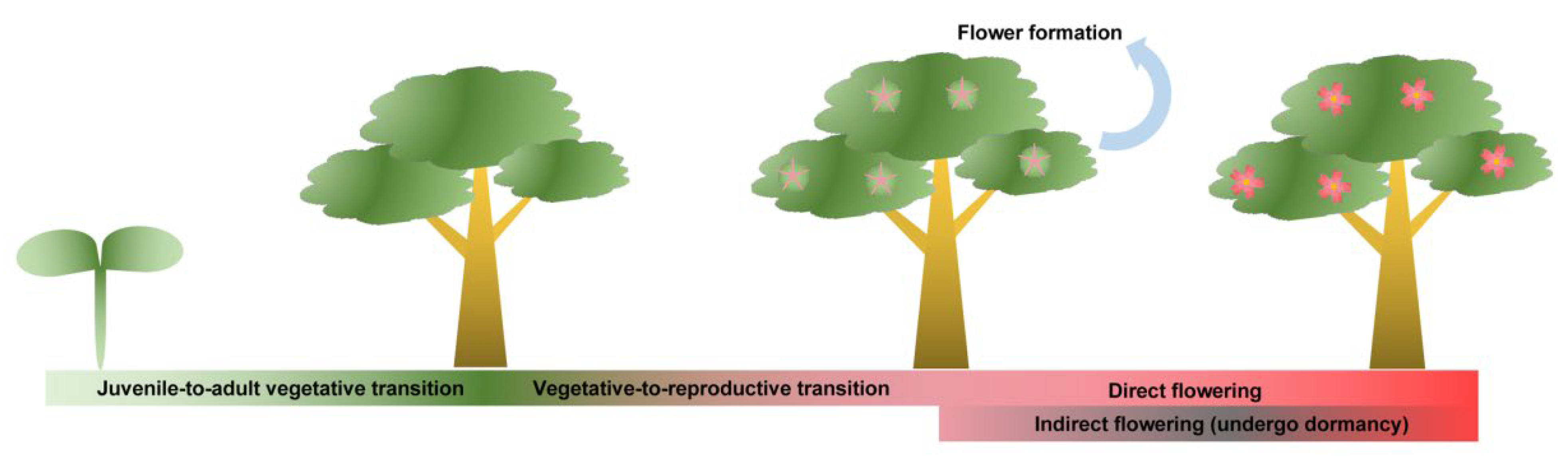
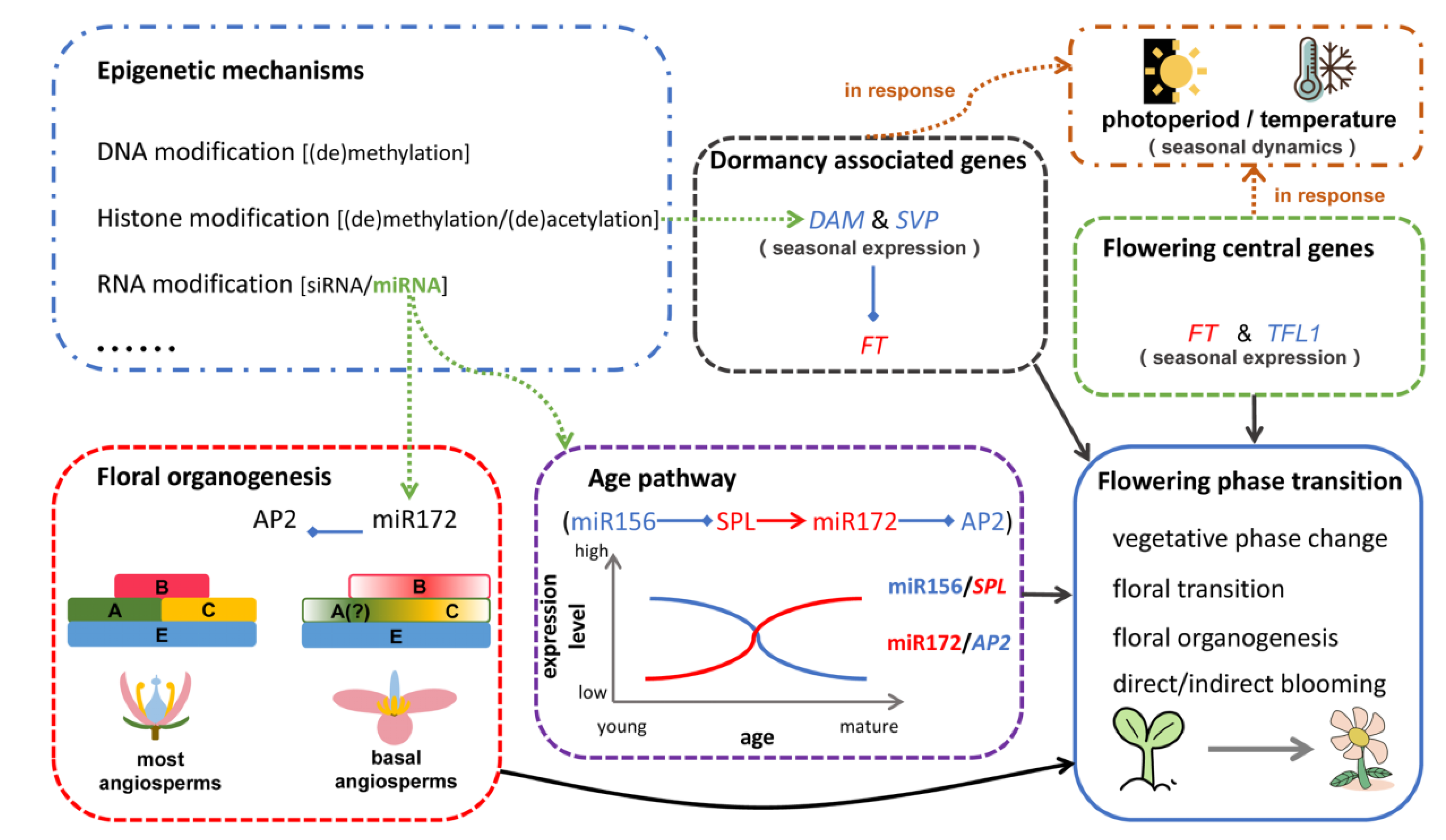
| Tree Species | Juvenile-to-Adult Vegetative Transition | Reference |
|---|---|---|
| Malus × domestica | 4–8 years | [15] |
| Camellia chrysantha | 6–8 years | [16] |
| Citrus spp. | 6–10 years | [17] |
| Eucalyptus globulus ssp. globulusis | 1–5 years | [18] |
| Jatropha curcas | 5 years in subtropical areas | [19] |
| Liriodendron Chinense | 8–10 years | [20] |
| Populus deltoides var. deltoides | 5–10 years | [21] |
| poplar T89 (Populus tremula L. × P. tremuloides) | 8–20 years | [22] |
| Rosa rugosa ‘Bao White’ | >3 years | [23] |
| Species | Technique | Regulators | Phase Transition | References |
|---|---|---|---|---|
| Actinidia chinensis | CRISPR/Cas9 | CEN *, CEN4 * | Juvenile–adult phase transition | [35] |
| Citrus sinensis | qRT-PCR | FT | Vegetative–reproductive phase transition | [39] |
| Citrus unshiu | qRT-PCR | FT | Vegetative–reproductive phase transition | [38] |
| Cocos nucifera | RNA-seq | FT | Juvenile–adult phase transition | [29] |
| Cornus spp. | Over-expression | TFL1 * | Juvenile–adult phase transition | [36] |
| Eucalyptus globulus | qRT-PCR | FT | Vegetative–reproductive phase transition | [14] |
| Fragaria vesca | Phenotyping | KSN | Vegetative–reproductive phase transition | [37] |
| Jatropha curcas | RNAi | FT * | Flowering transition | [27] |
| Juglans regia | RNA-seq | FT | Juvenile–adult phase transition | [25] |
| Liriodendron Chinense | RNA-seq | FT | Juvenile–adult phase transition; Perpetual flowering | [20] |
| Malus × domestica | Antisense expression | FT1 * | Juvenile–adult phase transition | [30] |
| VIGS | TFL1 * | Juvenile–adult phase transition | [33] | |
| qRT-PCR; Over-expression | TFL1-1 *, TFL1-2 * | Vegetative–reproductive phase transition | [41] | |
| Malus × domestica ‘Pinova’ | Over-expression; | FT1 * | Juvenile–adult phase transition | [28] |
| Antisense expression | FT1 * | Juvenile–adult phase transition; Vegetative–reproductive phase transition | [31] | |
| Paeonia suffruticosa | RNA-seq; qRT-PCR | FT | Reblooming | [43] |
| Populus deltoides | RT-PCR; Over-expression | FT2 * | Juvenile–adult phase transition; Vegetative–reproductive phase transition | [26] |
| Populus simonii | Over-expression | FT1*, FT2 * | Juvenile–adult phase transition | [22] |
| Populus trichocarpa | qRT-PCR; RNAi; Over-expression | CEN1 * | Juvenile–adult phase transition | [34] |
| Pyrus communis | RNAi | TFL1-1 *, TFL1-2 * | Juvenile–adult phase transition; Vegetative–reproductive phase transition | [32] |
| Pyrus pyrifolia | qRT-PCR | FT1; TFL1 | Vegetative–reproductive phase transition | [40] |
| Rosa spp. | (q)RT-PCR; Phenotyping | KSN | Vegetative–reproductive phase transition | [37] |
| Rosa hybrida | (q)RT-PCR | FT | Vegetative–reproductive phase transition | [12] |
| Vaccinium corymbosum | Over-expression | FT * | Perpetual flowering | [42] |
| Ziziphus jujuba | (q)RT-PCR | FT | Direct flowering | [11] |
| Species | Epigenetic Modification | Modified Site | Developmental Transition | Reference |
|---|---|---|---|---|
| Actinidia chinensis | H3K4me3; H3ac | AcSVP2 | Dormancy release | [140] |
| Rhododendron spp. | DNA methylation; H4ac | — | Floral transition | [130,131,132] |
| Castanea sativa | DNA methylation; Histone modification; H4ac | — | Bud dormancy | [128,129] |
| Citrus (‘Moncada’ mandarin) | H3K4me3 | CcMADS19 locus | Floral induction | [143] |
| Euphorbia esula | H3K4me3; H3K27me3 | DAM1 | Endodormancy release | [139] |
| Malus ×domestica | DNA methylation | — | Dormancy release | [119] |
| DNA methylation | SOC1, AP1, SPLs, etc. | Floral transition | [121] | |
| Histone methylation/acetylation | — | Flower induction | [133] | |
| Paeonia suffruticosa | DNA methylation | PsFT | Flowering | [124] |
| Paeonia suffruticosa ‘Luhehong’ | DNA methylation | — | Dormancy release | [120] |
| Populus tremula × Populus alba | Lysine acetylation | Metabolic enzymes | Dormancy release | [146] |
| Prunus avium | DNA methylation; siRNAs | PavMADS1, PavMADS2 | Bud dormancy and flowering | [125] |
| DNA methylation | — | Endodormancy | [126] | |
| H3K4me3 | PavDAM5 | Bud dormancy | [134] | |
| Prunus dulcis | DNA methylation | — | Dormancy release | [127] |
| Prunus persica | H3K4me3; H3K27me3; H3ac | DAM6 | Dormancy release | [136] |
| H3K27me3 | DAM1/4/5/6 | Bud dormancy release | [138] | |
| siRNAs; H3K27me3; CHH methylation | DAMs | Dormancy | [137] | |
| Endoplasmic reticulum stress; unfolded protein response | — | Endodormancy | [144] | |
| Pyrus pyrifolia ‘Kosui’ | H3K4me3 | PpMADS13-1 locus | Endodormancy phase transition | [135] |
| H3K4me3 | PpEBB | Bud break and flowering | [142] | |
| Salix viminalis | DNA methylation | — | Floral transition | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Nie, T.; Chen, Y.; Yin, Z. From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants. Int. J. Mol. Sci. 2022, 23, 10959. https://doi.org/10.3390/ijms231810959
Sun L, Nie T, Chen Y, Yin Z. From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants. International Journal of Molecular Sciences. 2022; 23(18):10959. https://doi.org/10.3390/ijms231810959
Chicago/Turabian StyleSun, Liyong, Tangjie Nie, Yao Chen, and Zengfang Yin. 2022. "From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants" International Journal of Molecular Sciences 23, no. 18: 10959. https://doi.org/10.3390/ijms231810959
APA StyleSun, L., Nie, T., Chen, Y., & Yin, Z. (2022). From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants. International Journal of Molecular Sciences, 23(18), 10959. https://doi.org/10.3390/ijms231810959






