Sensorineural Hearing Loss in Sjögren’s Syndrome
Abstract
:1. Pathology of Sjögren’s Syndrome (SS)
2. Clinical Manifestation of Sjögren’s Syndrome
2.1. Prevalence of Sjögren’s Syndrome
2.2. Characteristic Symptoms and the Diagnosis of Sjögren’s Syndrome
3. Autoimmune Inner Ear Disease (AIED)
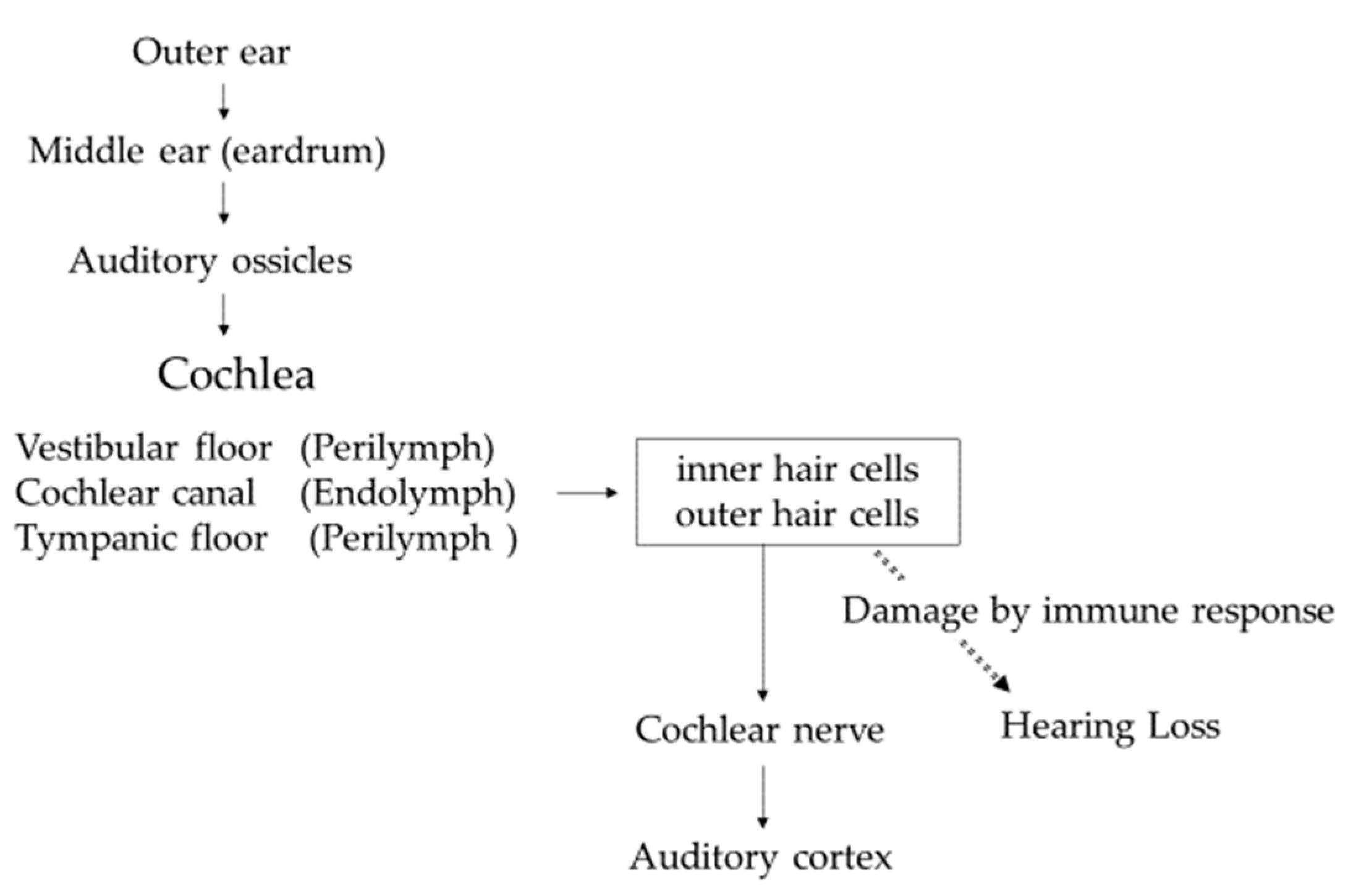
4. Histoanatomical Features of the Inner Ear [34]
5. Immunological Characteristics in the Inner Ear
6. Pathophysiology of Autoimmune Inner Ear Disease
- (a)
- Contribution of systemic circulating antibodies against inner ear antigens leading to antibody-dependent cell-mediated cytotoxicity with the activation of the complement system [40]. For example, IgG4-related disease caused inner ear involvement with cochlear ossification, and the presence of inner ear antibody was previously reported as the cause of inner ear dysfunction [41].
- (b)
- FcR-mediated activation of inflammatory reaction by immune complex deposition [42]. The immune complex deposition led to vasculitis of the inner ear vessels and determined atrophy of the stria vascularis. In a mouse model (by Iwai), abnormal helper T lymphocytes induced autoantibody production from B lymphocytes, and the resulting antigen–antibody complexes deposited in cochlear striae, causing hearing loss [43]. It is suspected that deposition of immune complexes reduced the blood flow in caliber of the auditory arteries and induced damage of the hair cells or the spiral ganglion by reactive oxygen species (ROS).
- (c)
- (d)
7. Treatment for Sensorineural Hearing Loss in Sjögren’s Syndrome
8. Future Direction
9. Strengths and Limitations of the Review
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Corazzi, V.; Bianchini, C.; Aimoni, C.; Pelucchi, S.; Skarzynski, P.H.; Hatzopoulos, S. Autoimmune inner ear disease (AIED): A diagnostic challenge. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418808680. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Acar-Denizli, N.; Zeher, M.; Rasmussen, A.; Seror, R.; Theander, E.; Li, X.; Baldini, C.; Gottenberg, J.-E.; Danda, D.; et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjögren’s syndrome at diagnosis in 8310 patients: A cross-sectional study from the Big Data Sjögren Project Consortium. Ann. Rheum. Dis. 2016, 76, 1042–1050. [Google Scholar] [CrossRef]
- Ba, N.K.B.; Varadarajan, V.V.; Sobel, E.S.; Haberman, R.S. Autoimmune inner ear disease: A systematic review of management. Laryngoscope 2020, 5, 1217–1226. [Google Scholar] [CrossRef]
- Loveman, D.M.; de Comarmond, C.; Cepero, R.; Baldwin, D.M. Autoimmune sensorineural hearing loss: Clinical course and treatment outcome. Semin. Arthritis Rheum. 2004, 34, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Bovo, R.; Aimoni, C.; Martini, A. Immune-mediated inner ear disease. Acta Oto-Laryngol. 2006, 126, 1012–1021. [Google Scholar] [CrossRef]
- Ralli, M.; D’Aguanno, V.; Di Stadio, A.; De Virgilio, A.; Croce, A.; Longo, L.; Greco, A.; De Vincentiis, M. Audiovestibular Symptoms in Systemic Autoimmune Diseases. J. Immunol. Res. 2018, 2018, 5798103. [Google Scholar] [CrossRef]
- Di Stadio, A.; Ralli, M. Systemic Lupus Erythematosus and hearing disorders: Literature review and meta-analysis of clinical and temporal bone findings. J. Int. Med. Res. 2017, 45, 1470–1480. [Google Scholar] [CrossRef]
- Abbasi, M.; Yazdi, Z.; Kazemifar, A.M.; Bakhsh, Z.Z. Hearing Loss in Patients with Systemic Lupus Erythematosus. Glob. J. Health Sci. 2013, 5, 102–106. [Google Scholar] [CrossRef]
- Roverano, S.; Cassano, G.; Paira, S.; Chiavarini, J.; Graf, C.; Rico, L.; Heredia, C. Asymptomatic Sensorineural Hearing Loss in Patients With Systemic Lupus Erythematosus. J. Clin. Rheumatol. 2006, 12, 217–220. [Google Scholar] [CrossRef]
- Lin, C.; Lin, S.-W.; Weng, S.-F.; Lin, Y.-S. Risk of Sudden Sensorineural Hearing Loss in Patients with Systemic Lupus Erythematosus: A Population-Based Cohort Study. Audiol. Neurotol. 2012, 18, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Maciaszczyk, K.; Durko, T.; Waszczykowska, E.; Erkiert-Polguj, A.; Pajor, A. Auditory function in patients with systemic lupus erythematosus. Auris Nasus Larynx 2011, 38, 26–32. [Google Scholar] [CrossRef] [PubMed]
- A Khalidi, N.; Rebello, R.; Robertson, D.D. Sensorineural hearing loss in systemic lupus erythematosus: Case report and literature review. J. Laryngol. Otol. 2008, 122, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Sperling, N.M.; Tehrani, K.; Liebling, A.; Ginzler, E. Aural Symptoms and Hearing Loss in Patients with Lupus. Otolaryngol. Neck Surg. 1998, 118, 762–765. [Google Scholar] [CrossRef]
- Dayal, V.S.; Ellman, M.H. Sensorineural hearing loss and lupus. Am. J. Otol. 1999, 26, 2065. [Google Scholar]
- Gomides, A.; Rosário, E.D.; Borges, H.; Gomides, H.; De Pádua, P.; Sampaio-Barros, P. Sensorineural dysacusis in patients with systemic lupus erythematosus. Lupus 2007, 16, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Karatas, E.; Onat, A.M.; Durucu, C.; Baglam, T.; Kanlikama, M.; Altunoren, O.; Buyukhatipoglu, H.; Kanlıkama, M. Audiovestibular disturbance in patients with systemic lupus erythematosus. Otolaryngol. Neck Surg. 2007, 136, 82–86. [Google Scholar] [CrossRef]
- Batuecas-Caletrío, A.; Del Pino-Montes, J.; Cordero-Civantos, C.; I Calle-Cabanillas, M.; Lopez-Escamez, J.A. Hearing and vestibular disorders in patients with systemic lupus erythematosus. Lupus 2013, 22, 437–442. [Google Scholar] [CrossRef]
- Gad, G.I.; Abdelateef, H. Function of the Audiovestibular System in Children with Systemic Lupus Erythematosus. Curr. Allergy Asthma Rep. 2014, 14, 446. [Google Scholar] [CrossRef]
- Compadretti, G.C.; Brandolini, C.; Tasca, I. Sudden Sensorineural Hearing Loss in Lupus Erythematosus Associated with Antiphospholipid Syndrome: Case Report and Review. Ann. Otol. Rhinol. Laryngol. 2005, 114, 214–218. [Google Scholar] [CrossRef]
- Cecatto, S.B.; Garcia, R.I.D.; Costa, K.S.; Anti, S.M.A.; Longone, E.; Rapoport, P.B. Perda auditiva sensorioneural no lúpus eritematoso sistêmico: Relato de três casos. Rev. Bras. Otorrinolaringol. 2004, 70, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Hisashi, K.; Komune, S.; Taira, T.; Uemura, T.; Sadoshima, S.; Tsuda, H. Anticardiolipin antibody-induced sudden profound sensorineural hearing loss. Am. J. Otolaryngol. 1993, 14, 275–277. [Google Scholar] [CrossRef]
- Raut, V.V.; Cullen, J.; Cathers, G. Hearing Loss in Rheumatoid Arthritis. J. Otolaryngol. 2001, 30, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, M.; Higaki, M.; Kinoshita, H.; Mizushima, Y.; Koizuka, I. Ear Involvement in Patients with Rheumatoid Arthritis. Otol. Neurotol. 2005, 26, 755–761. [Google Scholar] [CrossRef]
- Murdin, L.; Patel, S.; Walmsley, J.; Yeoh, L.H. Hearing difficulties are common in patients with rheumatoid arthritis. Clin. Rheumatol. 2007, 27, 637–640. [Google Scholar] [CrossRef]
- Rosenberg, J.N.; A Moffat, D.; Ramsden, R.T.; Gibson, W.P.; Booth, J.B. Middle ear function in rheumatoid arthritis. Ann. Rheum. Dis. 1978, 37, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Quatraro, C.; Silvestris, F. Sjögren’s syndrome: An autoimmune disorder with otoLaryngol.l involvement. Acta Otorhinolaryngol. Ital. 2005, 25, 139–144. [Google Scholar]
- Tumiati, B.; Casoli, P.; Parmeggiani, A. Hearing Loss in the Sjogren Syndrome. Ann. Intern. Med. 1997, 126, 450. [Google Scholar] [CrossRef]
- Ziavra, N.; Politi, E.N.; Kastanioudakis, I.; Skevas, A.; Drosos, A.A. Hearing loss in Sjögren’s syndrome patients. A comparative study. Clin. Exp. Rheumatol. 2000, 18, 725–728. [Google Scholar]
- Galarza-Delgado, D.A.; Gonzalez, M.J.V.; Torres, J.R.; Soto-Galindo, G.A.; Flores, L.M.; González, J.L.T. Early hearing loss detection in rheumatoid arthritis and primary Sjögren syndrome using extended high frequency audiometry. Clin. Rheumatol. 2017, 37, 367–373. [Google Scholar] [CrossRef]
- González, J.L.T.; Torres, J.R.; Ríos, Y.H.; González, M.J.V.; Saenz, M.A.M.; Soto-Galindo, G.A. Extended high-frequency audiometry as early detection of hearing loss in primary Sjögren syndrome. Clin. Rheumatol. 2017, 36, 2237–2241. [Google Scholar] [CrossRef]
- Gündüz, B.; Yildirim, N.; Güven, S.C.; Orhan, E.; Karamert, R.; Günendi, Z. Evaluation of Medial Olivocochlear Efferent System and Hearing Loss in Patients with Primary Sjögren’s Syndrome. Turk. J. Med Sci. 2019, 49, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, M.L.; Villarreal, I.M.; Moya, J.L.; García-Berrocal, J.R. Extended high frequency audiometry can diagnose sub-clinic involvement in a seemingly normal hearing systemic lupus erythematosus population. Acta Oto-Laryngol. 2016, 137, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hara, A. Actual Hearing Test, 4th ed.; Nanzando: Tokyo, Japan, 2017. (In Japanese) [Google Scholar]
- Wang, J.; Puel, J.-L. Presbycusis: An Update on Cochlear Mechanisms and Therapies. J. Clin. Med. 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, A.J.; Harris, J.P. Autoimmune Inner Ear Disease: A Retrospective Review of Forty-Seven Patients. Audiol. Neurotol. 2013, 18, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Mogi, G.; Lim, D.J.; Watanabe, N. Immunologic Study on the Inner Ear: Immunoglobulins in Perilymph. Arch. Otolaryngol. Head Neck Surg. 1982, 108, 270–275. [Google Scholar] [CrossRef]
- Okano, T. Immune system and resident macrophages in the inner ear. J. Jpn. Soc. Immunol. Allergol. Otolaryngol. 2018, 36, 223–238. (In Japanese) [Google Scholar] [CrossRef]
- Kariya, S.; Hizli, Ö.; Kaya, S.; Hizli, P.; Nishizaki, K.; Paparella, M.M.; Cureoglu, S. Histopathologic Findings in Peripheral Vestibular System from Patients with Systemic Lupus Erythematosus. Otol. Neurotol. 2015, 36, 1702–1707. [Google Scholar] [CrossRef]
- Greco, A.; Fusconi, M.; Gallo, A.; Marinelli, C.; Macri, G.; De Vincentiis, M. Sudden sensorineural hearing loss: An autoimmune disease? Autoimmun. Rev. 2011, 10, 756–761. [Google Scholar] [CrossRef]
- Tomiyama, S.; Harris, J.P. Elevation of Inner Ear Antibody Levels Following Direct Antigen Challenge of the Endolymphatic Sac. Acta Oto-Laryngol. 1989, 107, 202–209. [Google Scholar] [CrossRef]
- Rossini, B.A.A.; Penido, N.D.O.; Munhoz, M.S.L.; Bogaz, E.A.; Curi, R.S. Sudden Sensorioneural Hearing Loss and Autoimmune Systemic Diseases. Int. Arch. Otorhinolaryngol. 2016, 21, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Iwai, H. Prevention and treatment of progressive cochlear disorders by modifying systemic immune function-Examination by mouse model. Oto-Rhino-Laryngol. Tokyo 2018, 61, 12–23. (In Japanese) [Google Scholar]
- Wagner, W.; Fehrmann, A. Association of retinal vasculitis (Eales’ disease) and Meniere-like vestibulocochlear symptoms. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2005, 263, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef]
- Liu, W.; Danckwardt-Lillieström, N.; Schrott-Fischer, A.; Glueckert, R.; Rask-Andersen, H. Distribution of Immune Cells Including Macrophages in the Human Cochlea. Front. Neurol. 2021, 12, 781702. [Google Scholar] [CrossRef]
- Vambutas, A.; Pathak, S. AAO: Autoimmune and Autoinflammatory (Disease) in Otology: What is New in Immune-Mediated Hearing Loss. Laryngoscope Investig. Otolaryngol. 2016, 1, 110–115. [Google Scholar] [CrossRef]
- Sakano, H.; Harris, J.P. Emerging options in immune-mediated hearing loss. Laryngoscope 2018, 4, 102–108. [Google Scholar] [CrossRef]
- Green, L.; Miller, E.B. Sudden sensorineural hearing loss as a first manifestation of systemic lupus erythematosus: Association with anticardiolipin antibodies. Clin. Rheumatol. 2001, 20, 220–222. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Rubin, R.; Sataloff, R.T. Treatment-Refractory Autoimmune Sensorineural Hearing Loss: Response to Infliximab. Ear Nose Throat J. 2011, 90, 23–28. [Google Scholar] [CrossRef]
- Luetje, C.M. Theoretical and Practical Implications for Plasmapheresis in Autoimmune Inner Ear Disease. Laryngoscope 1989, 99, 1137–1146. [Google Scholar] [CrossRef]
- Almeida, R.S.; Oliveira, A.A.; Pego, P.M.; Abuowda, Y.; Gaspar, I.; Costa, J.M. Sensorineural hearing loss as the first manifestation of Sjögren’s syndrome. Rev. Assoc. Médica Bras. 2017, 63, 7–9. [Google Scholar] [CrossRef]
- Kadosono, O.; Saigusa, H.; Kusama, K.; Hiroyuki, I. A case of bilateral posterior labyrinthine deafness associated with Sjogren’s syndrome. Audiol. Jpn. 2016, 59, 375–376. (In Japanese) [Google Scholar]
- Kim, K.-S.; Kim, H.-S. Successful treatment of sensorineural hearing loss in Sjögren’s syndrome with corticosteroid. Korean J. Intern. Med. 2016, 31, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Okawa, Y.; Okanari, K.; Hirano, N.; Kawano, T.; Nishio, S.; Usami, S.; Maeda, T.; Ihara, K. Unilateral Sensorineural Hearing Loss in Children Associated with Sjögren’s Syndrome. Cureus 2021, 13, e18832. [Google Scholar] [CrossRef] [PubMed]
- Keiichi, S.; Shiro, M.; Tetsuya, K. McH-lpr/lpr-RA1 mice: A novel spontaneous mouse model of autoimmune sialadenitis. Immunol. Lett. 2021, 237, 3–10. [Google Scholar] [CrossRef]
| Reference [No.] | Disease | Number (Female/Male) | SNHL (%) | Frequency Band of SNHL |
|---|---|---|---|---|
| Tumiati B. et al. [28] | SS | 30 (30/0) | 46% | 2000–8000 Hz (12 patients) |
| Ziavra N. et al. [29] | SS | 45 (45/0) | 22.5% | 3000–8000 Hz (8 patients) |
| Galarza-Delgado DA. et al. [30] | SS | 60 (60/0) | 60% 70% 100% | 500–3000 Hz 4000–8000 Hz 10,000–16,000 Hz |
| González JLT. et al. [31] | SS | 63 (60/3) | 95.2% | 10,000–16,000 Hz |
| Gündüz B. et al. [32] | SS | 36 (36/0) | 52.77% | 9000–12,500 Hz |
| Galarza-Delgado DA. et al. [30] | RA | 117 (117/0) | 36.8% 68.4% 94.9% | 500–3000 Hz 4000–8000 Hz 10,000–16,000 Hz |
| Roverano S. et al. [10] | SLE | 30 (30/0) | 40% 13.3% 13.3% | 2000–4000 Hz 4000–8000 Hz 2000–8000 Hz |
| Lasso de la Vega M. et al. [33] | SLE | 77 (67/10) | 70% | 8000–18,000 Hz |
| Reference [No.] | Age (y)/Sex | Bilateral or Unilateral | Frequency Band (dB) | Treatment (Dose) | Effectiveness |
|---|---|---|---|---|---|
| Almeida RS. et al. [52] | 65/Female | Unilateral | 8000 Hz (70 dB) | PSL (1 mg/kg/day) | Effective, with recurrence |
| Kadosono O. et al. [53] | 46/Female | Bilateral | ND | mPSL (500 mg/day for 3 days) | Effective |
| Kim KS. et al. [54] | 62/Female | Unilateral | ND | mPSL (250 mg/day for 5 days) | Effective |
| Okawa Y. et al. [55] | 8/Female | Unilateral | Deaf | PSL (1 mg/kg/day for 10 days, tapered off for 4 days) | Not effective |
| Okawa Y. et al. [55] | 8/Male | Unilateral | Deaf | IVIG (1 g/kg) and PSL (1 mg/kg/day for 10 days, tapered off for 8 days) | Not effective |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawa, Y.; Ihara, K. Sensorineural Hearing Loss in Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 11181. https://doi.org/10.3390/ijms231911181
Okawa Y, Ihara K. Sensorineural Hearing Loss in Sjögren’s Syndrome. International Journal of Molecular Sciences. 2022; 23(19):11181. https://doi.org/10.3390/ijms231911181
Chicago/Turabian StyleOkawa, Yuko, and Kenji Ihara. 2022. "Sensorineural Hearing Loss in Sjögren’s Syndrome" International Journal of Molecular Sciences 23, no. 19: 11181. https://doi.org/10.3390/ijms231911181
APA StyleOkawa, Y., & Ihara, K. (2022). Sensorineural Hearing Loss in Sjögren’s Syndrome. International Journal of Molecular Sciences, 23(19), 11181. https://doi.org/10.3390/ijms231911181





