Defining the Role of Monocytes in Sjögren’s Syndrome
Abstract
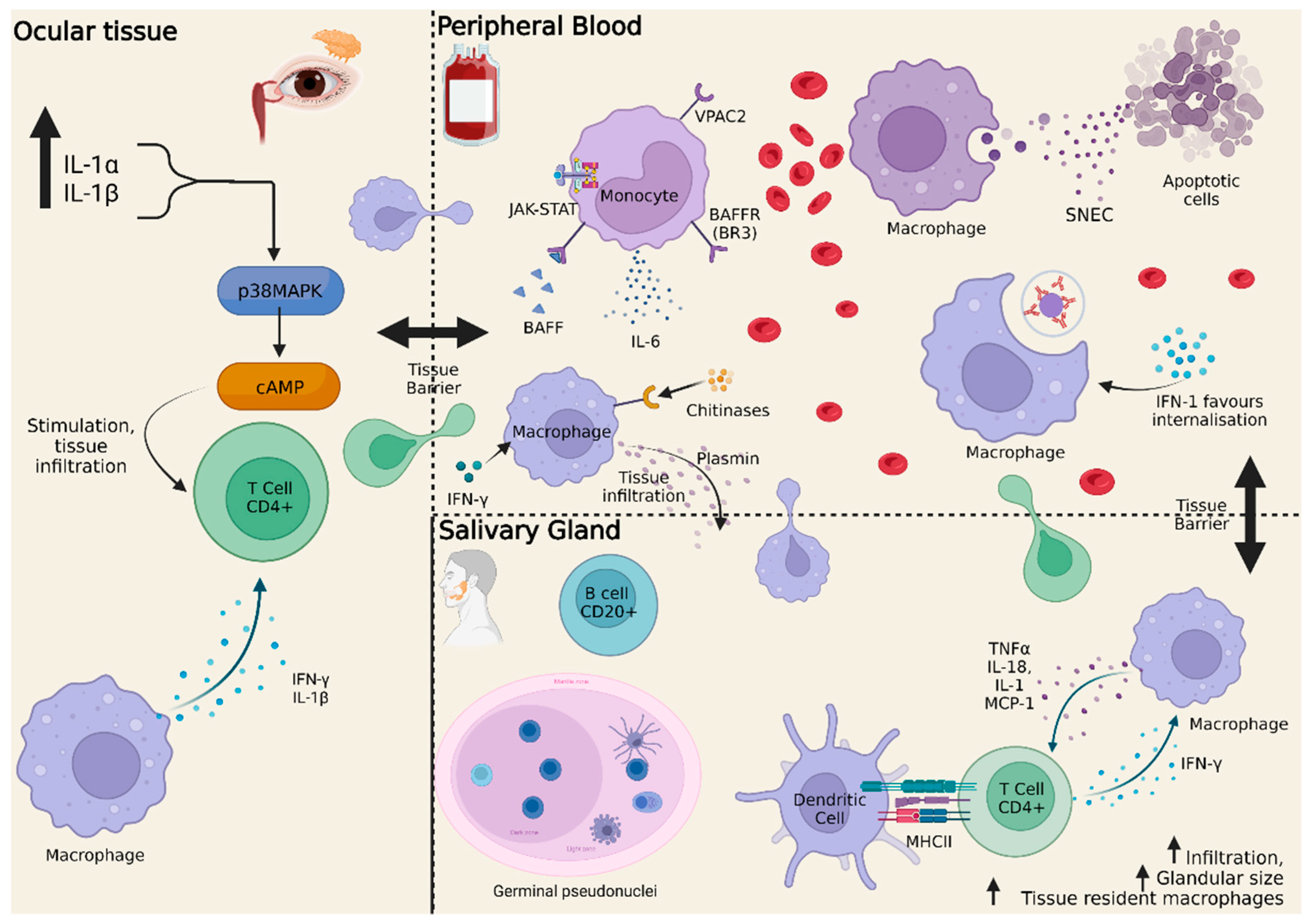
:1. Introduction
2. Monocytes
2.1. Classical (CD14++, CD16−)
2.2. Nonclassical (CD14−, CD16++)
2.3. Intermediate (CD14+, CD16+)
3. Monocyte Activation
4. Interferon Signature in Sjögren’s Disease
5. Monocytes in Sjögren’s Syndrome
5.1. Transcriptome Findings
5.2. Epigenetic Findings
6. Current and Future Treatments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chatzis, L.G.; Goules, A.V.; Tzioufas, A.G. Searching for the “X Factor” in Sjögren’s Syndrome Female Predilection. Clin. Exp. Rheumatol. 2021, 39, S206–S214. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Tzioufas, A.G.; Font, J. Primary Sjögren’s Syndrome: New Clinical and Therapeutic Concepts. Ann. Rheum. Dis. 2005, 64, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s Syndrome: A Systemic Autoimmune Disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, G.; Bursi, R.; Chatzis, L.G.; Fulvio, G.; Ferro, F.; Bartoloni, E.; Baldini, C. One Year in Review 2021: Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2021, 39, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, M.; Korpela, M.; Uusitalo, H.; Pukander, J.; Miettinen, A.; Helin, H.; Pasternack, A. Clinical Follow up Study of 87 Patients with Sicca Symptoms (Dryness of Eyes or Mouth, or Both). Ann. Rheum. Dis. 1999, 58, 423–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retamozo, S.; Acar-Denizli, N.; Rasmussen, A.; Horváth, I.F.; Baldini, C.; Priori, R.; Sandhya, P.; Hernandez-Molina, G.; Armagan, B.; Praprotnik, S.; et al. Systemic Manifestations of Primary Sjögren’s Syndrome out of the ESSDAI Classification: Prevalence and Clinical Relevance in a Large International, Multi-Ethnic Cohort of Patients. Clin. Exp. Rheumatol. 2019, 37, 97–106. [Google Scholar]
- Veenbergen, S.; Kozmar, A.; van Daele, P.L.A.; Schreurs, M.W.J. Autoantibodies in Sjögren’s Syndrome and Its Classification Criteria. J. Transl. Autoimmun. 2022, 5, 100138. [Google Scholar] [CrossRef]
- Kontny, E.; Lewandowska-Poluch, A.; Chmielińska, M.; Olesińska, M. Subgroups of Sjögren’s Syndrome Patients Categorised by Serological Profiles: Clinical and Immunological Characteristics. Reumatologia 2018, 56, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Braconi, D.; Bernardini, G.; Spiga, O.; Santucci, A. Leveraging Proteomics in Orphan Disease Research: Pitfalls and Potential. Expert Rev. Proteom. 2021, 18, 315–327. [Google Scholar] [CrossRef]
- Aronson, J.K. Rare Diseases and Orphan Drugs. Br. J. Clin. Pharm. 2006, 61, 243–245. [Google Scholar] [CrossRef]
- Nocturne, G.; Mariette, X. B Cells in the Pathogenesis of Primary Sjögren Syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T Cells in Primary Sjögren’s Syndrome: Targets for Early Intervention. Rheumatology 2019, 60, 3088–3098. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.-T.; Gao, F.; Gu, K.; Chen, D.-K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldini, C.; Ferro, F.; Elefante, E.; Bombardieri, S. Biomarkers for Sjögren’s Syndrome. Biomark. Med. 2018, 12, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Osca-Verdegal, R.; Pallardó, F.V.; García-Giménez, J.L. Epigenetic Regulation in the Pathogenesis of Sjögren Syndrome and Rheumatoid Arthritis. Front. Genet. 2019, 10, 1104. [Google Scholar] [CrossRef] [PubMed]
- Kapsogeorgou, E.K.; Papageorgiou, A.; Protogerou, A.D.; Voulgarelis, M.; Tzioufas, A.G. Low MiR200b-5p Levels in Minor Salivary Glands: A Novel Molecular Marker Predicting Lymphoma Development in Patients with Sjögren’s Syndrome. Ann. Rheum. Dis. 2018, 77, 1200–1207. [Google Scholar] [CrossRef]
- Wolf, A.A.; Yáñez, A.; Barman, P.K.; Goodridge, H.S. The Ontogeny of Monocyte Subsets. Front. Immunol. 2019, 10, 1642. [Google Scholar] [CrossRef] [Green Version]
- Orozco, S.L.; Canny, S.P.; Hamerman, J.A. Signals Governing Monocyte Differentiation during Inflammation. Curr. Opin. Immunol. 2021, 73, 16–24. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef] [Green Version]
- Canè, S.; Ugel, S.; Trovato, R.; Marigo, I.; de Sanctis, F.; Sartoris, S.; Bronte, V. The Endless Saga of Monocyte Diversity. Front. Immunol. 2019, 10, 1786. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.D.; Hamers, A.A.J.; Nakao, C.; Marcovecchio, P.; Taylor, A.M.; McSkimming, C.; Nguyen, A.T.; McNamara, C.A.; Hedrick, C.C. Human Blood Monocyte Subsets: A New Gating Strategy Defined Using Cell Surface Markers Identified by Mass Cytometry. Arter. Thromb. Vasc. Biol. 2017, 37, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Chevrot, M.; Poirier, H.; Passilly-Degrace, P.; Niot, I.; Besnard, P. CD36 as a Lipid Sensor. Physiol. Behav. 2011, 105, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, M.A. Structure and Function of the Leukocyte Adhesion Molecules CD11/CD18. Blood 1990, 75, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.D.; Rodriguez, P.C.; Koehn, B.H.; Baban, B.; Cui, Y.; Guo, G.; Shimoda, M.; Pacholczyk, R.; Shi, H.; Lee, E.-J.; et al. Activation of P53 in Immature Myeloid Precursor Cells Controls Differentiation into Ly6c+CD103+ Monocytic Antigen-Presenting Cells in Tumors. Immunity 2018, 48, 91–106.e6. [Google Scholar] [CrossRef] [Green Version]
- Askenase, M.H.; Han, S.-J.; Byrd, A.L.; Morais da Fonseca, D.; Bouladoux, N.; Wilhelm, C.; Konkel, J.E.; Hand, T.W.; Lacerda-Queiroz, N.; Su, X.; et al. Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity 2015, 42, 1130–1142. [Google Scholar] [CrossRef] [Green Version]
- Coillard, A.; Segura, E. Antigen Presentation by Mouse Monocyte-Derived Cells: Re-Evaluating the Concept of Monocyte-Derived Dendritic Cells. Mol. Immunol. 2021, 135, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Nakagawa, K.; Sugihara, F.; Kuwahara, R.; Ashihara, M.; Yamane, F.; Minowa, Y.; Fukushima, K.; Ebina, I.; Yoshioka, Y.; et al. Identification of an Atypical Monocyte and Committed Progenitor Involved in Fibrosis. Nature 2017, 541, 96–101. [Google Scholar] [CrossRef]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical Patrolling Monocyte Function in the Vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Gamrekelashvili, J.; Kapanadze, T.; Sablotny, S.; Ratiu, C.; Dastagir, K.; Lochner, M.; Karbach, S.; Wenzel, P.; Sitnow, A.; Fleig, S.; et al. Notch and TLR Signaling Coordinate Monocyte Cell Fate and Inflammation. Elife 2020, 9, e57007. [Google Scholar] [CrossRef]
- Carlin, L.M.; Stamatiades, E.G.; Auffray, C.; Hanna, R.N.; Glover, L.; Vizcay-Barrena, G.; Hedrick, C.C.; Cook, H.T.; Diebold, S.; Geissmann, F. Nr4a1-Dependent Ly6C(Low) Monocytes Monitor Endothelial Cells and Orchestrate Their Disposal. Cell 2013, 153, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Hamers, A.A.J.; Dinh, H.Q.; Thomas, G.D.; Marcovecchio, P.; Blatchley, A.; Nakao, C.S.; Kim, C.; McSkimming, C.; Taylor, A.M.; Nguyen, A.T.; et al. Human Monocyte Heterogeneity as Revealed by High-Dimensional Mass Cytometry. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Venneri, M.A.; de Palma, M.; Ponzoni, M.; Pucci, F.; Scielzo, C.; Zonari, E.; Mazzieri, R.; Doglioni, C.; Naldini, L. Identification of Proangiogenic TIE2-Expressing Monocytes (TEMs) in Human Peripheral Blood and Cancer. Blood 2007, 109, 5276–5285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Zhuang, H.; Lee, P.Y.; Li, M.; Yang, L.; Nigrovic, P.A.; Reeves, W.H. Differential Responsiveness of Monocyte and Macrophage Subsets to Interferon. Arthritis Rheumatol. 2020, 72, 100–113. [Google Scholar] [CrossRef]
- Coillard, A.; Segura, E. In Vivo Differentiation of Human Monocytes. Front. Immunol. 2019, 10, 1907. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Belge, K.U.; von Hundelshausen, P.; Draude, G.; Steppich, B.; Mack, M.; Frankenberger, M.; Weber, K.S.; Ziegler-Heitbrock, H.W. Differential Chemokine Receptor Expression and Function in Human Monocyte Subpopulations. J. Leukoc. Biol. 2000, 67, 699–704. [Google Scholar] [CrossRef]
- Pinho, V.; Italiani, P.; Mitroulis, I.; Kalafati, L.; Bornhäuser, M.; Hajishengallis, G.; Chavakis, T. Regulation of the Bone Marrow Niche by Inflammation. Front. Immunol. 2020, 11, 1540. [Google Scholar] [CrossRef]
- Seneviratne, A.N.; Edsfeldt, A.; Cole, J.E.; Kassiteridi, C.; Swart, M.; Park, I.; Green, P.; Khoyratty, T.; Saliba, D.; Goddard, M.E.; et al. Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis. Circulation 2017, 136, 1140–1154. [Google Scholar] [CrossRef]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The Immunology of Macrophage Activation Syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marketos, N.; Cinoku, I.; Rapti, A.; Mavragani, C.P. Type I Interferon Signature in Sjögren’s Syndrome: Pathophysiological and Clinical Implications. Clin. Exp. Rheumatol. 2019, 37, S185–S191. [Google Scholar]
- Brkic, Z.; Maria, N.I.; van Helden-Meeuwsen, C.G.; van de Merwe, J.P.; van Daele, P.L.; Dalm, V.A.; Wildenberg, M.E.; Beumer, W.; Drexhage, H.A.; Versnel, M.A. Prevalence of Interferon Type I Signature in CD14 Monocytes of Patients with Sjogren’s Syndrome and Association with Disease Activity and BAFF Gene Expression. Ann. Rheum. Dis. 2013, 72, 728–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgoin, P.; Biéchelé, G.; Ait Belkacem, I.; Morange, P.-E.; Malergue, F. Role of the Interferons in CD64 and CD169 Expressions in Whole Blood: Relevance in the Balance between Viral- or Bacterial-Oriented Immune Responses. Immun. Inflamm. Dis. 2020, 8, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Lochte, S.; Piehler, J.; Schreiber, G. IFNAR1 and IFNAR2 Play Distinct Roles in Initiating Type I Interferon-Induced JAK-STAT Signaling and Activating STATs. Sci. Signal. 2021, 14, eabe4627. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, Function, and Differentiation Potential of Human Monocyte Subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [Green Version]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef]
- Nordmark, G.; Eloranta, M.-L.; Ronnblom, L. Primary Sjögren’s Syndrome and the Type I Interferon System. Curr. Pharm. Biotechnol. 2012, 13, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Wildenberg, M.E.; van Helden-Meeuwsen, C.G.; van de Merwe, J.P.; Drexhage, H.A.; Versnel, M.A. Systemic Increase in Type I Interferon Activity in Sjögren’s Syndrome: A Putative Role for Plasmacytoid Dendritic Cells. Eur. J. Immunol. 2008, 38, 2024–2033. [Google Scholar] [CrossRef]
- Bao, M.; Liu, Y.J. Regulation of TLR7/9 Signaling in Plasmacytoid Dendritic Cells. Protein Cell 2013, 4, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; McNamara, N.A. Macrophages: Important Players in Primary Sjögren’s Syndrome? Expert Rev. Clin. Immunol 2014, 10, 513–520. [Google Scholar] [CrossRef]
- Lee, K.E.; Kang, J.H.; Yim, Y.R.; Kim, J.E.; Lee, J.W.; Wen, L.; Park, D.J.; Kim, T.J.; Park, Y.W.; Yoon, K.C.; et al. The Significance of Ectopic Germinal Centers in the Minor Salivary Gland of Patients with Sjögren’s Syndrome. J. Korean Med. Sci. 2016, 31, 190–195. [Google Scholar] [CrossRef]
- Florencia Quiroga, M.; Arbour, N.; Dario Motrich, R.; Ishimaru, N.; Ushio, A.; Arakaki, R.; Otsuka, K.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; et al. CCL22-Producing Resident Macrophages Enhance T Cell Response in Sjögren’s Syndrome. Front. Immunol. 2018, 9, 2594. [Google Scholar] [CrossRef]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and Anti-Inflammatory Forms of Interleukin-1 in the Tear Fluid and Conjunctiva of Patients with Dry-Eye Disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of Human Monocyte Subpopulations in Health and Disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Vakrakou, A.G.; Papadopoulou, A.; Germenis, A.; Kanavakis, E.; Moutsopoulos, H.M.; Manoussakis, M.N. Impaired Degradation and Aberrant Phagocytosis of Necrotic Cell Debris in the Peripheral Blood of Patients with Primary Sjögren’s Syndrome. J. Autoimmun. 2015, 56, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.E.; Janko, C.; Grossmayer, G.E.; Frey, B.; Voll, R.E.; Kern, P.; Kalden, J.R.; Schett, G.; Fietkau, R.; Herrmann, M.; et al. Remnants of Secondarily Necrotic Cells Fuel Inflammation in Systemic Lupus Erythematosus. Arthritis Rheum. 2009, 60, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Grossmayer, G.E.; Munoz, L.E.; Weber, C.K.; Franz, S.; Voll, R.E.; Kern, P.M.; Kalden, J.R.; Schett, G.; Herrmann, M.; Gaipl, U.S. IgG Autoantibodies Bound to Surfaces of Necrotic Cells and Complement C4 Comprise the Phagocytosis Promoting Activity for Necrotic Cells of Systemic Lupus Erythaematosus Sera. Ann. Rheum. Dis. 2008, 67, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Mavragani, C.P.; Crow, M.K. Activation of the Type I Interferon Pathway in Primary Sjogren’s Syndrome. J. Autoimmun. 2010, 35, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Greenwell-Wild, T.; Moutsopoulos, N.M.; Gliozzi, M.; Kapsogeorgou, E.; Rangel, Z.; Munson, P.J.; Moutsopoulos, H.M.; Wahl, S.M. Chitinases in the Salivary Glands and Circulation of Patients with Sjögren’s Syndrome: Macrophage Harbingers of Disease Severity. Arthritis Rheum. 2011, 63, 3103–3115. [Google Scholar] [CrossRef] [Green Version]
- Gliozzi, M.; Greenwell-Wild, T.; Jin, W.; Moutsopoulos, N.M.; Kapsogeorgou, E.; Moutsopoulos, H.M.; Wahl, S.M. A Link between Interferon and Augmented Plasmin Generation in Exocrine Gland Damage in Sjögren’s Syndrome. J. Autoimmun. 2013, 40, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Klinngam, W.; Janga, S.R.; Lee, C.; Ju, Y.; Yarber, F.; Shah, M.; Guo, H.; Wang, D.; MacKay, J.A.; Edman, M.C.; et al. Inhibition of Cathepsin S Reduces Lacrimal Gland Inflammation and Increases Tear Flow in a Mouse Model of Sjögren’s Syndrome. Sci. Rep. 2019, 9, 9559. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, K.; Edman, M.; Schenke-Layland, K.; MacVeigh-Aloni, M.; Janga, S.R.; Schulz, B.; Hamm-Alvarez, S.F. Increased Expression of Cathepsins and Obesity-Induced Proinflammatory Cytokines in Lacrimal Glands of Male NOD Mouse. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5019–5029. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Abad, C.; Martinez, C.; Leceta, J.; Gomariz, R.P. Vasoactive Intestinal Peptide Prevents Experimental Arthritis by Downregulating Both Autoimmune and Inflammatory Components of the Disease. Nat. Med. 2001, 7, 563–568. [Google Scholar] [CrossRef]
- Hauk, V.; Fraccaroli, L.; Grasso, E.; Eimon, A.; Ramhorst, R.; Hubscher, O.; Pérez Leirós, C. Monocytes from Sjögren’s Syndrome Patients Display Increased Vasoactive Intestinal Peptide Receptor 2 Expression and Impaired Apoptotic Cell Phagocytosis. Clin. Exp. Immunol. 2014, 177, 662–670. [Google Scholar] [CrossRef]
- Lodde, B.M.; Mineshiba, F.; Wang, J.; Cotrim, A.P.; Afione, S.; Tak, P.P.; Baum, B.J. Effect of Human Vasoactive Intestinal Peptide Gene Transfer in a Murine Model of Sjogren’s Syndrome. Ann. Rheum. Dis. 2006, 65, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Bruserud, Ø.; Oftedal, B.E.; Wolff, A.B.; Husebye, E.S. AIRE-Mutations and Autoimmune Disease. Curr. Opin. Immunol. 2016, 43, 8–15. [Google Scholar] [CrossRef]
- Mackay, F.; Groom, J.R.; Tangye, S.G. An Important Role for B-Cell Activation Factor and B Cells in the Pathogenesis of Sjögren’s Syndrome. Curr. Opin. Rheumatol. 2007, 19, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Tanaka, M.; Kojima, M.; Setoyama, Y.; Kameda, H.; Suzuki, K.; Tsuzaka, K.; Ogawa, Y.; Tsubota, K.; Abe, T.; et al. Regulatory Mechanisms for the Production of BAFF and IL-6 Are Impaired in Monocytes of Patients of Primary Sjögren’s Syndrome. Arthritis Res. 2011, 13, R170. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, K.; Suzuki, K.; Takei, E.; Ikeda, Y.; Takeuchi, T. Elevated Expression of BAFF Receptor, BR3, on Monocytes Correlates with B Cell Activation and Clinical Features of Patients with Primary Sjögren’s Syndrome. Arthritis Res. 2020, 22, 157. [Google Scholar] [CrossRef]
- Charras, A.; Arvaniti, P.; le Dantec, C.; Dalekos, G.N.; Zachou, K.; Bordron, A.; Renaudineau, Y. JAK Inhibitors and Oxidative Stress Control. Front. Immunol. 2019, 10, 2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The Role of JAK/STAT Signaling Pathway and Its Inhibitors in Diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Pertovaara, M.; Silvennoinen, O.; Isomäki, P. STAT-5 Is Activated Constitutively in T Cells, B Cells and Monocytes from Patients with Primary Sjögren’s Syndrome. Clin. Exp. Immunol. 2015, 181, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.; Cudrici, C.; Zernetkina, V.; Niculescu, F.; Rus, H.; Drachenberg, C.; Rus, V. TRAIL, DR4 and DR5 Are Upregulated in Kidneys from Patients with Lupus Nephritis and Exert Proliferative and Proinflammatory Effects. Clin. Immunol. 2009, 132, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellino, G.; Corallini, F.; Trotta, F.; Secchiero, P. Elevated Levels of TRAIL in Systemic Lupus Erythematosus Are Associated to the Presence of Anti-SSA/SSB Antibodies. Lupus 2007, 16, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Mun, S.; Kim, S.-M.; Shin, W.; Jung, W.; Paek, J.; Lee, J.; Hudson, E.; Reeves, W.H.; Han, K.; et al. The Inflammatory Signature in Monocytes of Sjögren’s Syndrome and Systemic Lupus Erythematosus, Revealed by the Integrated Reactome and Drug Target Analysis. Genes Genom. 2022, 44, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, R.; Zhang, M.; Wang, B.; Liao, Z.; Shi, G.; Li, Y. Abnormal Changes of Monocyte Subsets in Patients With Sjögren’s Syndrome. Front. Immunol. 2022, 13, 864920. [Google Scholar] [CrossRef]
- Matsumura, R.; Umemiya, K.; Kagami, M.; Tomioka, H.; Tanabe, E.; Sugiyama, T.; Sueishi, M.; Kayagaki, N.; Yagita, H.; Okumura, K. Expression of TNF-Related Apoptosis Inducing Ligand (TRAIL) on Infiltrating Cells and of TRAIL Receptors on Salivary Glands in Patients with Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2002, 20, 791–798. [Google Scholar]
- Chen, W.S.; Lin, K.C.; Chen, C.H.; Liao, H.T.; Wang, H.P.; Li, W.Y.; Lee, H.T.; Tsai, C.Y.; Chou, C.T. Autoantibody and Biopsy Grading Are Associated with Expression of ICAM-1, MMP-3, and TRAIL in Salivary Gland Mononuclear Cells of Chinese Patients with Sjogren’s Syndrome. J. Rheumatol. 2009, 36, 989–996. [Google Scholar] [CrossRef]
- Lopes, A.P.; Bekker, C.P.J.; Hillen, M.R.; Blokland, S.L.M.; Hinrichs, A.C.; Pandit, A.; Kruize, A.A.; Radstake, T.R.D.J.; van Roon, J.A.G. The Transcriptomic Profile of Monocytes from Patients with Sjögren’s Syndrome Is Associated with Inflammatory Parameters and Is Mimicked by Circulating Mediators. Front. Immunol. 2021, 12, 701656. [Google Scholar] [CrossRef]
- Maria, N.I.; Brkic, Z.; Waris, M.; van Helden-Meeuwsen, C.G.; Heezen, K.; van de Merwe, J.P.; van de Merwe, P.L.; Dalm, V.A.S.H.; Drexhage, H.A.; Versnel, M.A. MxA as a Clinically Applicable Biomarker for Identifying Systemic Interferon Type i in Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2014, 73, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Rose, T.; Szelinski, F.; Lisney, A.; Reiter, K.; Fleischer, S.J.; Burmester, G.R.; Radbruch, A.; Hiepe, F.; Grützkau, A.; Biesen, R.; et al. SIGLEC1 Is a Biomarker of Disease Activity and Indicates Extraglandular Manifestation in Primary Sjögren’s Syndrome. RMD Open 2016, 2, e000292. [Google Scholar] [CrossRef] [Green Version]
- Duroux-Richard, I.; Robin, M.; Peillex, C.; Apparailly, F. MicroRNAs: Fine Tuners of Monocyte Heterogeneity. Front. Immunol. 2019, 10, 2145. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory Network of MiRNA on Its Target: Coordination between Transcriptional and Post-Transcriptional Regulation of Gene Expression. Cell Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. Transcription and Processing of Human MicroRNA Precursors. Mol. Cell 2004, 16, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Boldin, M.P.; Taganov, K.D.; Rao, D.S.; Yang, L.; Zhao, J.L.; Kalwani, M.; Garcia-Flores, Y.; Luong, M.; Devrekanli, A.; Xu, J.; et al. MiR-146a Is a Significant Brake on Autoimmunity, Myeloproliferation, and Cancer in Mice. J. Exp. Med. 2011, 208, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Shibuya, H.; Nagata, Y.; Niimoto, T.; Ochi, M. The Inhibitory Effect of MicroRNA-146a Expression on Bone Destruction in Collagen-Induced Arthritis. Arthritis Rheum. 2011, 63, 1582–1590. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-KappaB-Dependent Induction of MicroRNA MiR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, G.; Zhang, L.; Zhao, M.; Huang, H. Decreased MicroRNA-181a and -16 Expression Levels in the Labial Salivary Glands of Sjögren Syndrome Patients. Exp. Med. 2018, 15, 426–432. [Google Scholar] [CrossRef]
- Williams, A.E.G.; Choi, K.; Chan, A.L.; Lee, Y.J.; Reeves, W.H.; Bubb, M.R.; Stewart, C.M.; Cha, S. Sjögren’s Syndrome-Associated MicroRNAs in CD14(+) Monocytes Unveils Targeted TGFβ Signaling. Arthritis Res. 2016, 18, 95. [Google Scholar] [CrossRef] [Green Version]
- Arsura, M.; Wu, M.; Sonenshein, G.E. TGF Beta 1 Inhibits NF-Kappa B/Rel Activity Inducing Apoptosis of B Cells: Transcriptional Activation of I Kappa B Alpha. Immunity 1996, 5, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Black, J.C.; van Rechem, C.; Whetstine, J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Smolle, M.; Workman, J.L. Transcription-Associated Histone Modifications and Cryptic Transcription. Biochim. Biophys. Acta 2013, 1829, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Imgenberg-Kreuz, J.; Sandling, J.K.; Almlöf, J.C.; Nordlund, J.; Signér, L.; Norheim, K.B.; Omdal, R.; Rönnblom, L.; Eloranta, M.L.; Syvänen, A.C.; et al. Genome-Wide DNA Methylation Analysis in Multiple Tissues in Primary Sjögren’s Syndrome Reveals Regulatory Effects at Interferon-Induced Genes. Ann. Rheum. Dis. 2016, 75, 2029–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Peng, Y.; Chen, Y.-Y.; Wang, A.-Q.; Deng, C.-W.; Peng, L.-Y.; Wu, Q.-J.; Zhao, Y.; Fei, Y.-Y.; Zhang, W. Genome-Wide DNA Methylation Patterns in Monocytes Derived from Patients with Primary Sjogren Syndrome. Chin. Med. J. 2021, 134, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, A.; Li, T.; Kerick, M.; Català-Moll, F.; Comet, N.R.; Rodríguez-Ubreva, J.; de La Rica, L.; Branco, M.R.; Martín, J.; Ballestar, E. TET2- and TDG-Mediated Changes Are Required for the Acquisition of Distinct Histone Modifications in Divergent Terminal Differentiation of Myeloid Cells. Nucleic Acids Res. 2017, 45, 10002–10017. [Google Scholar] [CrossRef] [PubMed]
- Gamrekelashvili, J.; Giagnorio, R.; Jussofie, J.; Soehnlein, O.; Duchene, J.; Briseño, C.G.; Ramasamy, S.K.; Krishnasamy, K.; Limbourg, A.; Kapanadze, T.; et al. Regulation of Monocyte Cell Fate by Blood Vessels Mediated by Notch Signalling. Nat. Commun. 2016, 7, 12597. [Google Scholar] [CrossRef] [Green Version]
- Ohishi, K.; Varnum-Finney, B.; Serda, R.E.; Anasetti, C.; Bernstein, I.D. The Notch Ligand, Delta-1, Inhibits the Differentiation of Monocytes into Macrophages but Permits Their Differentiation into Dendritic Cells. Blood 2001, 98, 1402–1407. [Google Scholar] [CrossRef] [Green Version]
- Vasamsetti, S.B.; Karnewar, S.; Kanugula, A.K.; Thatipalli, A.R.; Kumar, J.M.; Kotamraju, S. Metformin Inhibits Monocyte-to-Macrophage Differentiation via AMPK-Mediated Inhibition of STAT3 Activation: Potential Role in Atherosclerosis. Diabetes 2015, 64, 2028–2041. [Google Scholar] [CrossRef] [Green Version]
- Seror, R.; Nocturne, G.; Mariette, X. Current and Future Therapies for Primary Sjögren Syndrome. Nat. Rev. Rheumatol. 2021, 17, 475–486. [Google Scholar] [CrossRef]
- Gottenberg, J.-E.; Ravaud, P.; Puéchal, X.; le Guern, V.; Sibilia, J.; Goeb, V.; Larroche, C.; Dubost, J.-J.; Rist, S.; Saraux, A.; et al. Effects of Hydroxychloroquine on Symptomatic Improvement in Primary Sjögren Syndrome: The JOQUER Randomized Clinical Trial. JAMA 2014, 312, 249–258. [Google Scholar] [CrossRef]
- Winzer, M.; Aringer, M. Use of Methotrexate in Patients with Systemic Lupus Erythematosus and Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2010, 28, S156-9. [Google Scholar]
- Price, E.J.; Rigby, S.P.; Clancy, U.; Venables, P.J. A Double Blind Placebo Controlled Trial of Azathioprine in the Treatment of Primary Sjögren’s Syndrome. J. Rheumatol. 1998, 25, 896–899. [Google Scholar] [PubMed]
- Drosos, A.A.; Skopouli, F.N.; Galanopoulou, V.K.; Kitridou, R.C.; Moutsopoulos, H.M. Cyclosporin a Therapy in Patients with Primary Sjögren’s Syndrome: Results at One Year. Scand. J. Rheumatol. Suppl. 1986, 61, 246–249. [Google Scholar] [PubMed]
- Bikker, A.; van Woerkom, J.-M.; Kruize, A.A.; van der Wurff-Jacobs, K.M.G.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; van Roon, J.A.G. Clinical Efficacy of Leflunomide in Primary Sjogren’s Syndrome Is Associated with Regulation of T-Cell Activity and Upregulation of IL-7 Receptor α Expression. Ann. Rheum. Dis. 2012, 71, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Van Woerkom, J.M.; Kruize, A.A.; Geenen, R.; van Roon, E.N.; Goldschmeding, R.; Verstappen, S.M.M.; van Roon, J.A.G.; Bijlsma, J.W.J. Safety and Efficacy of Leflunomide in Primary Sjögren’s Syndrome: A Phase II Pilot Study. Ann. Rheum. Dis. 2007, 66, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Lin, J. Mycophenolate for the Treatment of Primary Sjögren’s Syndrome. J. Transl. Int. Med. 2020, 8, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.M.; Meiners, P.M.; Vissink, A.; Spijkervet, F.K.L.; Abdulahad, W.; Kamminga, N.; Brouwer, E.; Kallenberg, C.G.M.; Bootsma, H. Effectiveness of Rituximab Treatment in Primary Sjögren’s Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 2010, 62, 960–968. [Google Scholar] [CrossRef]
- Devauchelle-Pensec, V.; Mariette, X.; Jousse-Joulin, S.; Berthelot, J.-M.; Perdriger, A.; Puéchal, X.; le Guern, V.; Sibilia, J.; Gottenberg, J.-E.; Chiche, L.; et al. Treatment of Primary Sjögren Syndrome with Rituximab: A Randomized Trial. Ann. Intern. Med. 2014, 160, 233–242. [Google Scholar] [CrossRef]
- Bowman, S.J.; Everett, C.C.; O’Dwyer, J.L.; Emery, P.; Pitzalis, C.; Ng, W.-F.; Pease, C.T.; Price, E.J.; Sutcliffe, N.; Gendi, N.S.T.; et al. Randomized Controlled Trial of Rituximab and Cost-Effectiveness Analysis in Treating Fatigue and Oral Dryness in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2017, 69, 1440–1450. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Rivas, N.; Sang-Park, H.; Díaz Del Campo, P.; Fernández-Castro, M.; Corominas, H.; Andreu, J.L.; Navarro-Compán, V. Efficacy of Belimumab in Primary Sjögren’s Syndrome: A Systematic Review. Reum. Clin. 2021, 17, 170–174. [Google Scholar] [CrossRef]
- Chevalier, K.; Belkhir, R.; Seror, R.; Mariette, X.; Nocturne, G. Efficacity of a Sequential Treatment by Anti-CD 20 Monoclonal Antibody and Belimumab in Type II Cryoglobulinaemia Associated with Primary Sjögren Syndrome Refractory to Rituximab Alone. Ann. Rheum. Dis. 2020, 79, 1257–1259. [Google Scholar] [CrossRef]
- De Vita, S.; Quartuccio, L.; Salvin, S.; Picco, L.; Scott, C.A.; Rupolo, M.; Fabris, M. Sequential Therapy with Belimumab Followed by Rituximab in Sjögren’s Syndrome Associated with B-Cell Lymphoproliferation and Overexpression of BAFF: Evidence for Long-Term Efficacy. Clin. Exp. Rheumatol. 2014, 32, 490–494. [Google Scholar] [PubMed]
- Bowman, S.J.; Fox, R.; Dörner, T.; Mariette, X.; Papas, A.; Grader-Beck, T.; Fisher, B.A.; Barcelos, F.; de Vita, S.; Schulze-Koops, H.; et al. Safety and Efficacy of Subcutaneous Ianalumab (VAY736) in Patients with Primary Sjögren’s Syndrome: A Randomised, Double-Blind, Placebo-Controlled, Phase 2b Dose-Finding Trial. Lancet 2022, 399, 161–171. [Google Scholar] [CrossRef]
- Meiners, P.M.; Vissink, A.; Kroese, F.G.M.; Spijkervet, F.K.L.; Smitt-Kamminga, N.S.; Abdulahad, W.H.; Bulthuis-Kuiper, J.; Brouwer, E.; Arends, S.; Bootsma, H. Abatacept Treatment Reduces Disease Activity in Early Primary Sjögren’s Syndrome (Open-Label Proof of Concept ASAP Study). Ann. Rheum. Dis. 2014, 73, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; Brennan, M.T.; Kok, M.R.; Leakan, R.A.; Smith, J.A.; Manny, J.; Baum, B.J.; Pillemer, S.R. Etanercept in Sjögren’s Syndrome: A Twelve-Week Randomized, Double-Blind, Placebo-Controlled Pilot Clinical Trial. Arthritis Rheum. 2004, 50, 2240–2245. [Google Scholar] [CrossRef]
- Mariette, X.; Ravaud, P.; Steinfeld, S.; Baron, G.; Goetz, J.; Hachulla, E.; Combe, B.; Puéchal, X.; Pennec, Y.; Sauvezie, B.; et al. Inefficacy of Infliximab in Primary Sjögren’s Syndrome: Results of the Randomized, Controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis Rheum. 2004, 50, 1270–1276. [Google Scholar] [CrossRef]
- IL-6 Receptor Inhibition in Primary Sjögren Syndrome: Results from a Randomized Multicenter Academic Double Blind Placebo-Controlled Trial of Tocilizumab in 110 Patients-ACR Meeting Abstracts. Available online: https://acrabstracts.org/abstract/il-6-receptor-inhibition-in-primary-sjogren-syndrome-results-from-a-randomized-multicenter-academic-double-blind-placebo-controlled-trial-of-tocilizumab-in-110-patients/ (accessed on 27 September 2022).
- St.Clair, E.W.; Baer, A.N.; Wei, C.; Noaiseh, G.; Parke, A.; Coca, A.; Utset, T.O.; Genovese, M.C.; Wallace, D.J.; McNamara, J.; et al. Clinical Efficacy and Safety of Baminercept, a Lymphotoxin β Receptor Fusion Protein, in Primary Sjögren’s Syndrome: Result from a Phase II Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2018, 70, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Safety, Tolerability, Pharmacokinetics, and Therapeutic Efficacy of SAR441344 in Primary Sjögren’s Syndrome (PSjS) (PhaethuSA). US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT04572841 (accessed on 29 September 2022).
- Juarez, M.; Diaz, N.; Johnston, G.I.; Nayar, S.; Payne, A.; Helmer, E.; Cain, D.; Williams, P.; Devauchelle-Pensec, V.; Fisher, B.A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Study of Oral Seletalisib in Primary Sjögren’s Syndrome. Rheumatology 2021, 60, 1364–1375. [Google Scholar] [CrossRef]
- Safety of Tofacitinib, an Oral Janus Kinase Inhibitor, in Primary Sjogren’s Syndrome-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04496960 (accessed on 27 September 2022).
- Bai, W.; Liu, H.; Dou, L.; Yang, Y.; Leng, X.; Li, M.; Zhang, W.; Zhao, Y.; Zeng, X. Pilot Study of Baricitinib for Active Sjogren’s Syndrome. Ann. Rheum. Dis. 2022, 81, 1050–1052. [Google Scholar] [CrossRef]
- Narain, S.; Berman, N.; Furie, R. Biologics in the Treatment of Sjogren’s Syndrome, Systemic Lupus Erythematosus, and Lupus Nephritis. Curr. Opin. Rheumatol. 2020, 32, 609–616. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Anifrolumab Treatment for 24 Weeks in Patients with Primary Sjögren’s Syndrome (ANISE-II). US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT05383677 (accessed on 29 September 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequí-Sabater, J.M.; Beretta, L. Defining the Role of Monocytes in Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 12765. https://doi.org/10.3390/ijms232112765
Sequí-Sabater JM, Beretta L. Defining the Role of Monocytes in Sjögren’s Syndrome. International Journal of Molecular Sciences. 2022; 23(21):12765. https://doi.org/10.3390/ijms232112765
Chicago/Turabian StyleSequí-Sabater, Jose Miguel, and Lorenzo Beretta. 2022. "Defining the Role of Monocytes in Sjögren’s Syndrome" International Journal of Molecular Sciences 23, no. 21: 12765. https://doi.org/10.3390/ijms232112765
APA StyleSequí-Sabater, J. M., & Beretta, L. (2022). Defining the Role of Monocytes in Sjögren’s Syndrome. International Journal of Molecular Sciences, 23(21), 12765. https://doi.org/10.3390/ijms232112765




